CD26, which is a costimulatory molecule and peptidase, is responsible for the degradation of interferon (IFN)-γ-induced chemokines. To elucidate the immunopathological role of CD26 in allergic asthma, we investigated plasma soluble CD26 (sCD26) concentration and its cell surface expression on lymphocytes, monocytes, CD4+ T helper, CD8+ T suppressor plus cytotoxic T, invariant natural killer T (iNKT), and CD19+ B lymphocytes in allergic asthmatic patients. Plasma sCD26 was significantly elevated in asthmatic patients regardless of inhaled corticosteroid treatment (all P < 0.05). Cell surface expression of CD26 was significantly up-regulated on lymphocytes, especially on CD4+ and iNKT lymphocytes (all P < 0.05), but not on other cell types. Significant positive correlations were found between sCD26 and the percentage of eosinophils, Th2-related chemokines CCL5 and CCL22, and costimulatory molecule sCTLA-4 (all P < 0.05). In conclusion, the aberrant expression of CD26 may contribute to the inflammatory process and Th2 predominance in the immunopathogenesis of allergic asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Allergic asthma is characterized by infiltration into the airway submucosa of leukocytes, which contributes to airway obstruction and bronchial hyperresponsiveness (1). Its pathogenesis is centrally mediated by the activation, differentiation, and chemotaxis of T lymphocytes, which is regulated by cytokines and chemokines (2). The degradation of such cytokines by peptidases, such as CD13 and CD26, is an important mechanism to modulate peptide-mediated inflammation in asthma (3, 4).
CD26, a membrane-anchored ectoenzyme with dipeptidyl peptidase IV activity in its extracellular domain, is a 110-kDa transmembrane glycoprotein, which belongs to the serine protease family. It acts to selectively remove the N-terminal dipeptide from peptides with proline or alanine in the penultimate position (5). CD26 alone cannot directly degrade intact cytokines such as IL-1β, IL-2, and IL-6 under in vitro conditions. It can only degrade synthetic oligopeptides with sequences analogous to the N-terminal part of these natural or recombinant cytokines. For the hydrolysis of these cytokines, a concerted action of both CD26 and another CD26-related enzyme peptidase aminopeptidase N is needed (6). A number of chemokines are also substrates of CD26 including CXCL9 (monokine induced by γ-inteferon, MIG) and CXCL10 (interferon (IFN)-γ inducible protein-10, IP-10) (5). CD26 is expressed on a variety of tissues including endothelial, epithelial, and T-cells, with a preferential expression on the subset of CD4+ memory T-cells (5). It is up-regulated after T-cell activation and is highly expressed on activated T helper (Th) type 1 lymphocytes (7). CD26 also exists as a secreted isoform, soluble CD26 (sCD26), which lacks the cytoplasmic tail, transmembrane region, and circulates in plasma (8).
The cell surface expression of CD26 has been shown to positively correlate with the production of Th1 cytokines by T-cell clones. Also, its expression is induced by stimuli favoring the development of Th1 response (9–11). The number of CD26+ T lymphocytes is increased in the inflamed tissues, such as thyroid and synovial fluids, of patients with autoimmune diseases such as rheumatoid arthritis (12). Despite the substantial evidence on the involvement of CD26 in Th1-related diseases, its role as costimulatory factor and peptidase with regard to the pathogenesis of asthma is not yet fully understood.
Although the dual role of Th2 lymphocytes and eosinophils is widely accepted as being critical for the onset and progression of the pathogenesis of allergic asthma, recent studies have suggested a role of costimulatory molecule CD26 in the pathogenesis by enhancing Th2-dependent allergic inflammation (13). Originally described as a T-cell activation molecule, CD26 as a costimulatory molecule have been studied extensively in vitro (5). The optimal activation of T-cells require two signals—a primary signal delivered by the antigenic peptide presented by the major histocompatibility complex (MHC) molecules, and a nonspecific signal generated by the interaction of costimulatory molecules such as cytotoxic T-lymphocyte-associated antigen (CTLA)-4 and CD28 (14, 15). It has been suggested that allergen-sensitized CD26+ Th1 lymphocytes produce IFN-γ and Th1-polarized dendritic cells, which are later capable of augmenting allergen-induced pulmonary inflammation (13).
Natural killer T (NKT) cells is a subset of T-cells that exhibit both properties of natural killer cells and conventional T-cells. A majority of NKT cells expressing an invariant T-cell receptor, which is restricted to interactions with CD1d, are known as the invariant NKT (iNKT) cells. Upon activation, these cells produce a large amount of cytokines IL-2, tumor necrosis factors (TNF)-α, IL-4, and IFN-γ (16). Recent studies have shown activated iNKT cells causes exacerbation of allergic airway inflammation in mouse models, and in addition, the number of these cells are found to be increased in the lungs of patients with chronic asthma (17).
To elucidate the immunopathological role of CD26 in the pathogenesis of allergic asthma, we investigated the plasma concentration of sCD26 and cell surface expression of CD26 on lymphocytes, monocytes, CD4+ T helper, CD8+ T suppressor plus cytotoxic T lymphocytes, CD19+ B lymphocytes, and iNKT lymphocytes in patients with allergic asthma treated or not treated with inhaled corticosteroid treatment (ICS). Both Th2-related chemokine CCL5 (regulated upon activation normal T-cell expressed and secreted (RANTES)) and CCL22 (macrophage-derived chemokine (MDC)) can be truncated by CD26 (18, 19), and these Th2-related chemokines in patients with allergic asthma have been studied by our group. Our previous results indicated a significant elevation of plasma CCL5 and CCL22, and the elevation showed positive correlation with GINA disease severity score (20, 21). For assessing the role of CD26 in Th2 predominance in allergic asthma, the association between plasma concentration of sCD26 and other costimulatory molecules in plasma such as sCTLA-4 and sCD28 and Th2-related chemokines CCL5, CCL11 (eotaxin), and CCL22 were also investigated in this study cohort.
MATERIALS AND METHODS
Asthmatic Patients, Control Subjects,and Blood Samples
Fifty-one Chinese adult patients with allergic asthma were recruited from the Asthma Clinic of the Prince of Wales Hospital, Hong Kong. The atopic status of these patients was ascertained by positive serum-specific IgE assays to house dust mite (Dermatophagoides pteronyssinus 1, Der p 1), cat, dog, mixed cockroaches, and mixed moulds by fluorescence enzyme immunoassay (AutoCAP analyzer, Pharmacia Diagnostics AB, Uppsale, Sweden) (22). Diagnosis of asthma was based on the guidelines of the American Thoracic Society (23), and lung function of the subjects was assessed by spirometry (Model S, Vitalograph, Buckingham, UK) according to the American Thoracic Society standards (24). Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio were measured before and 15 min after the inhalation of salbutamol (Glaxo Operations Ltd., Greenford, UK). The results were compared with the local predicted age- and sex-matched values (25). The severity of asthma in these patients was assessed according to the Global Initiative for Asthma (GINA) guidelines (26, 27). All our studied asthmatic patients were on short acting bronchodilator as needed. Some of them were on inhaled corticosteroid such as beclomethasone dipropionate (Becloforte; Glaxo Wellcome, Research Triangle Park, NC, USA) or budesonide (Pulmicort; AstraZeneca, London, UK). All patients and control subjects had no oral intake of steroid or change of asthma medications for 4 weeks before recruitment of study. Thirty-five sex- and age-matched nonasthmatic healthy Chinese volunteers were recruited as control subjects. All subjects were nonsmokers and free from upper respiratory tract infection for 4 weeks before recruitment. Nine milliliters (mL) of venous peripheral ethylenediaminetetraacetic acid (EDTA) blood and 5 mL of clotted blood were collected from each participant. Aliquots of whole blood were processed immediately for peripheral blood mononuclear cell (PBMC) isolation. Plasma and serum samples were preserved at −70°C for subsequent assays. The above protocol was approved by the clinical research ethics committee of the Chinese University of Hong Kong—New Territories East Cluster Hospitals, and informed consent was obtained from all participants according to the Declaration of Helsinki.
Assays for sCD26, sCTLA-4, sCD28, CCL5, CCL22, and CCL11 in Plasma
Plasma sCD26, sCTLA-4, sCD28, CCL11, and CCL22 concentration was measured using enzyme-linked immunosorbant assay (ELISA) reagents from Bender Medsystems Diagnostics GmbH, Vienna, Austria and R&D Systems Inc., MN, USA, respectively. CCL5 was assayed by human chemokine cytometric bead array (BD Pharmingen, CA, USA) using flow cytometry (BD Calibur flow cytometer) (20, 28).
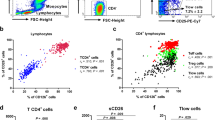
Cell Surface Expression of CD26 on CD4+, CD19+, CD8+, and iNKT Cells
PBMC were prepared by centrifugation of blood using a density gradient (Ficoll-Paque Plus; Amersham Pharmacia Biotech Ltd., Uppsala, Sweden). The cells were incubated with fluorescein isothiocyanate (FITC)-conjugated CD26-specific monoclonal antibody (mAb), followed by phycoerythrin (PE)-conjugated CD4, allophycocyanin (APC)-conjugated CD19 and peridinin-chlorophyll-protein (PerCP)-conjugated CD8 antibodies (Becton Dickinson Biosciences Pharmingen). FITC-conjugated mouse immunoglobulin (Ig) G was used as isotypic control. The typical forward and side scatter gates for lymphocytes were set to exclude contaminating cells from the analysis.
For the study of cell surface expression of CD26 on iNKT cells, PBMC were incubated with purified CD26-specific mAb (BD Pharmingen) followed by APC-conjugated secondary antibody (R&D Systems). These cells were subsequently incubated with PerCP-conjugated CD3 (BD Pharmingen), FITC-conjugated Vα24 and PE-conjugated Vβ11 antibodies (Beckman Coulter, Fullerton, CA) for the identification of iNKT cells.
The expression of CD26 on CD4+, CD19+, iNKT, and CD8+ cells was then analyzed by flow cytometry (BD FACSCalibur). Altogether, 5000 events were collected in the log mode and were expressed as mean fluorescence intensity (MFI).
Statistical Analysis
The Mann–Whitney U test was used to assess the differences of data between the asthmatic patients and the control subjects. The Kruskal–Wallis test followed, when significance arose, by Dunn’s test for pair-wise comparisons of data between asthmatic patients with or without receiving ICS and the control subjects. Spearman correlation test was used for correlation analysis between parameters. All analyses were performed using statistical software (SPSS for Windows, version 9.0, SPSS Inc., Chicago, IL). Probability (P) values of less than 0.05 were considered significant. Unless otherwise specified, results are expressed as the median (interquartile range (IQR)).
RESULTS
Asthma Severity and Atopic Status
The characteristics of the study groups are summarized in Table I. The mean ± SD FEV1 of all asthmatic patients was 2.5 ± 0.9 L/min (85.9 ± 16.9% of predicted normal values), while the FEV1/FVC ratio was 84.1 ± 8.2%. The severity of asthma in these patients according to GINA comprised: intermittent asthma 9 patients (17.7%), mild persistent asthma 6 patients (11.8%), moderate persistent asthma 18 patients (35.3%), and severe persistent asthma 18 patients (35.3%).
Plasma sCD26
As shown in Fig. 1, sCD26 was present in plasma. Its concentration was significantly higher in allergic asthmatic patients than control subjects [526 (390–658) versus 398 (316–504) ng/mL; P < 0.001] (Fig. 1A). It was significantly higher in both ICS-treated [515 (390–687) versus 398 (316 504) ng/mL; P < 0.01] and non-ICS-treated asthmatic patients than that of control subjects [542 (390–637) versus 398 (316–504) ng/mL; P < 0.05] (Fig. 1B).
Concentration of sCD26 in plasma. Box and whisker plots showing the comparison of sCD26 between control subjects and (A) all asthmatic patients, with differences determined by Mann–Whitney U Test, and (B) asthmatic patients receiving ICS and those not receiving therapy, with differences determined by Kruskal–Wallis test followed by Dunn’s test. Differences were considered significant when P < 0.05. * P < 0.05, ** P < 0.01, *** P < 0.001.
Plasma concentration of sCD26 and sCTLA-4 were found to have a strongly significant and positive correlation (r = 0.47, P < 0.01) (Table II). However, there were no significant correlations between sCD26 and sCD28 (P > 0.05). We previously found that plasma concentrations of both sCTLA-4 and sCD28 were elevated in asthmatic patients (28). We also observed that there was a significant and positive correlation between plasma concentration of sCD26 and the percentage of circulating eosinophils in white blood cells (r = 0.43, P < 0.01) (Table II).
There were significant and positive correlations between plasma sCD26 and Th2 chemoattractants CCL5 and CCL22 (CCL5: r = 0.25, P < 0.05; CCL22: r = 0.56, P < 0.05) (Table II). We have shown the elevated plasma concentration of CCL5 and CCL22 in asthmatic patients than control subjects (20, 21). Although the concentration of plasma CCL11 was significantly elevated in allergic asthmatic patients compared with control subjects [67.45 (51.39–87.28) versus 50.92 (43.19–65.95) pg/mL, P < 0.01], no significant correlation was found between plasma CCL11 and sCD26 (r = 0.096, P > 0.05).
In addition, we observed an increase in plasma sCD26 concentration along with the progression of disease severity. Asthmatic patients with GINA severity score of 1, in which 6% of these patients were treated with inhaled corticosteroids, showed a median sCD26 concentration of 423 pg/mL; while those patients with higher GINA severity scores of 2, 3, or 4, in which 33, 73, and 92% of these patients were treated with inhaled corticosteroids, showed median sCD26 concentrations of 576, 544, and 540 pg/mL, respectively, which were higher than that of patients with GINA severity score of 1.
Expression of CD26 on Cell Surface of Lymphocytes, Monocytes, CD4+, CD19+, CD8+, and iNKT Lymphocytes
As shown in Fig. 2, there were no significant differences in cell surface expression of CD26 on monocytes and CD8+ and CD19+ lymphocytes between allergic asthmatic patients and control subjects (P > 0.05). However, there was a significant up-regulation in cell surface expression of CD26 on total lymphocytes of asthmatic patients than that of control subjects [7.71 (6.51–8.79) versus 5.12 (3.90–6.19) MFI, P < 0.01]. Using immunophenotyping of CD4 T lymphocytes by flow cytometry, we observed that the CD4+ lymphocytes of allergic asthmatic patients showed significant up-regulation in cell surface expression of CD26 than that of control subjects [12.97 (11.80–16.45) versus 8.81 (6.81–9.81) MFI, P < 0.01]. Results, therefore, indicate that CD26 expression is significantly elevated on CD4+ lymphocytes of allergic asthmatic patients. The cell surface expression of CD26 on iNKT lymphocytes was also significantly higher in asthmatic patients than that of control subjects [26.49 (2.18–37.11) versus 2.03 (1.13–3.87) MFI, P < 0.05].
The cell surface expression of CD26 was analyzed by flow cytometry as MFI on 10,000 viable cells, which included both the significant increase of CD26 expression on individual cell and the percentage of cells expressing the CD26 (both P < 0.05).
DISCUSSION
This study demonstrated an up-regulation of plasma concentration and cell surface expression of CD26 in patients with allergic asthma. In addition, plasma sCD26 showed positive correlation with the percentage of circulating eosinophils, plasma sCTLA-4, CCL5, and CCL22.
The bioavailability of cytokines for mediating inflammation depends on their production, stability, and degradation. Therefore, the presence of peptidases plays a role in determining the availability of cytokines at the site of inflammation. This study is the first to show sCD26 in plasma is significantly elevated in patients with allergic asthma regardless of ICS treatment. Both the membrane-bound and soluble forms of CD26 can rapidly truncate Th1-related chemokine CXCL10; the resulting CXCL10 isoform contains no inflammatory activity (4). Truncation of chemokines may not affect receptor binding, but influencing their ability to induce signaling, can result in antagonist function (29). It is well recorded that leukocyte infiltration in the pathogenesis of allergic asthma is regulated by chemoattractants of eosinophils and Th2 lymphocytes such as eotaxin and CCL5 (30). It is also widely known that chemoattractants of Th1 and Th2 lymphocytes such as Th1-related CXCL9, CXCL10, and Th2-related CCL17 (thymus and activation regulated chemokine (TARC)), CCL22 counteract each other (20). Thus, the elevation of sCD26 suggests an enhanced truncation of the Th1 chemoattractants, which contributes to a diminished antagonizing effect on Th2 chemoattractants, and hence, the pathological process of Th2-related leukocyte infiltration. This suggestion is in concordance with the hypothesis that CD26 decreases Th1 chemokine receptor CXCR3 binding by processing the IFN-γ-induced chemokines such as CXCL9 and CXCL10, thus, abolishing the chemotaxis of Th1 cells (4). This is further supported by our previous findings that these IFN-γ-induced chemokines are diminished in expression in patients with allergic asthma (20). We found no significant correlation between plasma concentrations of sCD26 and Th1--related chemokine CXCL10 (r = 0.045, P = 0.699) and CXCL9 (r = 0.163, P = 0.16) (data not shown). Moreover, there was also no significant correlation between plasma concentrations of sCD26 and neutrophil-related chemokines CXCL8 (r = 0.138, P = 0.240) and CXCL5 (r = 0.204, P = 0.317) (data not shown). In addition, the association between plasma sCD26 and the Th2 chemoattractants CCL5 and CCL22 in this study further suggest a role of sCD26 in the Th2-mediated pathogenesis of asthma. Together with our previous results of the positive correlation of CCL5 and CCL22 with disease severity (20, 21), we, therefore, suggested that these two Th2 chemokines may serve as potential surrogate disease marker of allergic asthma. Together with the elevated cell surface CD26 expression on CD4+ T lymphocytes, it is likely that CD26 exert a role in regulating these chemokines in asthma.
Soluble CD26 may originate from various sources such as endothelial, epithelial cells from liver or kidney in addition to circulating leukocytes (5). Although, in this study, the origin of sCD26 in plasma is not completely elucidated, a previous study has shown that, by measuring the electrophoretic mobility of the enzymatic activity of CD26, the serum sCD26 coincides with that of lymphocyte CD26 in healthy subjects (5). However, it is still uncertain whether the activity of this enzyme will be changed in pathological conditions.
CD86, produced from antigen-presenting cells (APC), is one of the ligands for CTLA-4 and CD28, which has been shown to be involved in the exacerbation of allergic asthma (28). In this study, the expression of CD26 on cell surface was found to be specifically elevated on CD4+ T lymphocytes. Previous findings have shown that the expression of cell surface CD26 was increased after T-cell stimulation (31). The elevated expression of cell surface CD26 in our present study suggests an enhanced activation of CD4+ lymphocytes in patients with allergic asthma. In addition, previous studies have demonstrated an up-regulation of CD86 expression on APC by CD26 activation (32). This provides greater APC-T lymphocyte interaction, which can enhance T lymphocyte proliferation. The elevated expression of CD26 is in concordance with our previous study demonstrating an enhanced expression of CD86 in patients with allergic asthma (28). Other studies have suggested a role of CTLA-4 in Th2 activation in asthmatic patients (28, 33). Interestingly, sCD26 showed positive correlation with sCTLA-4 in this study, which suggests a role of CD26 in the Th2-mediated pathogenesis of asthma.
Box and whisker plots showing the cell surface expression of CD26 on lymphocytes, monocytes, CD4 +, CD8 +, and CD19 + cells. Comparisons were made between the control subjects and asthmatic patients of CD26 on (A) total lymphocytes, (B) total monocytes, (C) CD4 + T helper, (D) CD8+ T suppressor plus cytotoxic, (E) CD19 + B, and (F) iNKT lymphocyte populations, with differences determined by Mann–Whitney U Test. Differences were considered significant when P < 0.05. **P < 0.01.
iNKT cells, recently identified to play an essential role in the development of allergen-induced airway hyperreactivity in mouse models of allergic asthma, are found to be enriched in the lungs of asthmatic patients (17). The elevated expression of cell surface CD26 on iNKT cells suggested an enhanced activation of these cells, probably leading to an induction of Th2 cytokines, in patients with allergic asthma.
Moreover, it has been shown that CD26-deficient rats showed lower T-cell counts and serum-specific IgE levels (34), which is in line with our previous findings that patients with allergic asthma exhibited significantly higher serum IgE levels (20). In our present study, the significant positive correlation between sCD26 and the percentage of circulating eosinophils indicates that sCD26 maybe related to the exacerbation of allergic inflammation. This suggests that therapeutic drugs that target on sCD26 may alleviate the allergic inflammation. The aberrant expression of CD26 has been shown to be clinically relevant for various diseases such as the differentiated thyroid carcinoma, chronic hepatitis C virus infection, rheumatoid arthritis, diabetes, depression, and atopic dermatitis (5).
CD26 in BAL has been studied in rat asthma model, suggesting the role of CD26 in the pathogenesis of asthma via Th2-cell-dependent processes (34). Elevation of the expression of two CD26-related peptidases, amino-peptidase N and dipeptidyl peptidase IV has also been shown in human bronchus using immunohistochemical enzyme histochemical staining (3). Together with our present results using peripheral blood collected by noninvasive method, we could conclude that CD26 can contribute to the inflammatory process and Th2 predominance in the immunopathogenesis of allergic inflammation.
In conclusion, we have demonstrated that the expression of CD26 is elevated in allergic asthmatic patients and is associated with allergic inflammation. However, the exact pathophysiological function of CD26 requires further investigation.
REFERENCES
Yssel H, Groux H: Characterization of T cell subpopulations involved in the pathogenesis of asthma and allergic diseases. Int Arch Allergy Immunol 121:10–8, 2000
Larche M, Robinson DS, Kay AB: The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 111:450–63, 2003
Van DVV, Wierenga-Wolf AF, Driaansen-Soeting PW, et al.: Expression of aminopeptidase N and dipeptidyl peptidase IV in the healthy and asthmatic bronchus. Clin Exp Allergy 28:110–20, 1998
Proost P, Schutyser E, Menten P, et al.: Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 98:3554–61, 2001
Lambeir AM, Durinx C, Scharpe S, et al.: I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40:209–94, 2003
Hoffmann T, Faust J, Neubert K, et al.: Dipeptidyl peptidase IV (CD26) and aminopeptidase N (CD13) catalyzed hydrolysis of cytokines and peptides with N-terminal cytokine sequences. FEBS 336:61–4, 1993
Qin S, Rottman JB, Myers P, et al.: The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 101:746–54, 1998
Emanuele E, Minoretti P, Martinelli V, et al.: Circulating levels of soluble CD26 are associated with phobic anxiety in women. Prog Neuropsychopharmacol Biol Psychiatry 30:1334–6, 2006
Proost P, Struyf S, Loos T, et al.: Coexpression and interaction of CXCL10 and CD26 in mesenchymal cells by synergising inflammatory cytokines: CXCL8 and CXCL10 are discriminative markers for autoimmune arthropathies. Arthritis Res Ther 8:R107, 2006
Reinhold D, Bank U, Buhling F, et al.: Inhibitors of dipeptidyl peptidase IV induce secretion of transforming growth factor-beta 1 in PWM-stimulated PBMC and T cells. Immunology 91:354–60, 1997
Willheim M, Ebner C, Baier K, et al.: Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with T(H1) subsets. J Allergy Clin Immunol 100:348–55, 1997
Busso N, Wagtmann N, Herling C, et al.: Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol 166:433–42, 2005
Ohnuma K, Yamochi T, Hosono O, et al.: CD26 T cells in the pathogenesis of asthma. Clin Exp Immunol 139:13–6, 2005
Chambers CA: The expanding world of co-stimulation: The two-signal model revisited. Trends Immunol 22:217–23, 2001
Chen YQ, Shi HZ: CD28/CTLA-4–CD80/CD86 and ICOS–B7RP-1 costimulatory pathway in bronchial asthma. Allergy 61:15–26, 2006
Mercer JC, Ragin MJ, August A: Natural killer T cells: Rapid responders controlling immunity and disease. Int J Biochem Cell Biol 37:1337–43, 2005
Akbari O, Faul JL, Hoyte EG, et al.: CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med 354:1117–29, 2006
Proost P, DeMeester I, Schols D, et al.: Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Converstion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. J Bio Chem 273:7222–7, 1998
Proost P, Struyf S, Schols D, et al.: Truncation of macrophage-derived chemokine by CD26/dipeptidyl-peptidase IV beyond its predicted cleavage site affects chemotactic activity and CC chemokine receptor 4 interaction. J Bio Chem 274:3988–93, 1999
Lun SW, Wong CK, Ko FW, et al.: Aberrant expression of CC and CXC chemokines and their receptors in patients with asthma. J Clin Immunol 26:145–52, 2006
Leung TF, Wong CK, Lam CW, et al.: Plasma TARC concentration may be a useful marker for asthmatic exacerbation in children. Eur Respir J 21: 616–620, 2003
Lam CW, Fung HK, Vrijmoed LP, et al.: Aetiology of allergic rhinitis in Hong Kong. Allergol Int 47:23–28, 1998
American Thoracic Society: Guidelines for the evaluation of impairment/disability in patients with asthma. Am Rev Respir Dis 147:1056–61, 1993
American Thoracic Society: Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 152:1107–36, 1995
Lam KK, Pang SC, Allan WG et al.: Predictive nomograms for forced expiratory volume, forced vital capacity, and peak expiratory flow rate, in Chinese adults and children. Br J Dis Chest 77:390–396, 1983
National Asthma Education and Prevention Program: Expert panel report 2: Guidelines for the management of asthma. Bethesda, MD: National Institutes of Health, April 1997 Publication No. 97–4051
National Heart, Blood, and Lung Institute: Global strategy for asthma management and prevention: WHO/NHLBI workshop report. Bethesda, MD: National Heart, Lung, and Blood Institute, 1995; Publication No. 95–3659
Wong CK, Lun SW, Ko FW, et al.: Increased expression of plasma and cell surface co-stimulatory molecules CTLA-4, CD28 and CD86 in adult patients with allergic asthma. Clin Exp Immunol 141:122–9, 2005
Baggiolini M: Chemokines and leukocyte traffic. Nature 392:565–8, 1998
Pease JE: Asthma, allergy and chemokines. Curr Drug Targets. 7:3–12, 2006
Ishii T, Ohnuma K, Murakami A, et al.: CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc Natl Acad Sci U.S.A 98:12138–43, 2001
Ohnuma K, Yamochi T, Uchiyama M, et al.: CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol Cell Biol 25:7743–57, 2005
Lee SY, Lee YH, Shin C, et al.: Association of asthma severity and bronchial hyperresponsiveness with a polymorphism in the cytotoxic T-lymphocyte antigen-4 gene. Chest 122:171–6, 2002
Kruschinski C, Skripuletz T, Bedoui S, et al.: CD26 (dipeptidyl-peptidase IV)-dependent recruitment of T cells in a rat asthma model. Clin Exp Immunol 139:17–24, 2005
Author information
Authors and Affiliations
Corresponding author
Additional information
Equal first authors
Rights and permissions
About this article
Cite this article
Lun, S.W.M., Wong, C.K., Ko, F.W.S. et al. Increased Expression of Plasma and CD4+ T Lymphocyte Costimulatory Molecule CD26 in Adult Patients with Allergic Asthma. J Clin Immunol 27, 430–437 (2007). https://doi.org/10.1007/s10875-007-9093-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-007-9093-z