Previous studies have indicated that the infiltration of CD8+ T cells in colorectal cancer is an independent predictor of increased survival but clinical observations have suggested that the cytotoxic function of CD8+ T cells infiltrating colorectal cancer may often be limited. In this study, we have assessed the phenotype of colorectal cancer CD8+ tumor-infiltrating lymphocytes (TILs) isolated ex vivo from tumor tissue, and assessed the perforin content of TIL with respect to their location using immunohistochemistry. We found that CD8+ T cells TILs isolated from colorectal cancer are mainly composed of antigen-experienced cells of effector memory type (TEM, CD45RA-CCR7−, and CD27+/CD28− or CD27−/CD28−), and contain only minor proportions of terminally differentiated CD8+ T cells (TEMRA, CD45RA+CCR7−). The perforin content of these TILs, however, is significantly lower than that of antigen-experienced T cells in PBMCs due to the much lower levels of perforin found in the CD27-CD28− subset in TILs compared with CD8+ T cells of similar phenotype in PBMCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Colorectal carcinomas are solid epithelial tumors that have long been considered to be poorly immunogenic. However, along with the discovery of human T cell defined tumor associated- and specific-antigens (TAA/TSA), tumor antigens were also identified in colorectal cancers and both autologous cellular and/or humoral immune responses to specific tumor antigens, such as mutated TGF-β receptor II (1, 2), SART-1 (3), Ep-CAM (4) and NY-ESO-1 (5, 6), have been reported in some colorectal cancer patients. A large body of evidence has proven that the tumor-reactive CD8+ lymphocytes play a central role in cancer immunity. Recently, Naito et al. (7) have classified CD8+ tumor infiltrating T lymphocytes (TILs) in primary colorectal cancer into three groups: 1) peri-tumoral, consisting of cells distributed along the invasive margin of the tumor; 2) stromal, consisting of cells infiltrating the tumor stroma; and 3) intraepithelial, corresponding to cells that infiltrates cancer cell nests and are in direct contact with tumor cells. These authors reported that the existence of CD8+ TILs in the third group was a strong independent predictor of survival. In contrast, the presence of stromal TILs did not correlate with a better prognosis. Further study of the function of CD8+ TILs collected from the tumor sites of colorectal carcinomas is needed to unveil the clinical implications of these observations.
Upon antigen stimulation, naive CD8+ T cells proliferate and go through several differentiation stages that can be distinguished by the expression of surface markers, such as CD45RA/CCR7 (8) and/or CD27/CD28 (9). Their progeny include effector T cells and effector memory cells. Effector cells migrate to peripheral tissues and display immediately restrain invasion by pathogens or cancer cells, while memory cells travel through secondary lymphoid organs and can generate a new wave of effector cells upon a re-encounter with the stimulating antigen (8). In cancer tissues, TILs can be attracted to tumor environment by cancer cells themselves, the inflammatory signals caused by cancer, or naturally homing factors (10–12). Under any of the above circumstances, the differential phenotype and cytotoxic function of CD8+ T cells influence the clinical outcome dramatically by 1) direct killing of cancer cells, 2) angiostasis through acting on stromal cells, or 3) maintenance a cancer tolerant environment through regulatory T cells.
In this study, we examined the phenotype and cytotoxic status of CD8+ T lymphocytes from colorectal carcinomas. Generally, CD8+ T cells can be classified into two distinct effector cell types depending on their cytokine-secretion profiles following an antigen encounter (13, 14). Type 1 CD8+ T cells predominantly secrete IL-2 and IFN-γ, while type 2 CD8+ T cells produce IL-4, IL-5, and IL-10. Although the patterns of cytokine production are different, both populations of CD8+ T cells exert a cytotoxic function via the perforin pathway and have been demonstrated to contribute to the mediation of antitumor effects (14–16). Therefore, perforin was adopted here as the common cytotoxic indicator in the functional analysis of CD8+ TILs. We compared the differentiated phenotype and the perforin expression level in TILs and peripheral blood mononuclear cells (PBMCs) of human colorectal cancer patients. The results showed that tumor tissues of two-third of colorectal cancer patients were infiltrated by T lymphocytes. Among them, the majority of CD8+ infiltrating T lymphocytes located in tumor lesions were antigen-experienced effector memory cells (TEM) instead of terminally differentiated effector cells (TEMRA, CD45RA+T effector memory) (17), and that they express much less perforin than CD8+ T cells with similar phenotype in PBMCs. Furthermore, the function of the CD27-CD28− subset of CD8+ T cells in TILs was not represented by their phenotype as they were in PBMCs, indicating an antigen-experienced effector-memory phenotype and a functional tolerant state of low perforin expression of the CD8+ T cells that infiltrate colorectal cancer tissues. The location and expression of the CD8+ TILs were also determined by immunohistochemical staining.
MATERIALS AND METHODS
Sample Collection and Preparation
After obtaining informed consent and the approval of the Hospital Ethic Review Committee, 40 preoperative peripheral blood samples and their corresponding postoperative tumor tissues were collected from patients at Beijing Cancer Hospital, School of Oncology, Peking University. All tissues were pathologically confirmed as primary colorectal carcinomas. None of the patients enrolled in this study had been subjected to chemotherapy or any other therapy with immunosuppressive effects before lymphocyte isolation. The patients’ clinical characteristics are summarized in Table I. Tumor infiltrating lymphocytes were obtained via mechanical dispersion of tumor tissues into a single cell suspension in calcium-magnesium free HBSS (containing EDTA) within an hour of surgery, followed by Ficoll–Hypaque gradient centrifugation as described by Watanabe et al. (18). Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation using Ficoll–Hypaque as previously described, and the number of TILs in colorectal cancer tissues was counted. Flow cytometric analysis (FACS) was carried out when TILs numbered more than 0.2×106 cells. The number of TILs was scored as follows: 0, no TILs; +, 0.2–2×106 TILs; ++, more than 2×106 TILs. Paraffin embedded tumor tissue samples were prepared for immunohistochemical staining.
Flow Cytometric Detection
Antibodies and Associated Agents.
The following mAbs, the corresponding isotype control antibodies and other agents were purchased from BD PharMingen (San Diego, USA): anti-CD27-fluorescin isothiocyanate (FITC) and phycoerythrin (PE), anti-CD28-FITC and -PE-CY5, anti-CD3-PE, anti-CD8-PE-CY5, anti-CD107a-FITC, anti-perforin-FITC, Cytofix/CytopermTM, Perm/WashTM buffer. Other sources of mAbs were Beckman Coulter, Fullerton, USA (anti-CD8-ECD), Immunotech, Marseile, France (7-AAD, anti-CD45RA-ECD and isotype antibody), eBioscience, San Diego, USA (anti-TCR-αβ-PE and anti-TCR-γδ-FITC), and RD SYSTEMS, Tustin, USA (anti-CCR7-FITC).
Flow Cytometric Analysis.
After TILs or PBMCs were isolated, the cells were aliquoted and stained with anti-CD8 along with anti-CD27 and anti-CD28, anti-CD45RA and anti-CCR7 or isotype controls at 4°C for 30 min. The cells were then washed twice with phosphate buffered saline (PBS)/2% FCS. Intracellular levels of perforin were measured in freshly isolated CD8+ T lymphocytes without previous stimulation. In brief, after staining with the appropriate mAbs, cells were fixed with Cytofix/CytopermTM for 20 min at 4°C. After permeabilization, the cells were washed with Perm/WashTM buffer and labeled with anti-perforin mAb. Labeled cells were collected and analyzed by a COULTER EPICS XLTM with EXPO32 ADC software (Beckman Coulter, Fullerton, CA). Approximately 5000–10,000 CD8+ gated events were acquired for analysis. Intracellular perforin expression is represented as the normalized mean fluorescence intensity (NMFI), which is the mean fluorescence intensity (MFI) of cells stained with anti-perforin-FITC mAb divided by the MFI of cells stained with mouse IgG2b-FITC mAb (control). To exclude natural killer (NK) cells, FACS analysis and sorting was performed on gated CD8bright T cells. The cells were also stained with both anti-TCR-αβ-PE and anti-TCR-γδ-FITC to determine which subsets of T cells were present.
Immunohistochemistry.
Anti-CD3 (murine, clone PS1, 1:100), anti-CD8 (murine, clone 1A5, 1:40), and anti-perforin (murine, clone 5B10, 1:20) monoclonal antibodies were obtained from Novocastra Laboratories Ltd., Newcastle upon Tyne, UK. Following heat-based antigen retrieval in EDTA buffer (1 mmol/L, pH 8.0), paraffin-embedded specimens were incubated overnight with mAbs at 4°C. Detection of the primary antibody was performed with PowerVisionTM two-step histostaining reagent (Zhongshan Biotechnology, Beijing, China). Normal lymph nodes and T lymphoma were used as negative and positive controls.
The counting of intratumor intraepithelial CD3+, CD8+ and perforin+ cells was performed independently by two pathologists using a video-assisted measuring system (MOTICAM 2000, MOTIC IMAGES ADVANCED 3.2 software; Shamen, China). Nine areas of 670 μm×500 μm were chosen. The total numbers of positive cells were divided by 3 (1 mm3). Fields were chosen to contain the maximal amount of neoplastic cells with minimal necrotic debris. The cell numbers of intraepithelial or stromal CD3+ and CD8+ cells were graded as nil (no staining in 1 mm2), mild (1–19 positive cells/1 mm2), moderate (20–49 positive cells/1 mm2), or severe (>50 positive cells/1 mm2) according to the criterion proposed by Naito et al. (7). The number of intraepithelial or stromal perforin positive cells were counted and directly compared.
Statistical Analysis
Since most of the frequency data of PBMC and TIL subsets were normally distributed according to Kolmogorov–Smirnov statistics, the SPSS program (SSPS Inc., Chicago, USA) was adopted in analyzing the data using the paired-samples t-test. Data sets that did not exhibit a normal distribution were analyzed with the same software using the Wilcoxon test. Spearman’s non-parametric correlation coefficient (rho) was used to measure the degree of association between the two methods of evaluating TILs (FCM and immunohischemical staining) and the number of CD3+ and CD8+ cells. Statistical significance was accepted at p < 0.05 (two tailed).
RESULTS
Isolation and Assessment of CD8+ T Lymphocytes Infiltrating Colorectal Carcinomas
Lymphocytes infiltrating colorectal carcinoma tissues were isolated from fresh tumor tissue as detailed in the Methods section. From 12 of 40 tumor samples, we obtained only low numbers of TILs ( < 0.2×106) that were insufficient for analysis. The number of TILs obtained in the remaining 28 cases varied from 0.2 to 7×106 (median, 0.9×106) per gram of tumor tissue (Table I). All TILs were stained with 7-AAD and showed a content in viable cells >80% (not shown). The numbers of CD3+ T cells varied from 0 to 5×105 (median, 2.1×105) per gram of tumor tissue, which was in agreement with previous data by Watanabe et al. (17). CD8+ T cells represented between 12 and 58% of total T cells in these samples. Of those, >90% were CD8bright. The large majority (91–97%) of CD8bright cells in TILs were TCR-αβ positive, whereas significant proportions of TCR-γδ T cells were only detected in four samples, at frequencies lower than 5%. The presence and localization of CD3+ and CD8+ T lymphocytes in the tumors of 32 colorectal patients was assessed by immunohistochemistry. Both CD3+ and CD8+ infiltrating T lymphocytes were present in the majority of these samples either in the stroma and/or intraepithelial. Depending on the location of the infiltrating T lymphocytes, samples were classified into three groups: 1) those showing both stromal and intraepithelial lymphocyte infiltration (12 cases); 2) those showing lymphocyte infiltration only in the stromal tissues (17 cases); and 3) those where infiltrating lymphocytes were undetectable (3 cases) (Fig. 1). In general, most CD3+ and CD8+ T cells were detected in the stroma. For the 12 samples containing stromal and intraepithelial CD3+ T lymphocytes, the numbers of positive cells at both locations were counted and compared. The ratio of the CD3+ T cells in intraepithelial tissue versus stroma varied from 1/60to 2/5 (median of 1/30). The CD8 expression pattern was similar to that of CD3 (Table I). As expected, the number of CD8+ T cells in the stroma was lower than that of CD3+ T cells. However, we found a direct correlation between the numbers of CD3+ and CD8+ T cells (Spearman’s rho coefficiency = 0.78, p=0.001). We then compared the numbers of intraepithelial and stromal CD3+ or CD8+ TILs in paraffin embedded tumor tissues with the number of TILs isolated from fresh tumor tissues. We found a positive correlation between the number of isolated TILs and the number of stromal CD3+ T cells (correlation coefficiency = 0.514, p=0.003) or CD8+ T cells (correlation coefficiency = 0.481, p=0.006) found by immunohistochemical analysis. Thus, considering the distribution patterns of infiltrating lymphocytes in colorectal carcinomas, most CD8+ T infiltrating lymphocytes isolated from these tissues are likely to correspond to lymphocytes present in the tumor stroma.
CD3 expression in tumor tissues. Both CD3 and CD8 were detected in stroma or/and intraepithelial tumors, and can be categorized into three expression patterns: (A) lymphocytes infiltrating in both stromal and intraepithelial tumor tissue (from patient 18); (B) lymphocytes infiltrating only tumor stromal tissue (from patient 23), and undetectable lymphocytes (picture not shown).
Phenotypic Analysis of CD8+ T Lymphocytes in Ex Vivo Isolated TILs from Colorectal Carcinoma Samples
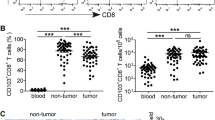
In order to assess the differentiation stage of CD8+ T lymphocytes infiltrating colorectal carcinomas, we stained TILs isolated ex vivo with antibodies specific for CD8 and either CD45RA and CCR7, or CD27 and CD28 as described previously (8, 9, 17). PBMC and TIL samples from the same patients were stained at the same time to allow an internal comparison between similar subsets in PBMCs and TILs (Fig. 2; Table II). As expected, co-staining of CD8+ T lymphocytes in patients’ PMBCs with anti-CD45RA and anti-CCR7 antibodies identified different subsets that included, according to the classification proposed by Sallusto et al. (8), a naïve (CD45RA+CCR7+, 10.2±6.6%) and an antigen-experienced population (CCR7−) comprising both TEMRA (CD45RA+CCR7−, 46.4±16.2%) and TEM, CD45RA-CCR7−, 39.4±14.1%) (17) in roughly similar proportions. In contrast, no significant proportions of naïve CD8+ T cells were detectable in TILs from colorectal carcinoma samples that contained predominantly antigen-experienced cells exhibiting for the largest part an effector-memory phenotype (TEM, CD45RA-CCR7−, 87.5±5%) and to a lesser extent an effector phenotype (TEMRA, CD45RA+CCR7−, 6.2±2.3%) (17). Staining of CD8+ T cells in PBMCs with antibodies against CD27 and CD28 identified four major subsets that, according to what previously described by Appay (9) and others (19), represents sequential differentiation stages: naïve and early differentiated (CD27+CD28+, 27.2±17.4%), intermediate (CD27+CD28−, 20.5±14%), and terminally differentiated (CD27-CD28−, 55.0±15.8%). In contrast, in TILs, only two of these subsets were found, in roughly similar proportions, namely CD27+CD28− (44.2±21.5%) and CD27-CD28− (47.6±20.1%).
Expression pattern in differentiated phenotypic classification of CD8+ T cells from TILs (B and D) and PBMCs (A and C) of patient 18. CD8+ T cells were stained with anti-CD45RA/anti-CCR7 (A and B) or anti-CD27/anti-CD28 (C and D). CD8+ T cells from TILs were mainly antigen-experienced cells or TEM (CD45RA−CCR7−, CD27+/−CD28−) and lacked terminally differentiated phenotypes or TEMRA (CD45RA+CCR7−, CD27−CD28−). Both pre-terminally differentiated subsets or TEM and terminally differentiated effector or TEMRA CD8+ T cells were found in PBMCs.
Assessment of Perforin Expression in Colorectal Carcinoma TILs with Respect to Their Phenotype Identifies a Major Effector-Memory Subset with Low Perforin Content
For 10 of these samples, in which the number of TILs was sufficient for further analysis, we examined the presence of the cytotoxic molecule perforin by intracellular staining using specific antibodies. Approximately 30–50% (34.6±2.1%) of total CD8+ T cells from PBMCs contained perforin, whereas only 0–13% (7.4±1.4.1%) of CD8+ T cells from TILs contained this molecule. In addition, the normalized mean fluorescence intensity (NMFI) of perforin staining was significantly lower in CD8+ T cells in TILs (TILs: 1.4±0.4) than in PBMCs (3.8±0.9; p=0.001; Fig. 3). It is been previously reported that the expression level of perforin increases along with the stage of differentiation of CD8+ T cells (8, 20), and negatively correlates with the expression of CD27 (20). Therefore, we analyzed perforin expression in TILs with respect to their differentiation stage by co-staining with perforin-, CD27-, and CD28-specific antibodies. As expected, low levels of perforin were found in the CD27+CD28− subset from both PBMCs (NMFI: 2.1±1.5) and TILs (NMFI: 1.2 ± 0.3). However, perforin levels in the CD27−CD28− subset in TILs (MFI: 1.5±0.8) was found to be similar to the level of CD27+CD28− subsets ( p=0.586), and significantly lower than that of the corresponding subset in PBMCs (p=0.002) (Fig. 3). The low intracellular level of perforin in CD8+ T cells at the tumor site could, in principle, result from the degranulation occurring after interaction of cytotoxic T cells with tumor cells. To address this possibility, we assessed the expression of CD107a, a lysosomal integral membrane protein in cytotoxic granules, which is a marker of degranulation after stimulation (21). No CD107a was detected on the surface of cells with low intracellular perforin levels (Fig. 3), indicating that low perforin expression in CD8+ TILs was most likely not due to degranulation. To further exclude the possibility that the procedure of lymphocyte isolation might have resulted in partial degranulation of CD8+ T cells or in the degradation of perforin, perforin expression was further assessed by in situ immunohitochemistry analysis of six samples containing perforin expressing CD8+ cells both stromal and intraepithelial revealed that perforin expression was less frequent in the stromal as compared to the intraepithelial lymphocytes (stroma: 16±6%; intraepithelial: 59 ± 22%, t=−4.274, p=0.01; Fig. 4). In addition, analysis of an additional seven cases where lymphocytes were only present in the stroma revealed that less than 10% of total CD8+ T cells present at this location express perforin (Table III).
CD8+ T cells were stained with anti-CD27, anti-CD28, and anti-perforin or anti-CD107a (from patient 18). A low level of perforin was found in the CD27+CD28− subset, with a higher level in the CD27−CD28− CD8+ T cells from PBMCs. These results were in accordance with previous studies that the phenotypic classification of human CD8+ T cells reflected their function in PBMCs (20). In contrast, the perforin level in the CD27−CD28− subset was found to be similar to that in the CD27+CD28− subset from CD8+ TILs. No CD107a was detected on the surface of cells from TILs with low intracellular perforin expression.
Perforin expression detected by immunohistochemistry. (A) Perforin expressed in both intraepithelial and stromal tumor tissues (400×). (B) Comparison of the numbers of perforin positive cells in the stroma and intraepithelium from six samples in which perforin was expressed in both sites. The frequency of CD8+ cells expressing perforin in the stroma (white bar) was significantly lower than that in the intraepithelial tissues (black bar) (stromal: 16±6%; intraepithelial tumor tissues: 59±22%; p=0.01).
DISCUSSION
Although some studies have suggested that the number of CD8+ tumor infiltrating lymphocytes is a good prognostic marker in colorectal cancer patients (7, 22), many patients with obviously infiltrating lymphocytes nevertheless have a short survival time. Other reports do not support a protective role for infiltrating lymphocytes (23). In several clinical trials, no survival benefit was found after adoptive therapy of TILs to treat colorectal cancer patients (24–27). Thus, elucidating the functional state of TILs in colorectal cancer in vivo is critical in interpreting the conflicting data. To our knowledge, this study is the first attempt to detect the differentiated phenotype and cytotoxic function (represented by perforin) of TILs ex vivo of colorectal cancer. Fresh TILs were mechanically isolated from colorectal cancer tissues without the processes of enzyme digestion (28) or cryopreservation (29), which can change TIL phenotypes. Hence, the results reflect the actual phenotype and function status of the TILs in vivo.
The γδ T lymphocytes comprise 25–37% of intraepithelial lymphocytes residing in normal intestinal epithelium. However, the tumor-infiltrating lymphocytes in colorectal cancer tumor sites were mainly αβ T lymphocytes (18). Our results also showed that more than 90% of CD8+ TILs were αβ T lymphocytes. This phenomenon is consistent with previous reports (7, 18), and indicates that during tumor development, local inflammatory responses and/or tumor antigens themselves attract antigen-specific T cells to tumor sites.
Although several studies have confirmed that tumor-specific cellular responses do exist in colorectal cancer patients, the colorectal carcinoma-associated/specific antigens are not well defined. Therefore, it is difficult to obtain epitope-specific T lymphocytes to analyze their phenotype and function in the TILs of the majority of colorectal cancer patients. On the other hand, TILs isolated from tumor sites consist of multiple antigen-specific T lymphocytes responding to a cluster of various antigenic challenges. Either they act on cancer cells directly, or on cancer stromal cells, cytotoxic TILs will impact on cancer growth. In this situation, the TILs analyzed by this study were more representative of an evaluation of local immune status of the tumors.
According to Sallusto et al. (8) and Gupta (17), antigen-experienced CD8+ T cells in PBMCs can be classified into three subsets representing the differentiation stages: naïve (CD45RA+CCR7+), effector or TEMRA (CD45RA+CCR7−), and effector memory or TEM (CD45RA−CCR7−) cells. In our study, CD8+ T lymphocytes isolated from tumor sites were mostly antigen-experienced T cells with an effector memory (TEM) phenotype (88%), while in PBMCs, the majority of antigen-experienced CD8+ T cells were composed of both TEMRA (46%) and effector memory (TEM) (39%) cells. It was reported that although both types of antigen-experienced cells are functional in target cell killing, effector memory cells are less effective (30). These results indicated that CD8+ T lymphocytes in colorectal cancer sites were phenotypically cytotoxic, though their efficacy might not be as strong as that of effector cells.
On the other hand, when CD8+ TILs were analyzed according to the T-cell differentiation model defined by Appay et al. [9], two major differentiated phenotypes, CD27+CD28− and CD27-CD28−, were displayed that were different from CD8+ T cells in PBMCs. These results confirmed that the TILs we isolated were not cells from blood vessels but were tumor site infiltrators. The differentiation phenotypes were also distinct from those of metastatic lymph nodes, where the two major subsets of CD27+CD28+ and CD27−CD28−, were detected (11). We first revealed that the phenotype of CD8+ T cells from colorectal cancer tumor sites was a pre-terminally differentiated stage (CD45RA−CCR7−CD27+/−CD28−). Despite the fact that the T cells were infiltrating into a tumor site, they lacked the terminally differentiated subset of CD45RA+CCR−CD27−CD28− (TEMRA). In studies of chronic virus infections, it was found that distinct CD8+ T cell populations representing early to terminally differentiated stages were established corresponding to the immunogenicity of the viruses (9, 30). We postulate that the pre-terminally differentiated phenotype of CD8+ T cells in TILs from colorectal cancer could be the result of low immunogenicity of the tumor. As a result, immunosuppressive factors such as IL-10 (10), TGF-β (10), and regulatory T cells (31) in the local microenvironment of the tumor may influence the differentiation and function of T cells.
Our results further showed that CD8+ T cells from tumor sites expressed less perforin than those from PBMCs. Interestingly, we found that the low expression of perforin in these cells is the result of low or no expression by their CD27−CD28− subsets. The low level of perforin was not caused by the release of a cytotoxic molecule, since expression of the CD107a marker of degranulation was not detected (21). A previous study reported that the quantity of CD27 expression in CD8+ T cells was reversely correlated with perforin expression, as well as with cytotoxic functions (20). Our results in CD8+ T cells from PBMCs are in agreement with this opinion, but perforin expression in CD8+ T cells from tumor sites did not increase, while the quantity of CD27 decreased. These results suggest that the function of the CD27−CD28− subset of CD8+ T cells in TILs is not represented by their phenotype as is the case with PBMCs, and indicated by a deviation in the status of phenotype and function in these cells—they were antigen-experienced effector-memory CD8+ T (TEM) cells that were functionally tolerant. Further study will be needed to clarify the function of these T cells, including if they are regulatory T cells, or they are in a state of anergy only; and if the tolerant state can be reversed by immune interference in vitro or in vivo.
Immunohistochemistry labeling showed that the number of intraepithelial lymphocytes was much lower there than in the stroma, indicating that the majority of TILs isolated from fresh tumor tissues were stromal TILs. While stained by CD45RA antibody, CD45RA was not detected in the intraepithelial lymphocytes, suggesting that those cells were pre-terminally differentiated effector-memory (TEM) cells (data not shown). However, perforin expression was found to be relatively higher in intraepithelial tumors than in stromal tissues. Recently, some evidences have shown that cancer stromal cell killing is critical in cancer tissue eradication (32, 33). The tolerant state of colorectal cancer stroma CD8+ TILs may explain the irrelative of these cells with clinical prognosis of colorectal cancer.
By typing the differentiated phenotypes and functional molecules of infiltrating CD8+ T cells in colorectal cancer, we found that although the CD8+ T cell immune response did exist in tumor sites, most of the cells lacked cytotoxic molecule expression, despite being in the intermediate differentiated stage. The deviation of molecular function from the differentiation phenotype could explain the observed clinical phenomenon in which infiltrating lymphocytes do not inhibit cancer growth. Further studies on the mechanism and immunological intervention of TIL function are needed.
REFERENCES
Saeterdal I, Bjorheim J, Lislerud K, et al.: Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA 98:13255–13260, 2001
Linnebacher M, Gebert J, Rudy W, et al.: Frameshift peptide-derived T-cell epitopes: A source of novel tumor-specific antigens. Int J Cancer 93:6–11, 2001
Ito M, Shichijo S, Miyagi Y, et al.: Identification of SART3-derived peptides capable of inducing HLA-A2-restricted and tumor-specific CTLs in cancer patients with different HLA-A2 subtypes. Int J Cancer 88:633–639, 2000
Nagorsen D, Keilholz U, Rivoltini L, et al.: Natural T-cell response against MHC class I epitopes of epithelial cell adhesion molecule, her-2/neu, and carcinoembryonic antigen in patients with colorectal cancer. Cancer Res 60:4850–4854, 2000
Scanlan MJ, Welt S, Gordon CM, et al.: Cancer-related serological recognition of human colon cancer: Identification of potential diagnostic and immunotherapeutic targets. Cancer Res 62:4041–4047, 2002
Li M, Yuan YH, Han Y, Liu YX, Yan L, Wang Y, Gu J: Expression profile of cancer-testis genes in 121 human colorectal cancer tissue and adjacent normal tissue. Clin Cancer Res 11:1809–1814, 2005
Naito Y, Saito K, Shiiba K, et al.: CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494, 1998
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A: Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712, 1999
Appay V, Dunbar PR, Callan M, et al.: Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 8:379–385, 2002
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD: Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol 3:991–998, 2002
Mortarini R, Piris A, Maurichi A, et al.: Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res 63:2535–2545, 2003
Zippelius A, Batard P, Rubio-Godoy V, et al.: Effector function of human tumor-specific CD8 T cells in melanoma lesions: A state of local functional tolerance. Cancer Res 64:2865–2873, 2004
Sprent J, Surh CD: T cell memory. Annu Rev Immunol 20:551–579, 2002
Hishii M, Kurnick JT, Ramirez-Montagut T, Pandolfi F: Studies of the mechanism of cytolysis by tumour-infiltrating lymphocytes. Clin Exp Immunol 116:388–394, 1999
Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA, et al.: Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 192:755–760, 2000
Smyth MJ, Street SE, Trapani JA: Cutting edge: Granzymes A and B are not essential for perforin-mediated tumor rejection. J Immunol 171:515–518, 2003
Gupta S: Molecular mechanisms of apoptosis in the cells of the immune system in human aging. Immunol Rev 205:114–129, 2005
Watanabe N, Hizuta A, Tanaka N, Orita K: Localization of T cell receptor (TCR)-γδ+ T cells into human colorectal cancer: Flow cytometric analysis of TCR-γδ expression in tumor-infiltrating lymphocytes. Clin Exp Immunol 102:161–173, 1995
Tomiyama H, Matsuda T, Takiguchi M: Differentiation of human CD8+ T cells from a memory to memory/effector phenotype. J Immunol 168:5538–5550, 2002
Tomiyama H, Takata H, Matsuda T, Takiguchi M: Phenotypic classification of human CD8+ T cells reflecting their function: Inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol 34:999–1010, 2004
Rubio V, Stuge TB, Singh N, et al. Ex vivo identification and analysis of tumor-cytolytic T cells. Nat Med 9(11):1377–1382, 2003
Guidoboni M, Gafa R, Viel A, et al.: Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol 159:297–304, 2001
Neilsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesggaard F: Independent prognotic value of eosiniphil and mast cell infiltration in colorectal cancer. J Pathol 189:487–495, 1999
Yannelli JR, Hyatt C, McConnell S, et al.: Growth of tumor-infiltrating lymphocytes from human solid cancers: Summary of a 5-year experience. Int J Cancer 65:413–421, 1996
Hawkins MJ, Atkins MB, Dutcher JP, et al.: A phase II clinical trial of interleukin-2 and lymphokine-activated killer cells in advanced colorectal carcinoma. J Immunother 15:74–78, 1994
Fabbri M, Ridolfi R, Maltoni R, et al.: Tumor infiltrating lymphocytes and continuous infusion interleukin-2 after metastasectomy in 61 patients with melanoma, colorectal and renal carcinoma. Tumori 86:46–52, 2000
Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S: Tumor as elusive targets of T-cell-based active immunotherapy. Trends Immunol 24(6):334–341, 2003
Diederichsen ACP, Zeuthen J, Christensen PB, Kristensen T: Characterisation of tumor infiltrating lymphocytes and correlation with immunological surface molecules in colorectal caner. Eur J Cancer 35(5):721–726, 1999
Costantinia A, Mancinia S, Giuliodoroa S, Butinia L, Regnerya CM, Silvestrib G, Montronia M: Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods 278:145–155, 2003
Champagne P, Ogg FS, King AS, et al.: Skewed maturation of memory HIV-specific CD8 T lymphocyte. Nature 410:106–111, 2001
Unitt E, Rushbrook SM, Marshall A, et al.: Compromised lymphocytes infiltrate hepatocellular carcinoma: The role of T-regulatory cells. Hepatology 41:722–730, 2005
Spiotto MT, Rowley DA, Schreiber H: Bystander elimination of antigen loss variants in established tumors. Nat Med 10:294–298, 2004
Spiotto MT, Schreiber H: Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens crosspresented by stromal cells. Cancer Immun 5:8–15, 2005
ACKNOWLEDGEMENTS
This study was supported by Designed Grant of Cancer Research Institute (New York, NY), New Star Program of Science and Technology of Beijing Science and Technology Committee (H020821070130), “211” Program of Peking University, and Science Foundation of Beijing Cancer Hospital
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
YE, SW., WANG, Y., VALMORI, D. et al. Ex-Vivo Analysis of CD8+ T Cells Infiltrating Colorectal Tumors Identifies a Major Effector-Memory Subset with Low Perforin Content. J Clin Immunol 26, 447–456 (2006). https://doi.org/10.1007/s10875-006-9040-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-006-9040-4