Sarcoidosis (SA) is a granulomatous disorder of an unknown etiology. Mycobacterium tuberculosis heat shock proteins (Mtb-hsp), considered as causative agents, play an important role in apoptosis. A role for apoptosis has been proposed in pathogenesis of SA and tuberculosis (TB) granuloma formation but results remain controversial. Differences in Mtb-hsp-induced apoptosis between SA, TB, and healthy subjects found in this study might put some light on the etiology of SA. Early apoptotic peripheral blood mononuclear cells (PBMC) were determined in 22 SA patients, 20 TB patients, and 20 healthy volunteers by flow cytometry (Annexin-V-FITC). Our results revealed that spontaneous apoptosis of monocytes and CD8+ T-cells was comparable between tested groups. Apoptosis of unstimulated CD4+ T-cells was significantly lower in TB versus controls and insignificantly lower versus SA. Mtb-hsp- and PHA (Phytohemagglutinin)-induced monocytes apoptosis was significantly lower in TB versus controls and SA. Mtb-hsp-induced CD4+ T-cell apoptosis was significantly lower in TB versus controls and SA. There were no differences of PHA-induced CD4+ T-cell and CD8+ T-cell apoptosis between tested groups. Apoptosis of Mtb-hsp-induced CD8+ T-cells was significantly lower in TB and SA versus controls. Analysis of PBMC apoptosis before and after stimulation in each tested group revealed that, in contrast to TB, sarcoid monocytes were resistant to Mtb-hsp- and PHA-induced apoptosis and CD4+ T-cells were resistant to PHA- but not Mtb-hsp-induced apoptosis. CD8+ T-cell apoptosis, before and after Mtb-hsp or PHA stimulation, was significantly increased in all tested groups. It seems likely that dysregulated apoptosis of CD4+ T-cells and resistant apoptosis monocytes may be involved in pathogenesis of SA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sarcoidosis (SA) is a multisystem granulomatous disorder of an unknown etiology commonly affecting young adults and frequently associated with biliteral hilar lymphadenopathy and pulmonary infiltrations. The diagnosis is firmly established when clinico-radiological findings are supported by histological evidence of noncaseating epithelioid cell granulomas found on a tissue biopsy (1). Because tuberculous granulomatous inflammation is the histological hallmark of sarcoidosis, investigators continue to improve and apply modern diagnostic tools in the search for infectious agents such as mycobacteria, including antigens, e.g., Mycobacterium tuberculosis heat shock proteins (Mtb-hsp) in blood (2) and in affected tissue (3–7).
The Mtb-hsp70, -hsp65, -hsp16 molecules are expressed on the surface of monocytes/macrophages and presented effectively to T cells to induce immunity (8). Recently, it has been demonstrated that hsp plays an important role in apoptosis (9–12). In some studies, apoptosis is considered as a factor in pathogenesis of sarcoid and tuberculous granulomas (15–21).
Apoptosis, or programmed cell death, is a physiological, genetically controlled, cellular response to external and internal stimuli whose purpose is to eliminate unwanted cells, including infected cells, while preventing damage to surrounding cells or tissue (3). Apoptosis can be stimulated or inhibited by different signals or cytokines. Some of these regulators are pro-apoptotic (Bax, Bik, Bak, Bad, Bcl-Xs, TNF/TNFR1, Fas/FasL, caspases family, NFκB, ATP, H2O2) and some are anti-apoptotic (Bcl-2 family of genes, hsp, proteins-inhibitor of apoptosis) (23, 24).
The balance between pro-apoptotic and anti-apoptotic factors influences the resolution of inflammatory process or development of disease (25).
Aberrations in signaling, in receptors or alterations in the mechanism of apoptosis have been revealed in vitro in tuberculosis (TB) (17, 26–36) but few clinical studies (16, 30, 37) have been conducted among patients with TB. To date, only a small number of experimental and clinical studies on alternations of apoptosis of peripheral (18, 38) and local mononuclear cells (15, 19–21, 38–40) have been conducted in sarcoidosis and the results have been controversial. The essential role of the mycobacterial hsp70, hsp65, and hsp16 in apoptosis of the PBMC has thus far never been analyzed in sarcoidosis.
Therefore, the aim of this study was to compare spontaneous and mycobacterial heat shock protein-induced apoptosis of blood mononuclear cells in pulmonary sarcoidosis, tuberculosis, and in healthy controls.
PARTICIPANTS, MATERIALS, AND METHODS
Participants
Patients with Sarcoidosis.
Twenty-two patients (mean age 36.6 ± 8.8 years; range 23–56 years; 6 women, 16 men) with pulmonary sarcoidosis at the Pulmonology Hospital in Sopot and Wejherowo, Poland, were included in the study. The diagnosis of SA was based on histological (scalenobiopsy of the lymph nodes), clinical and radiological evidence. High-resolution computed tomography was used to diagnose Stage I (bilateral hilar lymphadenopathy—14 patients) and Stage II (bilateral hilar lymphadenopathy and diffuse pulmonary infiltrations—8 patients) of sarcoidosis. None of the patients had extrapulmonary SA. A negative PPD skin-test was an additional criterion for inclusion in the study group. Microbiological and cytological examination of the lymph nodes and sputum samples revealed no acid-fast bacilli (PCR, culture of M. tuberculosis), fungi, or atypical cells.
Patients with Tuberculosis.
Twenty unrelated patients (mean age 45.9 ± 16.7 years; range 30–60 years; 5 women, 15 men) with newly detected active pulmonary tuberculosis at the Pulmonology Hospital in Sopot and Wejherowo, Poland, were included in the study. A diagnosis of TB was established using standard clinical, radiographic, and bacteriological criteria. The patients were at a similar clinical stage and with similarly localized disease on the initial chest radiographs (CXRs). The diagnosis of TB was confirmed by demonstration of acid-fast bacilli in sputum smears and by positive sputum culture of M. tuberculosis. A positive PPD skin-test was an additional criterion included in the studied group. Patients were also classified according to their response to chemotherapy. Those who responded to the first line treatment (rifampin, isoniazid, ethambutol, pyrazinamide), were classified as drug responders.
Controls.
The control samples studied consisted of 20 unrelated healthy blood donor volunteers (mean age 33.9 ± 11 years; range 20–54 years; 10 women, 10 men) originating from the region of Gdańsk in northern Poland. They showed normal results of CXRs and blood and serum analysis and no acid-fast bacilli in sputum smears and in the sputum culture of M. tuberculosis. None of them showed a positive result in a PPD skin-test.
None of the controls, TB, or SA patients had a familial history of tuberculosis, sarcoidosis, or autoimmune disease. Testing was performed for HIV infection in all groups. All patients and the controls (non-smokers as well as smokers) originated from the region of Gdańsk in northern Poland and were vaccinated with Mycobacterium bovis bacillus Calmette-Guerin (BCG). In Poland, since 1955, BCG vaccinations at birth and again at 7, 12, and 18 years of age have been mandatory.
Ethical approval for the study was obtained from the Independent Bioethics Committee for Scientific Researches, Medical University of Gdańsk, Poland. Informed consent was obtained from both patients and controls.
Materials and Methods
The investigation was conducted before treatment in both tested groups of patients.
Specimen Collection and Preparation.
Venous blood samples (15 mL) were collected into tubes with anticoagulant (Becton Dickinson Company, USA) for PBMC cultures.
Reagents and Antibodies.
NUNC, Denmark, was the supplier of single-use plastic equipment; RPMI 1640 medium, inactivated foetal calf serum (FCS), and other media were purchased from Gibco, Life Technologies Inc., USA. We used phytohemagglutinin (Pharmacia, Sweden) or recombinant M. tuberculosis heat shock proteins for in vitro stimulation. Recombinant mycobacterial hsp (hsp70-batch: L70-01-2, lot 1; hsp65-batch: L65-99-1, lot 6; hsp16-batch: L16-00-1, lot 1) were obtained from LIONEX Diagnostics & Therapeutics GmbH, Braunschweig, Germany. The following monoclonal antibodies (mAb) were used in the flow cytometric studies: anti-CD3 (IgG1κ PE mAb, clone: UCHT1), anti-CD4 (IgG1κ CyChrome mAb, clone: RPA-T4), anti-CD8 (IgG1κ CyChrome mAb, clone: RPA-T8), anti-CD14 (IgG2aκ CyChrome mAb, clone: M5E2), anti-CD45 (IgG1κ FITC mAb, clone: HI30), and surface isotype controls (IgG1κ FITC, PE, Cychrome mAbs, clone: MOPC-21; IgG2aκ PE mAb, clone: G155-178) (all form BD Bioscience, PharMingen, Germany). Annexin-V-FITC was purchased at Biosource, USA.
Isolation of PBMC and Cell Cultures.
PBMC were isolated from the peripheral blood samples, by Ficoll-Paque gradient centrifugation. The population of PBMC, lymphocytes, and monocytes together was chosen to mimic conditions occurring in vivo, including possible cell-to-cell interactions between different PBMC subpopulations. After two washings with phosphate buffered saline, PBMC were suspended in RPMI 1640 culture medium supplemented with 5% FCS; 1×106 cells were then diluted with 1 mL medium (RPMI 1640 + 5% FBS) and cultured in triplicate in plastic 24-well plates. The cultures were stimulated with 1 μg/mL of PHA (Phytohemagglutinin) or 1 μg/mL of Mtb-hsp. All the cultures were incubated for 24 h at 37°C, in a humidified atmosphere containing 5% CO2. The controls consisted of non-stimulated cultures in order to estimate the amplitude of response to applied stimuli and to correct for the non-specific binding of the reagent.
Staining and Analysis of CD4+ and CD8+ T-cells with Annexin V.
The binding of annexin-V-FITC was used as a sensitive measurement of monocytes and CD4+ T-cells, CD8+ T-cells apoptosis. Aliquots of PBMC from the cultures were put into 12 mm×75 mm tubes (1×105 cells per tube), washed twice with cold PBS, stained with the following combinations of antibodies: CD3/CD4, CD3/CD8, CD3/CD14, or the appropriate isotype control antibodies (20 μg/test) and were incubated for 15 min in the dark at room temperature. The cells were then washed with PBS and resuspended in a solution containing 10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2. As the next step, 2 μL of annexin-V-FITC was added to the appropriate samples and the tubes were incubated for 15 min in the dark. Thus, tri-color combinations of fluorescence dyes in the following configurations were obtained in the samples: CD3/CD4/annexin-V, CD3/CD8/annexin-V, and CD3/CD14/annexin-V. To acquire flow cytometry data after incubation, the EPICS XL flow cytometer (Coulter, USA) was used. The data were analyzed using the WinList 5.0 software (Verity, USA). As the first step, dot plots of anti-CD3 versus side scatter (SSC) were acquired. Then, annexin-V-FITC versus CD14 Cychrome dot plots from CD3− region and annexin-V-FITC versus CD4 or CD8 Cychrome dot plots were generated. Typically, 10,000 events were acquired in the gated regions. The threshold level for annexin-V-positive cells was set for each sample at the intersection of the histogram curves obtained from unstained cells and the specific fluorescence.
Statistical Analysis.
Resultant data were analyzed using the program STATISTICA® for Windows v. 6.0 (StatSoft, USA). Descriptive statistic ANOVA/MANOVA test was used for repeated measurements and the least significant difference test (LSD) as a post hoc test. Only significant differences are presented in the full form. The level of significance was p≤0.05.
RESULTS
White blood cells (WBC) (see Table I). The analysis of the total number of WBC revealed significant differences among tested groups: Controls, SA patients, TB patients (p=0.009). The significantly higher total number of WBC was seen in TB patients in comparison to the healthy individuals (p=0.028) and the SA patients (p=0.003).
Monocytes (see Table I). The percentage of monocytes in SA patients was significantly higher than in controls and TB patients (p=0.002). Total number of blood monocytes was comparable between SA and TB patients but higher than in the control (p=0.07).
Apoptosis of unstimulated monocytes (%) in PBMC cultures (see Table II). The spontaneous apoptosis of monocytes was lower in TB patients than in controls (p=0.08) and in SA patients (p=0.052).
Mtb-hsp-induced apoptosis of monocytes (%) in PBMC cultures (see Table II and Fig. 1). The percentage of apoptotic monocytes after Mtb-hsp stimulation was significantly lower in the TB patients than in the control or SA patients (in the MANOVA test: Rrao = 3,34; p=0.042; in the LSD test: p=0.02 for TB versus controls, p=0.04 for TB versus SA). The analysis (in the two-tail paired t test) of apoptosis of monocytes before and after Mtb-hsp stimulation in each tested group revealed the increase of apoptosis in TB and in the controls (p=0.012, p=0.037, respectively), whereas there were no differences in the group of SA patients.
PHA-induced apoptosis of monocytes (%) in PBMC cultures (see Table II). The percentage of apoptotic PHA-induced monocytes was significantly lower in TB patients than in the controls or SA patients (in the MANOVA test: Rrao = 4.63; p=0.015; in the LSD test: p=0.004 for TB versus controls; p=0.056 for TB versus SA). The analysis (in the two-tail paired t test) of apoptosis of monocytes before and after PHA stimulation in each tested group revealed increased apoptosis in TB and in the controls (p=0.010, p=0.011, respectively), whereas there were no differences in the group of SA patients.
T Lymphocytes (see Table I). The percentage of T lymphocytes in SA patients was significantly higher than in the controls or TB patients (in the MANOVA test: Rrao = 5.08; p=0.008; in the LSD test p=0.043) but the total number of blood monocytes was comparable between tested groups of patients and the control (in the ANOVA test: F=2.13; p=0.127). The percentage of lymphocytes was insignificantly lower in the healthy group than in SA and TB patients (in the LSD test: p=0.28, p=0.36, respectively).
Apoptosis of unstimulated CD4+T lymphocytes (%) in PBMC cultures (see Table II). The spontaneous apoptosis of CD4+ T cells was significantly lower in TB patients than in the controls (p=0.006) and insignificantly lower than in SA patients (p=0.12).
Mtb-hsp-induced apoptosis of CD4+T lymphocytes (%) in PBMC cultures (see Table II and Fig. 1). The percentage of apoptotic CD4+ T-cells after hsp stimulation was significantly lower in TB patients than in the controls or SA patients (in the MANOVA test: Rrao = 3.76; p=0.032). The analysis (in the two-tail paired t test) of apoptosis of CD4+ T-cells before and after Mtb-hsp stimulation in each tested group revealed increased apoptosis in the group of SA patients (p=0.041), whereas no difference between TB and controls was observed.
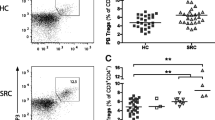
The comparative analysis of Mtb-hsp-induced apoptosis of peripheral blood mononuclear cells (PBMC) in tested groups. Three columns of dot plots from left to right represent healthy subject, tuberculosis patient, and sarcoidosis patient, respectively. The upper row dot plots were obtained from CD3− gate to show CD14+ monocytes and the middle and the bottom rows were obtained from CD3+ gate to show CD4+ and CD8+ T cells, respectively. The lines within dot plots represent the thresholds between negative and positive staining established using isotype controls. AnnexinV-positive cells in analyzed subsets are placed in upper right quadrants of the dot plots. The numbers in the upper right quadrants are mean ± SD from the entire group of patients.
PHA-induced apoptosis of CD4+T lymphocytes (%) in PBMC cultures (see Table II). There was no difference in PHA-induced apoptosis of CD4+ T-cells between tested groups (in the MANOVA test: Rrao = 1.48; p=0.24). The analysis (in the two-tail paired t test) of apoptosis of CD4+ T-cells before and after PHA stimulation in each tested group revealed increase of apoptosis in the TB patients and the control groups (p=0.018, p=0.014, respectively). There was no difference between SA patients and control group.
Apoptosis of unstimulated CD8+T lymphocytes (%) in PBMC cultures (see Table II). Spontaneous apoptosis of the CD8+ T-cells was comparable in all tested groups.
Mtb-hsp-induced apoptosis of CD8+T lymphocytes (%) in PBMC cultures (see Table II and Fig. 1). Percentage of apoptotic CD8+ T-cells after Mtb-hsp stimulation was higher in the control than in TB and SA groups (in the MANOVA test: Rrao = 2.81; p=0.067; in the LSD test: p=0.055 for controls versus TB, p=0.032 for controls versus SA). The analysis (in the two-tail paired t test) of apoptosis of CD8+ T-cells before and after Mtb-hsp stimulation in each tested group revealed a significant increase in apoptosis in all tested groups.
PHA-induced apoptosis of CD8+T lymphocytes (%) in PBMC cultures (see Table II). There were no differences in PHA-induced apoptosis of CD8+ T-cells between all tested groups (in the MANOVA test: Rrao = 0.44; p=0.64). The analysis (in the two-tail paired t test) of apoptosis of CD8+ T-cells before and after PHA stimulation in each tested group revealed a significant increase in apoptosis in all tested groups.
DISCUSSION
It has been suggested that low-virulence strains of intracellular pathogens with the capacity to persist in host macrophages for prolonged periods may generate production of abundant quantities of both host and microbial hsps that can then be expressed on the surface of macrophages and presented effectively to T-cells to induce immunity, granuloma (41, 42).
Studies on monocyte/macrophage infected with intracellular pathogens have shown the involvement of host hsp65 in prevention of apoptosis of phagocytes (41, 43, 44). Some authors (9, 14, 26) have suggested that hsp65 and hsp70 protect cells from apoptosis by maintaining the integrity of the cell mitochondria and/or bind to the apoptotic protease-activating factors, c-Jun N-terminal kinase, BAG-1 and Bcl-2 (9–13). Hsp70 overexpression reduced Fas-, TNF-α-, nitric oxide-, ceramide-, and caspase-induced apoptosis (14, 28). Mtb- hsp70 and -hsp65 induced release of pro-apoptotic cytokines IL-1β, IL-6, IL-8, TNF-α, NO, and anti-apoptotic IL-10 from human mononuclear cells (10, 11, 13).
Expression of small hsp16 was shown to be essential for preventing differentiating cells from undergoing apoptosis by different pathways, such as decreasing the intracellular level of reactive oxygen species (45).
Apoptosis of Monocytes
In this study, we have shown that spontaneous, PHA- and Mtb-hsp induced apoptosis of monocytes from SA patients was comparable to the control but was higher than in tuberculosis. PHA- and Mtb-hsp-induced apoptosis of monocytes only in tuberculosis, whereas the percentage of apoptotic monocytes between controls and SA patients was comparable. Only peripheral blood monocytes from SA patients were resistant to apoptosis induction by either PHA or mycobacterial hsp.
In sarcoidosis, the analysis of apoptosis of monocytes/macrophages has shown conflicting results. Authors (21, 39) found increased apoptosis of macrophages in SA. In contrast, Xaus et al. (19) showed that macrophages were unable to undergo apoptosis. Rutherford et al. (18) found that in SA patients the apoptosis-related gene products in PBMC, in particular, the Bcl-2 family and growth factors genes, were up-regulated consistent with a pro-survival profile. Others (19) found no difference between pro-apoptotic (Bax, Bcl-Xs, TNFR1) and anti-apoptotic genes (Bcl-2, Bcl-XL) in the sarcoid granuloma.
There are no reports on in vivo apoptosis of peripheral blood monocytes in patients with TB. However, it was shown in vitro that infection by M. tuberculosis promotes human alveolar macrophage apoptosis (16, 28, 35). Also, an in vivo study conducted by Placido et al. (37) revealed increased programmed cell death of alveolar macrophages in TB, whereas in vitro M. tuberculosis-induced apoptosis of monocyte-derived macrophages could not be observed. Some studies (33, 34) conducted in cell culture models of mycobacterial infection suggest that monocyte/macrophage apoptosis is a very early event strictly dependent on the M. tuberculosis amount via regulation of surface CD14 expression on monocytes. Other studies (16, 26–32) suggest that, due to prevalence of anti-apoptotic cytokines (IL-4, IL-10, TGF-β) with subsequent decrease an expression of NO synthetase and formation of inactive TNF-α-TNFR2 complexes, the virulent M. tuberculosis strain induces substantially less macrophage apoptosis than the attenuated strain.
Apoptosis of CD4+ T and CD8+ T Lymphocytes
Our results showed significant reduction of spontaneous (p=0.052) and Mtb-hsp-induced apoptosis of CD4+ T-cells from TB patients in comparison with the control and SA patients. The apoptosis of CD4+ T-cells was increased after Mtb-hsp stimulation only in sarcoidosis, whereas PHA stimulation showed decreased apoptosis of sarcoid CD4+ T lymphocytes and increased in TB patients and in controls. Results of the current study did not demonstrate any differences in spontaneous apoptosis of CD8+ T-cells between the investigated groups. The percentage of apoptotic CD8+ T-cells after Mtb-hsp stimulation was higher in our controls than TB and SA patients, whereas PHA-induced apoptosis was increased in all groups tested.
In sarcoidosis, the analysis of apoptosis of T lymphocytes has shown conflicting results. Stridh et al. (20) found that BAL fluid lymphocytes from patients with sarcoidosis display a non-apoptotic morphology associated with endogenous caspase-3 activity. Other studies (38, 39) report the increased apoptosis of CD4+ T- and CD8+ T-cells. Comparative analysis of the apoptosis of CD4+ T and CD8+ T lymphocytes demonstrated an increased apoptosis of CD4+ T-cells compared to CD8+ T-cells and higher apoptosis of T lymphocytes from peripheral blood than BAL fluid lymphocytes in sarcoidosis (38).
Also in tuberculosis, the analysis of apoptosis of T lymphocytes has shown conflicting results. The data obtained by Hirsch et al. (29) revealed increased spontaneous as well as M. tuberculosis-stimulated CD4+ T-cell apoptosis in TB patients. It has been also reported (16) that mycobacterial infection causes increased Fas expression and decreased bcl-2 expression in CD4+ T-cells. Other authors (30) have shown no difference in spontaneous apoptosis of CD4+ T lymphocytes between healthy subjects and tuberculosis patients. Comparison of unstimulated and stimulated PBMC cultures indicated that M. tuberculosis increased the CD4+ T-cell apoptosis in TB patients due to increased serum levels of anti-apoptotic TGF-β and IL-10 (32, 46).
It is possible that differences in the mechanism of cell death involved in individual experimental systems (mycobacterial preparation, doses, duration of culture), the different M. tuberculosis strains used, various ethnic origins of tested individuals, and the differential susceptibility of infected cells to apoptosis may explain these discordant results.
To our knowledge, this is the first study on the analysis of mycobacterial hsp-induced apoptosis of peripheral monocytes in sarcoidosis and tuberculsosis. In conclusion, the differences in apoptosis of monocytes and CD4+ T lymphocytes in SA and TB may suggest different mechanisms of pathogenesis in these diseases. In contrast to TB, sarcoid monocytes were resistant to PHA- and Mtb-hsp-induced apoptosis, whereas CD4+ T-cells were resistant to PHA- but not to Mtb-hsp-induced apoptosis.
REFERENCES
American Thoracic Society: Statement on sarcoidosis. Am J Respir Crit Care Med 160:736–755, 1999
Dubaniewicz A, Kämpfer S, Singh M: Serum anti-mycobacterial heat shock proteins antibodies in sarcoidosis and tuberculosis. Tuberculosis (Edinb) Dec 10;86(1):60–67, 2005 [E-pub ahead of print]
Lillebaek T, Thomsen VO: A patient with suspected sarcoidosis died from miliary tuberculosis. Scand J Infect Dis 32:218–220, 2000
Mangiapan G, Hance AJ: Mycobacteria and sarcoidosis: An overview and summary of recent molecular biological data. Sarcoidosis 12:20–37, 1995
Staton JM, Dench JE, Currie B, et al.: Expression and immune recognition of stress proteins in sarcoidosis and other chronic interstitial lung diseases. Immunol Cell Biol 73:23–32, 1995
Raja A, Uma Devi KR, Ramalingam B, Brennan PJ: Immunoglobulin G, A, and M responses in serum and circulating immune complexes elicited by the 16-kilodalton antigen of Mycobacterium tuberculosis. Clin Diagn Lab Immunol 9:308–312, 2002
Jaskiewicz K, Rzepko R, Dubaniewicz A, et al.: Pregranulomayous phase of sarcoidosis: Immunohistochemical diagnosis. Acta Histochem Oct 24, 2005 [E-pub ahead of print]
Zügel U, Kaufmann SHE: Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev 12:19–39, 1999
Stuart JK, Myszka DG, Joss L, et al.: Characterization of interactions between the anti-apoptotic protein BAG-1 and Hsc70 molecular chaperones. J Biol Chem 273:22506–22514, 1998
Friedland JS, Shattock R, Remick DG, Griffin GE: Mycobacterial 65-kD heat shock protein induces release of proinflammatory cytokines from human monocytic cells. Clin Exp Immunol 91:58–62, 1993
Wang Y, Kelly CG, Singh M, et al.: Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. Immunol 169:2422–2429, 2002
Lang D, Hubrich A, Dohle F, et al.: Differential expression of heat shock protein 70 (hsp70) in human monocytes rendered apoptotic by IL-4 or serum deprivation. J Leukoc Biol 68:729–736, 2000
Detanico T, Rodrigues L, Sabritto AC, et al.: Mycobacterial heat shock protein 70 induces interleukin-10 production: Immunomodulation of synovial cell cytokine profile and dendritic cell maturation. Clin Exp Immunol 135:336–342, 2004
Sreedhar AS, Csermly P: Heat shock proteins in the regulation of apoptosis: New startegies in tumor therapy. A comprehensive review. Pharmacol Ther 101:227–257, 2004
Cree IA, Nurbhai S, Milne G, Beck JS: Cell death in granulomata: The role of apoptosis. J Clin Pathol 40:1314–1319, 1987
Klingler K, Tchou-Wong KM, Brandli O, et al.: Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect Immun 65:5272–5278, 1997
Fratazzi C, Arbeit RD, Carini C, et al.: Macrophage apoptosis in mycobacterial infections. J Leukoc Biol 66:763–764, 1999
Rutherford RM, Kehren J, Staedtler F, et al.: Functional genomics in sarcoidosis-reduced or increased apoptosis? Swiss Med Wkly 131:459–470, 2001
Xaus J, Besalduch N, Comalada M, et al.: High expression of p21Waf1 in sarcoid granulomas: A putative role for long-lasting inflammation. J Leukoc Biol 74:295–301, 2003
Stridh H, Planck A, Gigliotti D, et al.: Apoptosis resistant bronchoalveolar lavage (BAL) fluid lymphocytes in sarcoidosis. Thorax 57:897–901, 2002
Dai H, Guzman J, Costabel U: Increased expression of apoptosis signalling receptors by alveolar macrophages in sarcoidosis. Eur Respir J 13:1451–1454, 1999
Behnia M, Robertson KA, Martin WJ: Role of apoptosis in host defense and pathogenesis of disease. Chest 117:1771–1777, 2000
Ashkenazi A, Dixit VM: Death receptors: Signaling and modulation. Science 281:305–308, 1999
Cohen JJ, Duke RC, Fadok VA, Sellins KS: Apoptosis and programmed cell death in immunity. Ann Rev Immunol 10:267–293, 1992
Savill J: Apoptosis in resolution of inflammation. J Leukoc Biol 61:375–380, 1997
Thoma-Uszynski S, Stenger S, Modlin RL: CTL-mediated killing of intracellular M. tuberculosis is independent of target cell nuclear apoptosis. J Immunol 165:5773–5779, 2000
Beltan E, Horgen L, Rastogi N: Secretion of cytokines by human macrophages upon infection by pathogenic and non-pathogenic mycobacteria. Microb Pathog 28:313–318, 2000
Balcewicz-Sablinska MK, Gan H, Remold HG: Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-α activity. J Infect Dis 180:1230–1237, 1999
Hirsch CS, Toossi Z, Johnson JL, et al.: Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J Infect Dis 183:779–788, 2001
Hertoghe T, Wajja A, Ntambi L, et al.: T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB). Clin Exp Immunol 122:350–359, 2000
Rojas M, Barrera LF, Puzo G, Garcia LF: Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: Role of nitric oxide and mycobacterial products. J Immunol 159:1352–1361, 1997
Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ: Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J Immunol 154:465–473, 1995
Heidenreich S, Schmidt M, August C, Cullen P, Rademaekers A, Pauels HG: Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol 159:3178–3188, 1997
Santucci MB, Amicosante M, Cicconi R, et al.: Mycobacterium tuberculosis-induced apoptosis in monocytes/macrophages: Early membrane modifications and intracellular mycobacterial viability. J Infect Dis 181:1506–1509, 2000
Keane J, Balcewicz-Sablinska K, Remold HG, et al.: Infection by M. tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun 65:298–304, 1997
Bingisser R, Stey C, Weller M, Groscurth P, Russi E, Frei K: Apoptosis in human alveolar acrophages is induced by endotoxin and is modulated by cytokines. Am J Respir Cell Mol Biol 15:64–70, 1996
Placido R, Mancino G, Amendola A, et al.: Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J Pathol 181:31–38, 1999
Herry I, Bonay M, Bouchonnet F, et al.: Extensive apoptosis of lung T-lymphocytes maintained in vitro. Am J Respir Cell Mol Biol 15:339–347, 1996
Kunitake R, Kuwano K, Miyazaki H, Hagimoto N, Nomoto Y, Hara N: Apoptosis in the course of granulomatous inflammation in pulmonary sarcoidosis. Eur Respir J 13:1329–1337, 1999
Domagała-Kulawik J, Droszcz P, Kraszewska I, et al.: Expression on cell in then the Fas antigen in the cells from bronchoalveolar lavage fluid (BALF). Folia Histochem Cytobiol 38:185–188, 2000
Hisaeda HI, Sakai T, Ishikawa H, et al.: Heat shock protein 65 induced by γδ Tcells prevents apoptosis of macrophages and contributes to host defense in mice infected with Toxoplasma gondii. J Immunol 159:2375–2381, 1997
Zügel U, Kaufmann SHE: Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev 12:19–39, 1999
Sakai T, Hisaeda H, Ishikawa H, et al.: Expression and role of heat shock protein 65 (hsp65) in macrophages during Trypanosoma cruzi infection: Involvement of hsp65 in prevention of apoptosis of macrophages. Microbes Infect 1:419–1427, 1999
Zhang M, Hisaeda H, Sakai T, et al.: CD4+ T cells are required for HSP65 expression in host macrophages and for protection of mice infected with Plasmodium yoelii. Parasitol Int 50:201–209, 2001
Arrigo AP: Small stress proteins: Chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem 379:19–26, 1998
Bonecini-Almeida MG, Ho JL, Boéchat N, et al.: Down-modulation of lung immune responses by interleukin-10 and transforming growth factor β (TGF-β) and analysis of TGF-β receptors I and II in active tuberculosis. Infect Immun 72:2628–2634, 2004
ACKNOWLEDGMENTS
The authors thank Dr. S.E. Jamieson (Cambridge Institute for Medical Research, Wellcome Trust/MRC Building, University of Cambridge School of Clinical Medicine, Addenbrookes Hospital, Cambridge, UK) for the English language improvements. This study was funded by the Polish State Committee for Scientific Researches, Grant No. 3PO5B 15522 (to A.D.1)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubaniewicz, A., Trzonkowski, P., Dubaniewicz-Wybieralska, M. et al. Comparative Analysis of Mycobacterial Heat Shock Proteins-Induced Apoptosis of Peripheral Blood Mononuclear Cells in Sarcoidosis and Tuberculosis. J Clin Immunol 26, 243–250 (2006). https://doi.org/10.1007/s10875-006-9011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-006-9011-9