Abstract
Emissions of biogenic volatile organic compounds (BVOCs) from mechanical wounding of leaves and branches of plants can contribute to the atmospheric burden of volatile organic compounds (VOCs) in both (a) urban airsheds (from urban garden maintenance) and (b) the global atmosphere (from large scale forest harvesting). These emissions of BVOCs are poorly understood and quantified, and their role in urban and global emissions inventories neglected. This paper presents measurements of the magnitude, duration and composition of emissions of BVOCs, carbon dioxide (CO2) and methane (CH4) from freshly cut leaf mulch and wood chips derived from a common eucalypt tree, Eucalyptus sideroxylon (red ironbark), found in southeastern Australian forests and gardens. The emissions of BVOCs from freshly cut and shredded leaves and wood of E. sideroxylon were found to be 2.3 ± 0.6 and 0.05 ± 0.04 mg g-1 DM (Dry Mass) from leaf mulch and wood chips respectively and to last typically for 1 day following cutting. Three sampling techniques were used for VOC speciation and the 12 most abundant BVOCs released from the mulch materials were identified. The specific BVOCs emitted in order of decreasing abundance from leaf mulch are: (a) stored plant oils, 1,8-cineole, α–pinene and o-cymene which make up the major part of the emissions, (b) a minor contribution from chemicals associated with environmental stress and wound defence, (Z)–3–hexenyl acetate, (E)-2-hexenal and (Z)-3-hexen-1-ol, and (c) a second minor contribution from metabolic products, acetaldehyde and acetone. The observed integrated emissions of BVOCs from leaves following mulching are equivalent to more than half and perhaps all of the likely stored plant oils in the leaves. For the two comparable studies available, one of a plant with stored oils (this study) and one of a plant without stored plant oils, the emissions of leaf wound defence BVOCs are in the same range for both plants. In the plant with stored plant oils, the plant oil emissions are about a factor of 11 larger in emission rate than the plant wound defence BVOCs. A compilation of available leaf wounding BVOC emission studies indicates that for plants with stored plant oils, plant oil emissions dominate, whereas with other plants, leaf wound defence BVOCs dominate the emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plants produce a myriad of biogenic volatile organic compounds (BVOCs) including many hydrocarbons and oxygenated organic compounds. Global emissions of BVOCs were estimated by Guenther et al. (1995) to account for approximately 86% of the total of VOCs emitted to the atmosphere, anthropogenic VOCs making up the balance. Emissions of BVOCs from intact trees and woody shrubs have been reviewed by Scholes et al. (2003) who identified the dominant compounds emitted under unperturbed conditions as isoprene, monoterpenes, sesquiterpenes and methanol. These compounds are emitted through leaf stomata, intact plant surfaces and damaged plant surfaces during the various stages of plant growth, plant injury and plant decay (Scholes et al. 2003). He et al. (2000) and Winters et al. (2009) specifically studied emissions from undisturbed Australian eucalyptus species. These studies showed similar emission rates and speciation of BVOCs from intact plants as were indicated by Scholes et al. (2003).
An emerging research issue concerns the effect of plant injury and environmental stress on the type and rate of BVOCs emitted from plants (Copolovici et al. 2011; Hu et al. 2008, 2011; Lucas-Barbosa et al. 2011; Niinemets et al. 2010; Piesik et al. 2010; Teuber et al. 2008). In this work, the focus is on plant injury. The suggestion that enhanced emissions of BVOCs arise from disturbance of vegetation was put forward by Rasmussen (1970). The identification of wound defence compounds, hexenyl acetate, hexenals, hexenols and n-hexanal as key BVOCs, was made in laboratory studies by Hatanaka et al. (1978). Juuti et al. (1990) demonstrated enhancement of monoterpene emissions after repeated rough handling of the plants in-situ. The work of Arey et al. (1991), Winer et al. (1992) and Konig et al. (1995) established that emissions to the atmosphere of (Z)-3-hexen-1-ol increased significantly as the result of mechanical damage to many broad-leafed plants. Subsequent work demonstrated that plant wounding causes episodic emissions of C6 aldehydes, esters and alcohols (Kirstine et al. 1998; Fall et al. 1999; Perera et al. 2002; Kirstine and Galbally 2004; Mayland et al. 1997). Kirstine and Galbally (2004) showed that the C6 compounds emitted from cut grass are likely to be significant contributors to VOCs in urban airsheds.
The work that has been reported on mechanical cutting and wounding of leaves and wood includes studies of BVOC emissions from leaf and needle cutting (Fall et al. 1999; Loreto et al. 2000; Litvak et al. 2002; Pasqua et al. 2002; Su et al. 2009), vegetative litter left after thinning of a forest (Goldstein et al. 2004), mulching of leaves and wood from Grevillea robusta (Fedele et al. 2007) and the kiln drying process during the preparation of sawn timber for commercial use (Englund and Nussbaum 2000; Strömvall and Petersson 1993). All of these studies show significantly enhanced emissions of BVOCs associated with plant mechanical wounding.
In previous work (Fedele et al. 2007), we reported on emissions from mulched fresh leaves and wood chips taken from the Australian native tree, Grevillea robusta. In this work, a further exploratory study, we report on the results of another common Australian native, Eucalyptus sideroxylon (red ironbark) which differs significantly from G.robusta in that its leaves have resin ducts containing stored oils. As with the previous study, the magnitude, duration and composition of BVOCs emitted from freshly mulched leaves and wood chips were measured using a flux chamber.
The results from these studies will aid in estimating the contribution of BVOC emissions from mulching to the atmospheric load of VOCs, and hence contribute information required for air quality modelling.
2 Materials and methods
The methods adopted for this project have been used in similar experiments involving cut grass (Kirstine et al. 1998) and wood chips and leaf mulch (Fedele et al. 2007).
Eucalyptus sideroxylon (red ironbark) is an Australian native tree, widely distributed in Victoria and New South Wales (Australian Native Plants Society 2003). E. sideroxylon grows as a medium-sized tree (10–30 m) in woodland areas and like most eucalypts its leaves are rich in essential oils stored in resin ducts. There are two varieties of E. sideroxylon, var. sideroxylon and var. tricarpa (Bramwells and Wiffen 1984). The tree studied here was identified as E. sideroxylon var. sideroxylon.
The term wood chips is defined as in our previous study (Fedele et al. 2007). It refers to the chips made from the combination of the wood and bark, since this is the common procedure in making wood chips for garden mulch.
2.1 Chipping and mulching
Mulching of leaves and branches was performed using an 1800 watt mulcher (shredder) manufactured by Talon Tools Australia: (no load speed: 2900 rpm, weight 22 kg and branch capacity 35 mm). Secateurs were used to cut the branches from the tree.
Branches were cut from a mature E. sideroxylon var sideroxylon tree located in the grounds of the CSIRO Aspendale Laboratory. The leaves were stripped off the branches and then the stripped leaves were fed through the mulcher. This was then repeated using the product mulch to get more finely mulched leaves. The leaves were 2 to 3 cm in length and about 0.4 mm thick. It appeared that each leaf was cut into about 4 pieces. The stripping and mulching of approximately 500 g of leaves occurred on the 26th of July and the 2nd, 16th, and 30th of August, 2004.
Wood chips were prepared from woody branches. Prior to mulching, the leaves, flowers and nuts were stripped off the branches. The woodchips ranged in size from 0.4 mm to 7 cm in length and generally were about 0.3 mm thick. Mulching of approximately 1 kg of wood (including bark) from the tree branches occurred on the 28th of July, the 16th of August and the 2nd of September 2004.
As soon as a leaf mulch or wood chip sample was prepared, a quantity of the sample was weighed (either 0.5 kg of fresh leaf mulch or 1 kg of wood chips) and placed as a 1 cm deep layer in the base of the flux measurement chamber (described in Fedele et al. (2007)) for the BVOC emission measurement. The time from commencement of the first mulching to commencement of the emission measurements was less than 30 min.
To determine the fraction of moisture in the mulch, a separate leaf mulch or wood chip sample of about 10 to 15 g was placed in an oven tray and weighed. The sample was dried in a LABLEC oven (Lablec Equipment Australia) at ~70°C for approximately 24 h. After being dried, the sample was reweighed to determine the ratio of fresh mass to dry mass (DM).
2.2 Chamber system and emission estimation
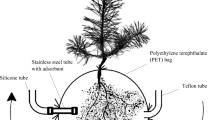
The full details of the chamber system used in this study were described in Fedele et al. (2007). The flux measurement chamber consists of two sections, the top, commonly regarded as the chamber, and the base which has a solid bottom and is used to contain the chips or mulch. The top is essentially an open bottomed mainly transparent box, 490 mm wide by 690 mm long by 400 mm tall. The chamber is made of an anodized aluminium frame and perspex windows lined with Tedlar® sheets on the inside. The chamber top has two pneumatically operated lids. When the lids were up, two fans, one in each opening, were activated (one fan drives air into the chamber, the other fan extracts air from the chamber) to flush the chamber and return the air in the chamber to as near to ambient conditions as possible. When the chamber lids were open, the concentrations of VOCs and CO2 in the chamber air were virtually indistinguishable from ambient air. When the lids were closed, the chamber has a closed constant volume of air. The chamber is not completely sealed, it has a small leak tube to prevent the build up of pressure differences between inside and outside the chamber.
The chamber was housed inside the laboratory at constant temperature of 20–21°C. Air was continuously drawn from the flux chamber at approximately 1 litre per minute and was monitored for the concentrations of VOCs, CO2 and CH4. The chambers were operated in an automatic static mode, closing once per hour for 20 min, followed by 40 min open. This pattern of closing and opening the chamber was repeated each hour for a period of approximately 48 h for each leaf mulch and wood chip experiment. The observed rate of change of concentration of VOCs, CO2 and CH4 in the chamber air for the 20 min periods following each chamber closure was transformed into an emission rate using conservation of mass and corrections for sampling and leakage (Fedele et al. 2007).
2.3 Continuous measurement of VOCs, CO2 and CH4 in the chamber
CO2 concentrations were measured using a non-dispersive infrared (NDIR) gas analyser (GasHound Model LI-800, LI-COR, Inc., Lincoln, Nebraska, USA). This instrument has an accuracy of 2% of the reading for CO2 concentrations >350 ppmv, as specified by the manufacturer. The calibration of the concentration of CO2 was based on the internationally accepted World Meteorological Organisation X93 CO2 mole fraction calibration scale.
CH4 and VOC concentrations were measured using a total hydrocarbon analyser (TEI Model 55°C, Thermo Environmental Instruments, TEI, Franklin MA, USA). This instrument is a gas chromatograph with flame ionisation detection system (GC-FID). We have ascertained from previous studies, including measurements of a range of non methane hydrocarbons (NMHC) and oxygenated VOC gas standards, (Fedele et al. 2007), that the TEI instrument responds to oxygenated VOCs and other substituted organics. The empirical calibration applied in this study for BVOC measurement is described below.
The TEI 55°C analyser was initially calibrated for CH4, and nominally calibrated for VOCs using a National Institute of Standards and Technology Standards Reference Material 1660a certified as 3.94 ± 0.02 μmol/mol (ppm) CH4 and 0.972 ± 0.005 μmol/mol (ppm) propane in air. Then for a set of VOC concentrations generated during the experiments by leaf mulch in the chamber, the nominal VOC response of the TEI 55°C was compared with the total VOC concentrations from active samples collected simultaneously by adsorbent tubes and analysed for the integrated concentration VOC species in the range of C2 to C10 by GC-FID. The analysis of the adsorbent tubes by GC-FID-MSD is described in section 2.6. The nominal VOC concentrations from the TEI 55°C continuous analyser were determined during this study to be a factor of 2.0 ± 0.2 less than the full VOC GC-FID analysis. This diminished response arises because the TEI 55°C system has a decreasing response to (a) higher molecular weight VOCs including both alkanes and aromatic compounds and (b) oxygenated VOCs. The diminished response is due in part to the failure of the TEI to adequately integrate tailing peaks from these higher molecular weight hydrocarbons and oxygenated VOCs. The subsequent presentations of VOC concentrations and emission estimates determined by the TEI instrument have been corrected by this factor to bring them in line with the sum of the concentrations of VOCs obtained by the GC-FID analysis. The emissions calculated here apply to the VOC compounds of 10 carbon atoms and less.
2.4 Adsorbent tube sampling
The thermal desorption tubes used in this study contained a stratified packing of Tenax TA/CarbosieveSIII (Markes International, United Kingdom) which is suitable for sampling a wide range of VOCs.
For each experiment, adsorbent tubes were used both while the chamber lids were open and when they were closed to trap VOCs from the chamber air for later analysis by GC-FID-MSD. The adsorbent tube sampling was done primarily during the first hour after the leaf mulch or wood chips were placed in the chamber. Adsorbent tube samples, defined as “background” air samples, were taken when the chamber lids were open to determine background concentrations of VOCs. The chamber lids were then closed and the chamber left for 15 min after which a second batch of air samples was taken from inside the closed chamber where the emissions from the mulch had accumulated. These samples were described as “emission” air samples. The composition of the emitted gases was determined by subtracting the background analysis from the emission analysis for the two tubes sampled during the one experiment. Tube samples were also taken as blanks in the same way as described above, except that there was no leaf mulch or wood chips in the chamber at the time of taking the air samples. These blanks were a precaution to monitor against extraneous VOC contamination. The sampling rate was 76 mL min-1 for 5 min, after which the tubes were disconnected from the system and immediately sealed. The subsequent GC-FID-MS analysis generally occurred on either the same day or the day subsequent to sample collection.
2.5 Solid Phase Microextraction Vial sampling
Solid Phase Microextraction (SPME) sampling for VOC emissions from leaves and wood chips was carried out using headspace sampling of mulch contained within 10 mL vials. Poly-dimethylsiloxane (PDMS) (thickness: 100 μm) and Carboxen/Poly-dimethylsiloxane (CAR/PDMS) (thickness: 75 μm) fibres were used to adsorb the volatile species. PDMS fibres generally adsorb non-polar compounds such as alkanes because the coating is non-polar. The combined Carboxen/PDMS (CAR/PDMS) fibre traps a wider range of compounds including most alkanes as well as polar compounds (Pawliszyn 1997).
Approximately 2 g of leaves or 3 g of wood were chopped and placed into separate vials, which were sealed with caps. The samples were left for 30 min to allow enough time for VOCs to accumulate in the head space. The SPME needle was then inserted through an access septum and the headspace compounds were exposed to the fibre for 20 min. The accumulation and extraction time were decided based on previous experiments (Perera et al. 2002). The fibre was directly injected to the heated injection port of the GC-FID-MSD for desorption.
2.6 Chemical Analysis of Adsorbent tubes and SPME fibres
Adsorption tube samples were analysed on an Agilent 6890 gas chromatograph (GC) fitted with two detectors operated in parallel, an Agilent 5973 N mass selective detector (MSD) and a flame ionization detector (FID). The tube samples, and gas standards used for calibration, were desorbed at 240°C and cryogenically concentrated for injection onto a capillary column using an Entech 7100 preconcentrator. The GC column was an Alltech AT-1 of 60 m length with a 320 μm internal diameter and a 1 μm film thickness. The temperature program was −50°C (6 min), 15°C/min to −10°C, 5°C/min to 150°C then 25°C min-1 to 240°C. The column flow rate was held constant at 2 mL min-1 over the 47 min analysis time. The software used for instrument control and data acquisition was ChemStation version DA (Agilent). The MSD was tuned using the ChemStation Standard Tune method and the mass range scanned was from 33–250 amu. A fixed volume of a 4-component internal standard (10 ppm of each compound of bromochloromethane, 1,4-difluorobenzene, chlorobenzene-d5 and 1-bromo-3-fluorobenzene) was introduced with each sample to monitor the response of the MSD. The internal standards showed consistent peak areas over the set of analyses presented here.
For SPME analyses, the same GC-FID-MSD system as described above was used. The operating conditions were: injection mode: splitless (holding 1 min); initial temperature: 45°C; final temperature: 240°C; increasing at 5°C min-1, flow rate: 2 mL min-1.
Compounds were identified using the MS library NBS75K.L (G1033a Rev C.00.00 NIST/EPA/NIH Mass Spectral Database). A compound was considered for acceptance if the match was above 85%. Those compounds with a match below 85% were only considered for acceptance if upon manual inspection their spectra matched.
A semi-quantitative analysis of the VOC speciation was undertaken using the mass spectral total ion count (TIC). The TIC areas of the chromatographic peaks were used to calculate the percentage contribution from each peak to the whole of the VOC emissions detected for that sample. Because of issues of calibration, the SPME results are not included in the attribution of final emissions.
3 Results
3.1 Leaf mulch emissions
Leaves were mulched and placed in the chamber on four occasions as described above. The concentrations of BVOCs and CO2 from leaves versus time after mulching showed a build up in concentrations of these gases in the closed chamber similar to that shown in Fedele et al. (2007). During the first chamber closure after leaf mulching, the concentrations increased to more than 360 ppmC of BVOCs and 750 ppm of CO2 (this CO2 concentration includes ambient CO2). No detectable amount of CH4 was released from leaves after mulching. The concentration of gases when the chambers were opened dropped to near ambient levels within a few minutes. Emissions were calculated from the concentration changes during the period when the chamber was closed.
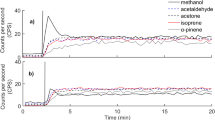
The emissions of BVOCs and CO2 from leaf mulch are plotted for each hour following the mulching in Fig. 1(a) and (b), respectively. The BVOC and CO2 emissions were a maximum immediately after mulching and decreased to near zero within 20 to 30 h of mulching. A comparison of the four separate leaf sample emission rates for BVOCs and CO2 shows that the results were very similar. The average total (integrated) mass emissions of BVOCs and CO2 were 2.3 ± 0.6 and 6 ± 1 mg g-1 DM of leaf mulch, respectively see Table 1. The emission of methane from the leaf mulch was below the limit of detection of ~1 × 10-11 g g-1 DM s-1.
3.2 Wood chip Emissions
Wood was mulched and placed in the chamber, also on four occasions. During the first chamber closure, the concentrations increased to more than 10.7 ppmC of BVOCs and 694 ppm of CO2; the former is much lower than the equivalent emissions from leaves. The emissions from wood chips were at their greatest immediately after mulching and rapidly decreased to near zero around a day after chipping for BVOCs and 2 days after chipping for CO2. The average total mass emissions of BVOCs and CO2 were 0.05 ± 0.04 and 2.4 ± 0.7 mg g-1 DM of wood chips, respectively (Table 1). The emission of methane from the wood chips was below the limit of detection of ~1 × 10-11 g g-1 DM s-1.
3.3 Speciation of the leaf mulch and wood chip emissions of BVOCs
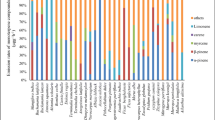
The 12 most abundant biogenic volatile organic compounds released from leaf mulch and wood chips as identified by adsorbent tubes and SPME are presented in Table 2. The selection for each compound (analyte) was based on the analyte making up more than 1% of the total signal in one or more of the analyses. There were three further compounds tentatively identified in the SPME analyses, the sesquiterpenes: allo-aromadendrene, aromadendrene and β-caryophyllene. The fit of the mass spectra for these had a median quality of 99%, however without direct standards the identification must be tentative. These compounds made up 0 to 1.5% of the total signal.
One question is how well the different sampling and analysis techniques capture the BVOCs emitted in this study? Table 2 indicates that the adsorption tubes/thermal desorption GC-FID-MSD used in this study were effective for sampling and analysis of BVOCs with boiling points (BPs) in the range 20–180°C; this includes simple organic compounds through to monoterpenes. The SPME fibres/thermal desorption GC-FID-MSD used were effective for sampling BVOCs with BPs from 150 to 260°C, which covers the range from C6 compounds, through monoterpenes and several sesquiterpenes.
Table 2, as well as presenting the composition of BVOC emissions, also presents a classification, according to Scholes et al. (2003), of whether the individual BVOCs either are compounds: arising from normal metabolic activity (MP), stored in the plant (plant oils, PO), stress induced and for wound defence (S&WD), or from other sources (O). Further, Table 2 presents the fractional composition of essential oils stored in the leaves of E. sideroxylon var sideroxylon from Bignell et al. (1997) for the species observed in this study. As well as the species listed in Table 2, Bignell et al. (1997) identified many other compounds including the monoterpene limonene that made up 6% of the composition of plant oils in his samples, but was not identified in our results. We did on one occasion sample the leaves of a juvenile E. sideroxylon tree growing alongside the main tree sampled and the juvenile tree’s leaves contained 4% limonene as well as the 1,8-cineole etc. Thus variability in leaf oil content may be significant and poorly quantified. Bignell et al. (1997) also identified that the sesquiterpene limonene that made up 6% of the composition of plant oils in his samples, but we would not expect to detect it in this study because of it high boiling point of 267–268°C, which was outside the qualitative recovery range (as determined by boiling point) of our SPME collection system.
4 Discussion
4.1 The total integrated emission rates of BVOCs from leaf mulch and wood chips
Emissions of BVOCs from mechanical wounding of E. sideroxylon prepared as leaf mulch and wood chips persist for up to 1 day after the mulching and chipping process, and CO2 emissions persist for up to 2 days, although more than 95% of the BVOCs and about 75% of the CO2 had been released in the first 8 h (Fig. 1). It is worth noting that the emissions of CO2 per mass of dry material from E. sideroxylon (2.4 ± 0.7 mg g-1 DM and 6 ± 1 mg g-1 DM) are very similar to the results previously reported for G Robusta (Fedele et al. 2007) ((2.5 ± 0.4 mg g-1 DM and 6.3 ± 0.6 mg g-1 DM).
The first question here is how do the emission rates compare between leaf mulch and wood chips? Emission rates of BVOCs of E. sideroxylon (red ironbark) leaf mulch and wood chips were significantly different with values of 2.3 ± 0.6 and 0.05 ± 0.04 mg g-1 DM of mulch material respectively. Total (integrated) emissions of BVOCs of leaf mulch were 46 times more than those of wood chips. The ratio of BVOC to CO2 emissions is around 0.4 for leaf mulch and 0.02 for wood chips. It is clear that wood chips emit far fewer BVOCs per unit of CO2 emitted than leaf mulch. This presumably relates to (a) the storage of plant oils in the leaves and (b) perhaps some functional differences between the living cells in leaves compared to plant stems.
The second question is how does the integrated BVOC emission from leaves following mulching compare with the total amount of plant oils stored in the leaves? The oil content of leaves of E. sideroxylon (based on dry weight) is 0.4% (Bignell et al. 1997), which translates to an oil content of 4 mg g-1 DM. The observed BVOC leaf emissions in this study following mulching are 2.3 ± 0.6 mg g-1 DM, which is equivalent to more than half of the likely stored plant oils in the leaves. Note that there was an unavoidable time delay (due to the several activities required) of less than 30 min between the commencement of the first mulching and the commencement of the emission measurement and there will be uncounted BVOCs lost in this period. Based on the highest observed initial flux, an estimate of this lost unobserved BVOC emission is 20% of the observed integrated emissions. Thus the total emissions will be slightly larger than that observed, and it is reasonable to assume that most of the stored plant oils are released to the atmosphere following leaf mulching.
The third question is how do these emissions compare with those from other plant species? There are few measurements with comparable methods and we are confined to a comparison with the preceding study of Fedele et al. (2007). Integrated emissions of BVOCs of E. sideroxylon were significantly greater than those of the G. robusta presented in Fedele et al. (2007) . Average integrated emissions of BVOCs of E. sideroxylon and G. robusta leaf mulch were 2.3 ± 0.6 and 0.38 ± 0.05 mg g-1 DM of mulch material respectively. Average integrated emissions of BVOCs of E. sideroxylon and G. robusta wood chips were 0.05 ± 0.04 and 0.022 ± 0.002 mg g-1 DM of mulch material respectively. The key differences between these plant species are that E. sideroxylon has oil storage ducts while Grevillea robusta does not, and the BVOC emissions from E. sideroxylon are largely plant oils while BVOC emissions from Grevillea robusta are other compounds. This will be explored further in the following section.
Finally, given some evidence of CH4 emissions from plants living in aerobic conditions (Keppler et al. 2006), are there indications of significant CH4 production from this tree, following mechanical wounding? The answer to this question is clearly “no” since any CH4 emissions from the leaf mulch and wood chips from E. sideroxylon examined here, were below the measured detection limit of 1 × 10-11 g g-1 DM s-1.
4.2 The composition of BVOC emissions from E. sideroxylon
The identities and fractions of total amount of BVOCs emitted from the leaf mulch and wood chips are presented in Table 2 along with the fraction that these compounds make up of plant oils analysed in other studies of E. sideroxylon.
The major BVOCs emitted from mechanical plant wounding captured by the adsorbent tubes can be classified according to their fractional occurrence (determined semi-quantitatively). These are: 1,8-cineole (75–88%), acetaldehyde (0–10%), α–pinene (5–6%), (Z)-3-hexen-1-ol (0–6%),(E)-2-hexenal (2–3%), o-cymene (0–2%), acetone (0–1%) and (Z)–3–hexenyl acetate (0–0.7%), (Table 2).
There are four possible processes that could cause the observed emissions of BVOCs. They are the:
-
release of BVOCs that are already stored within the plant material,
-
ongoing living processes of the plant material of photosynthesis and/or respiration,
-
plant response to the mechanical trauma, and
-
death of the plant material.
Each will be discussed in turn.
BVOCs that are already stored in the plant (called both essential oils and plant oils) will be released to the atmosphere via this mechanical damage to the plant structure. When the resin ducts, within which the essential oils are stored, are disrupted by mulching, these compounds are released. In the case of E. sideroxylon var sideroxylon, there is only one analysis available of the plant oil composition (Bignell et al. 1997) and this was obtained via freezing, grinding and vacuum distilling the leaves. The major BVOC constituents were: 1,8 cineole (~60%), α-pinene (~14%), limonene (~6%) and bicyclogermacrene (~6%) and the total oil yield was 0.4% by dry mass. In this study, as shown in Table 2, the major fraction (80–90%) of BVOCs emitted from mechanical plant wounding of E. sideroxylon, consisted of mainly 1,8-cineole and α–pinene with some o-cymene (plant oils). As shown in the previous section, these emissions correspond to the release of about half or more of the plant oils stored in the leaves.
The emissions of BVOCs as part of the plant wound defence mechanism (Heldt and Heldt 1997; Matsui 2006) includes the compounds (Z)-3-hexenyl acetate, (Z)-3-hexen-1-ol, (E)-2-hexenal and hexanal. In this study, Table 2, 1 to 10% of the VOC emissions from mechanical plant wounding came from these stress and wound defence compounds (Z)-3-hexenyl acetate and (E)-2-hexenal. The SPME analysis would indicate some (Z)-3-hexen-1-ol also.
There is an indication of ongoing metabolic activity in this plant material (after mechanical wounding) as indicated by approximately 1 to 10% of the plant emissions being acetaldehyde and acetone.
There is evidence of emissions of nonane and decane in these data. While these compounds did appear as contaminants in the background chamber measurements, they were present in higher concentrations in the emission measurements. Savage et al. (1996) observed alkanes in plant tissue including nonane and undecanes. Isidorov and Jdanova (2002) observed nonane and decane production in leaf litter.
Some simple organic compounds, described as phytohormones, including ethylene, jasmonic acid and methyl jasmonate, salicylic acid and methyl salicylate and abscisic acid are associated with the programmed cell death of leaves (Lim et al. 2007; Trobacher 2009). However, none of these were detected in this study.
In summary, the major BVOCs (Table 2), emitted in the first hour following mechanical wounding of the leaves of E. sideroxylon and the plant mechanism associated with them, in order of decreasing abundance are: (a) compounds arising from plant oils stored in the leaves and wood, 1,8-cineole, α–pinene and o-cymene, which make up the major fraction of the emissions, (b) a minor contribution from chemicals associated with environmental stress and wound defence, (Z)–3–hexenyl acetate, (E)-2-hexenal and (Z)-3-hexen-1-ol, and (c) a second minor contribution from compounds which are associated with normal plant metabolic processes acetaldehyde and acetone.
The major BVOCs identified in emissions occurring in the first hour following mechanical wounding of plants from other studies are listed in Table 3. It is evident from Table 3 that following mechanical wounding, for plants with oil storage ducts (eucalypts and pine) the major BVOCs released are the plant oils, whereas in grasses, silky oak and poplar, where oil storage is absent, the emissions are dominated by the wound defence BVOCs. The plant wound defence mechanism is ubiquitous in plants. Therefore it is reasonable to ask whether the release (emission) of plant oils substitutes for the emissions of normal wound defence compounds or whether the two processes are independent. The BVOC wound defence BVOC emissions from E. sideroxylon are typically 2–10% of the total emissions from leaves, or 0.04–0.23 mg g-1 DM and from G. Robusta (Fedele et al. 2007) are around 48% or 0.18 mg g-1 DM. Thus it appears that there may be no difference in the emissions of wound defence BVOCs from a plant either with stored plant oils in the leaves and one without. The additional emissions from the stored plant oils are 11 times those from the wound defence mechanism.
Ethanol and acetaldehyde are normally associated with anaerobic glycolysis in plants (Lehninger et al. 1993). All woody plants examined so far have the capacity to produce ethanol (Kimmerer and MacDonald 1987). While it is generally thought that ethanol is metabolized to acetaldehyde within the leaves, Jardine et al. (2009) provided evidence that it may also be formed from peroxidation reactions with the fatty acids released immediately after cutting. Ethanol and acetaldehyde made up typically half the BVOCs found in the leaf mulch and wood chip emissions from Grevillia robusta by Fedele et al. (2007). However, while acetaldehyde was observed in the emissions from E. sideroxylon, particularly from the wood chips (Table 2), ethanol was only observed in trace amounts (<1%). Information is needed on the rate of oxidation of ethanol within various plants before this observation can be explained.
5 Conclusion
The emissions of BVOCs from mechanically wounded (freshly mulched) Eucalyptus sideroxylon (red ironbark) leaves and wood have been measured and found to last typically for 1 day following mulching. Mass emissions of BVOCs from leaves and wood chips were calculated to be 2.3 ± 0.6 and 0.05 ± 0.02 mg g-1 DM of mulch materials respectively. The most abundant volatile organic compounds released from mulch materials (fractions determined semi-quantitatively) included: 1,8-cineole (75–88%), acetaldehyde (0–10%), α–pinene (5–6%), (Z)-3-hexen-1-ol (0–6%),(E)-2-hexenal (2–3%), o-cymene (0–2%), acetone (0–1%) and (Z)–3–hexenyl acetate (0–0.7%). These emissions arise from the release of plant oils stored in the leaves, the plant wound defence mechanisms and normal metabolic activity. A compilation of available leaf wounding BVOC emission studies indicates that for plants with stored plant oils, plant oil emissions dominate, whereas with other plants, leaf wound defence BVOCs dominate the emissions. For the two comparable studies available, one of a plant with (this study) and one plant without stored plant oils (Fedele et al. 2007), the emissions of leaf wound defence BVOCs are in the same range for both plants. The plant oil emissions, where present, are about a factor of 11 greater than the plant wound defence BVOCs. These emissions from mechanical wounding represent a currently unaccounted source of BVOCs in urban air that will contribute to both urban photochemistry and secondary organic aerosol formation.
References
Arey, J., Winer, A.M., Atkinson, R., Aschmann, S.M., Long, W.D., Morrison, C.L.: The emission of (Z)-3-hexen-1-ol, (Z)-3-hexenylacetate and other oxygenated hydrocarbons from agricultural plant species. Atmos. Environ. Gen. Top. 25A(5–6), 1063–1075 (1991)
Australian Native Plants Society: Eucalyptus sideroxylon. http://farrer.riv.csu.edu.au/ASGAP/e-sider.html (2003). Accessed 2nd October 2011
Bignell, C.M., Dunlop, P.J., Brophy, J.J., Jackson, J.F.: Volatile leaf oils of some Queensland and Northern Australian Species of the Genus Eucalyptus. (Series II). Part I. Subgenus Symphyomyrtus, Section Adnataria: (a) Series Oliganthae, (b) Series Ochrophloiae, (c) Series Moluccanae, (d) Series Polyanthemae, (e) Series Paniculatae, (f) Series Melliodorae and (g) Series Porantheroideae. Flavour Frag. J. 12, 19–27 (1997)
Bramwells, H.W., Wiffen, T.: Patterns of variation in eucalyptus sideroxylon A. Cunn. ex Woolls. I variation in adult morphology. Aust. J. Bot. 32, 263–281 (1984)
Copolovici, L., Kännaste, A., Remmel, T., Vislap, V., Niinemets, U.: Volatile emissions from Alnus glutionosa induced by herbivory are quantitatively related to the extent of damage. J. Chem. Ecol. 37(1), 18–28 (2011)
Englund, F., Nussbaum, R.M.: Monoterpenes in Scots Pine and Norway Spruce and their emission during kiln drying. Holzforschung 54(5), 449–456 (2000)
Fall, R., Karl, T., Hansel, A., Jordan, A., Lindinger, W.: Volatile organic compounds emitted from leaf wounding: On-line analysis by proton transfer-reaction mass spectrometry. J. Geophys. Res. 104(D13), 15963–15974 (1999)
Fedele, R., Galbally, I.E., Porter, N., Weeks, I.A.: Biogenic VOC emissions from fresh leaf mulch and wood chips of Grevillea robusta (Australian Silky Oak). Atmos. Environ. 41(38), 8736–8746 (2007)
Goldstein, A.H., McKay, M., Kurpius, M.R., Schade, G.W., Lee, A., Holzinger, R., Rasmussen, R.A.: Forest thinning experiment confirms ozone deposition to forest canopy is dominated by reaction with biogenic VOCs. Geophys. Res. Lett. 31, L22106 (2004)
Guenther, A., Hewitt, C.N., Erickson, D., Fall, R., Geron, C., Graedel, T., Harley, P., Klinger, L., Lerdau, M., McKay, W.A., Pierce, T., Scholes, B., Steinbrecher, R., Tallamraju, R., Taylor, J., Zimmerman, P.: A global model of natural volatile organic compound emissions. J. Geophys. Res. 100(D5), 8873–8892 (1995)
Hatanaka, A., Sekiya, J., Kajiwar, T.: Distribution of an enzyme system producing cis-3-hexenal and n-hexanal from linolenic and linoleic acids in some plants. Phytochemistry 17, 869–872 (1978)
He, C., Murray, F., Lyons, T.: Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmos. Environ. 34(4), 645–655 (2000)
Heldt, H.W., Heldt, F.: Plant Biochemistry and Molecular Biology. Oxford University Press, Michegan (1997)
Hu, Z., Leng, P., Shen, Y., Wang, W.: Emissions of saturated C6-C10 aldehydes from poplar (Populus simonii × P. pyramidalis ‘Opera 8277’) cuttings at different levels of light intensity. J. For. Res. 22(2), 233–238 (2011)
Hu, Z.H., Shen, Y.B., Luo, Y.Q., Shen, F.Y., Gao, H.B., Gao, R.F.: Aldehyde volatiles emitted in succession from mechanically damaged leaves of poplar cuttings. J Plant Biol 51(4), 269–275 (2008)
Isidorov, V.A., Jdanova, M.: Volatile organic compounds from leaves litter. Chemosphere 48, 975–979 (2002)
Jardine, K., Karl, T., Lerdau, M., Harley, P., Guenther, A., Mak, J.E.: Carbon isotope analysis of acetaldehyde emitted from leaves following mechanical stress and anoxia. Plant Biol. 11(4), 591–597 (2009)
Juuti, S., Arey, J., Atkinson, R.: Monoterpene emission rate measurements from a Monterey pine. J. Geophys. Res. 95(D6), 7515–7519 (1990)
Keppler, F., Hamilton, J.T.G., Braβ, M., Röckmann, T.: Methane emissions from terrestrial plants under aerobic conditions. Nature 439, 187–191 (2006)
Kimmerer, T.W., MacDonald, R.C.: Acetaldehyde and ethanol biosynthesis in plants. Plant Physiol. 84, 1204–1209 (1987)
Kirstine, W., Galbally, I., Ye, Y., Hooper, M.: Emissions of volatile organic compounds (primarily oxygenated species) from pasture. J. Geophys. Res. 103(D9), 10605–10619 (1998)
Kirstine, W.V., Galbally, I.E.: A simple model for estimating emissions of volatile organic compounds from grass and cut grass in urban airsheds and its application to two Australian cities. J. Air Waste Manag. Assoc. 54(10), 1299–1311 (2004)
Konig, G., Brunda, M., Puxbaum, H., Hewitt, C.N., Duckham, S.C., Rudolph, J.: Relative contribution of oxygenated hydrocarbons to the total biogenic VOC emissions of selected mid-European agricultural and natural plant species. Atmos. Environ. 29(8), 861–874 (1995)
Lehninger, A.L., Nelson, D.L., Cox, M.M.: Principles of Biochemistry, 2nd edn. Worth Publishers, New York (1993)
Lim, P.O., Kim, H.J., Nam, H.G.: Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136 (2007)
Litvak, M.E., Constable, J.V.H., Monson, R.K., Litvak, M.E., Constable, J.V.H., Monson, R.K.: Supply and demand processes as controls over needle monoterpene synthesis and concentration in Douglas fir Pseudotsuga menziesii(Mirb.) Franco. Oecologia 132(3), 382–391 (2002)
Loreto, F., Nascetti, P., Graverini, A., Mannozzi, M.: Emission and content of monoterpenes in intact and wounded needles of the Mediterranean Pine, Pinus pinea. Funct. Ecol. 14(5), 589–595 (2000)
Lucas-Barbosa, D., Van Loon, J.J.A., Dicke, M.: The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry 72(13), 1647–1654 (2011)
Matsui, K.: Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 9, 274–280 (2006)
Mayland, H.F., Flath, R.A., Shewmaker, G.E.: Volatiles from fresh and air-dried vegetative tissues of tall fescue (Festuca arundinacea Schreb.): relationship to cattle preference. J. Agric. Food Chem. 45(6), 2204–2210 (1997)
Niinemets, Ü., Arneth, A., Kuhn, U., Monson, R.K., Peñuelas, J., Staudt, M.: The emission factor of volatile isoprenoids: Stress, acclimation, and developmental responses. Biogeosciences 7(7), 2203–2223 (2010)
Pasqua, G., Monacelli, B., Manfredini, C., Loreto, F., Perez, G.: The role of isoprenoid accumulation and oxidation in sealing wounded needles of Mediterranean pines. Plant Science 163(2), 355–359 (2002)
Pawliszyn, J.: Solid Phase Microextraction (Theory and Practice). Wiley-VCH, Inc, New York (1997)
Perera, R.M., Marriott, P.J., Galbally, I.E.: Headspace solid-phase microextraction - comprehensive two-dimensional gas chromatography of wound induced plant volatile organic compound emissions. Analyst 127, 3–9 (2002)
Piesik, D., Wenda-Piesik, A., Lamparski, R., Tabaka, P., Ligor, T., Buszewski, B.: Effects of mechanical injury and insect feeding on volatiles emitted by wheat plants. Entomol. Fennica 21(2), 117–128 (2010)
Rasmussen, R.A.: Isoprene: identified as a forest-type emission to the atmosphere. Environ. Sci. Tech. 4, 667–671 (1970)
Savage, T.J., Hristova, M.K., Croteau, R.: Evidence for an elongation/reduction/C1-elimination pathway in the biosynthesis of n-heptane in xylem of Jeffrey pine. Plant Physiol. 111(4), 1263–1269 (1996)
Scholes, M.C., Matrai, P.A., Andreae, M.O., Smith, K.A., Manning, M.R., Artaxo, P., Barrie, L.A., Bates, S.S., Butler, J.H., Ciccioli, P., Cieslik, S.A., Delmas, R.J., Dentener, F.J., Ducce, R.A., Erickson, D.J., Galbally, I.E., Guenther, A.B., Jaenicke, R., Jäjne, A.J., Kienee, R.P., Lacaux, J.P., Liss, P.S., Malin, G., Matsonm, P.A., Mosier, A.R., Neue, H.U., Paerl, H.W., Platt, U.F., Quinn, P.K., Seiler, W. and Weiss, R.F.: Biosphere-Atmosphere Interactions. Atmospheric Chemistry in a Changing World: An Integration and Synthesis of a Decade of Tropospheric Chemistry Research: The International Global Atmospheric Chemistry Project of the International Geosphere-Biosphere Programme Global Change the IGBP series. Springer, Berlin (2003)
Strömvall, A.-M., Petersson, G.: Photooxidant-forming monoterpenes in air plumes from kraft pulp industries. Environ. Pollut. 79(3), 219–223 (1993)
Su, J.W., Zeng, J.P., Qin, X.W., Ge, F.: Effect of needle damage on the release rate of Masson pine (Pinus massoniana) volatiles. J. Plant Res. 122(2), 193–200 (2009)
Teuber, M., Zimmer, I., Kreuzwieser, J., Ache, P., Polle, A., Rennenberg, H., Schnitzler, J.P.: VOC emissions of Grey poplar leaves as affected by salt stress and different N sources. Plant Biol. 10(1), 86–96 (2008)
Trobacher, C.P.: Ethylene and programmed cell death in plants. Botany-Botanique 87(8), 757 (2009)
Winer, A.M., Arey, J., Atkinson, R., Aschmann, S.M., Long, W.D., Morrison, C.L., Olszyk, D.M.: Emission rates of organics from vegetation in California's central valley. Atmos. Environ. Gen. Top. 26A(14), 2647–2659 (1992)
Winters, A.J., Adams, M.A., Bleby, T.M., Rennenberg, H., Steigner, D., Steinbrecher, R., Kreuzwieser, J.: Emissions of isoprene, monoterpene and short-chained carbonyl compounds from Eucalyptus spp. in southern Australia. Atmos. Environ. 43(9), 3035–3043 (2009)
Acknowledgments
This work was part of Leesun Kim’s Honours year project and was carried out at the Aspendale Laboratory of CSIRO Marine and Atmospheric Research. We thank Mick Meyer, CSIRO Marine and Atmospheric Research for the botanical identification and other assistance, Terry Elms, Philip Marriott, Frank Antolasic and Paul Morrison, RMIT University, for their assistance with this project and Wayne Kirstine, Monash University, for his helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, L., Galbally, I.E., Porter, N. et al. BVOC emissions from mechanical wounding of leaves and branches of Eucalyptus sideroxylon (red ironbark). J Atmos Chem 68, 265–279 (2011). https://doi.org/10.1007/s10874-012-9221-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-012-9221-x