Abstract
This study elucidates the characteristics of ambient PM2.5 (fine) and PM1 (submicron) samples collected between July 2009 and June 2010 in Raipur, India, in terms of water soluble ions, i.e. Na+, NH +4 , K+, Mg2+, Ca2+, Cl−, NO −3 and SO 2−4 . The total number of PM2.5 and PM1 samples collected with eight stage cascade impactor was 120. Annual mean concentrations of PM2.5 and PM1 were 150.9 ± 78.6 μg/m3 and 72.5 ± 39.0 μg/m3, respectively. The higher particulate matter (PM) mass concentrations during the winter season are essentially due to the increase of biomass burning and temperature inversion. Out of above 8 ions, the most abundant ions were SO 2−4 , NO −3 and NH +4 for both PM2.5 and PM1 aerosols; their average concentrations were 7.86 ± 5.86 μg/m3, 3.12 ± 2.63 μg/m3 and 1.94 ± 1.28 μg/m3 for PM2.5, and 5.61 ± 3.79 μg/m3, 1.81 ± 1.21 μg/m3 and 1.26 ± 0.88 μg/m3 for PM1, respectively. The major secondary species SO 2−4 , NO −3 and NH +4 accounted for 5.81%, 1.88% and 1.40% of the total mass of PM2.5 and 11.10%, 2.68%, and 2.48% of the total mass of PM1, respectively. The source identification was conducted for the ionic species in PM2.5 and PM1 aerosols. The results are discussed by the way of correlations and principal component analysis. Spearman correlation indicated that Cl− and K+ in PM2.5 and PM1 can be originated from similar type of sources. Principal component analysis reveals that there are two major sources (anthropogenic and natural such as soil derived particles) for PM2.5 and PM1 fractions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the past two decades there has been a rapid increase in urbanization and industrialization in many cities of India. With this has come a dramatic increase in the number and density of motor vehicles (Taneja et al. 2008). With rapid urbanization and corresponding increase in traffic, there has been growing evidence that ambient concentration level of particulate matter (PM) is also rising up (He et al. 2001; Wang et al. 2005). Airborne PM is a mixture of solid and liquid particles suspended in the air. These particles are important in terms of their impact on the environment and public health. Hence, international policy on air quality, protection of human health and the protection of the environment requires research on ambient air particulate pollution (Hellebust et al. 2010). The various possible impacts caused by PM depend on their size, chemical composition, concentrations, accumulation time and exposure time (Mkoma et al. 2009). In recent years in Asian countries, such as India and China, the aerosol problem has become increasingly acute due to elevated loadings of atmospheric pollutants from increasing vehicular and industrial emissions as well as from increasing energy for domestic uses (Srivastava et al. 2008; Tsang et al. 2008; Zhang et al. 2008). Recently, many Indian studies have focused on PM measurement and their characterization for health and local air quality assessment covering urban areas (Katiyar et al. 2002; Sastry et al. 2004; Gadgil and Jadhav 2004; Ramachandran 2005; Ganguly et al. 2006; Karar and Gupta 2006; Chowdhury et al. 2007; Badrinath et al. 2007; Srivastava and Jain 2007; Bhaskar et al. 2008: Kulshrestha et al. 2009; Bhaskar and Mehta 2010; Deshmukh et al. 2010). However, more measurements and detailed chemical characterization of aerosols are needed for highly industrialized and densely populated metropolitan city of eastern central India.
The source characteristics and health hazards of the PM10, coarse (PM2.5–10; 2.5 < d p < 10 μm), fine (PM2.5; d p < 2.5 μm) and submicron (PM1; d p < 1 μm) particulate matter are distinct. It is admitted that the impact of PM2.5 and PM1 particles is larger than the impact of PM2.5–10 because these particles go deeper in the respiratory tract and become trapped on it, which affects the lung function (Fang et al. 2007).
In many urban and rural areas water soluble ionic species (Na+, K+, NH +4 , Mg2+, Ca2+, Cl−, NO −3 and SO 2−4 ) accounts for large fractions of atmospheric particle mass and are associated with adverse effects on human health and acidification of the environment (Grantz et al. 2003; Jung and Kim 2006). Determining their composition is essential to understand their properties and reactivity and hence their environmental effects (Singh et al. 2010). The major components of PM are sulfate (SO 2−4 ), nitrate (NO −3 ), ammonia (NH +4 ), organic carbon (OC), and elemental carbon (EC) (Lee et al. 2001). Sufficient data on PM10 (Kyotani and Iwatsuki 2002; Shi et al. 2003; Dan et al. 2004; Samburova et al. 2005; Johnson et al. 2006; Nicolas et al. 2009; Khan et al. 2010; Pateraki et al. 2010) and PM2.5 (Niemi et al. 2005; Wang et al. 2006; Chen et al. 2008; Raman et al. 2008; Mkoma et al. 2009; Mena et al. 2010; Tai et al. 2010) are now available worldwide that emphasize on characteristics and sources. Nowadays, in developed countries, attention has been paid for PM2.5 and PM1 particles. However, much less is known, and even less has been done about PM1. Although some literature (Cabada et al. 2004; Shen et al. 2009) reported that the major components of PM1 and PM2.5 originate from the same sources and they concluded the investigation of PM1 did not yield significant new information in with that obtained from the PM2.5, it is clear that the PM1 was a better indicator for anthropogenic sources than PM2.5, because compared with PM2.5 and PM10, it minimized interference from natural sources (Lee et al. 2006).
Therefore, this study was carried out to characterize PM2.5 and PM1 and water soluble ions associated with these size fractions in the eastern central India.
2 Experimental
2.1 The study area
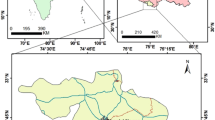
Continuous sampling of aerosols was conducted in Raipur (22o 33′ to 21o14′N Latitude and 82o 6′ to 81o38′E Longtitude). Figure 1 shows the geographical location of the sampling site. The atmospheric air collections were performed at the terrace, i.e. at an altitude of approximately 15 m from the ground level, of the building School of Studies in Chemistry, Pt. Ravishankar Shukla University Raipur, India. It is the largest institution for higher education in this region of eastern central India. The national highway connecting to the University has heterogeneous and heavy traffic flow. It is a congested area with heavy traffic density. The climate is tropical with four distinct seasons, viz., fall (July–Sept), winter (October–January), spring (February–March), and summer (April–June).
2.2 Sample collection
Size-segregated aerosol samples were collected between July 2009 and June 2010 by 8-Stage cascade impactor type aerosol sampler (TE 20–800 Tisch Air Pollution Monitoring Equipment, USA, flow rate 28.3 ALPM) at sampling site Raipur, India in four different seasons (fall, winter, spring and summer). During each season, the PM2.5, and PM1 samples were collected twice in a week for the period of 24 h. Table 1 gives an overview of the sampling period. Power failure during sampling hours was added for additional sampling time period. The sampling were performed at a rate of 1.7 m3 h−1 and in total 40.8 m3 day−1 of air passed through each filter. Total 120 samples were collected at sampling site. The samples were collected on Whatmann 41 glass filters. Each set of samples consist of eight different filters for various size ranges. These size ranges are as follows—stage 1: 10.0–9.0 μm, stage 2: 9.0–5.8 μm, stage 3: 5.8–4.4 μm, stage 4: 4.4–2.5 μm, stage 5: 2.5–2.0 μm, stage 6: 2.0–1.0 μm; stage 7: 1.0–0.7 μm and stage 8: 0.7–0.4 μm. Subsequently, these eight size ranges particles were divided into two broad categories, viz. PM2.5, and PM1. PM2.5 particles presented the sum total of particles of size ranges 2.5–2.0, 2.0–1.0, 1.0–0.7 and 0.7–0.4 μm and PM1 particles presented the sum total of particles of size ranges 1.0–0.7 and 0.7–0.4 μm.
The filters were kept in vacuum desiccators for 24 h to remove moisture content before mounting them on the air sampler. After sampling the filters were immediately transferred to vacuum desiccators to again de-moisture in the same manner. Before and after sampling, the filters were weighed using an analytical balance (Sartorius CP225D) with a reading precision of 10 μg after stabilizing under a constant temperature (20 ± 5°C) and humidity (40 ± 2%). All weight measurements were repeated three or more times to ensure reliability. To ensure the quality of weighing, another blank filter was dealt with simultaneous using the same method as the samples. The exposed filters and field blanks were placed into polyethylene bottles and kept frozen at −20°C during storage and transported cool for analysis. All the procedures were strictly quality-controlled to avoid possible contaminations of the samples.
2.3 Chemical analysis
One-fourth of the filter samples were extracted into 10 ml ultrapure water (specific resistance: 18 MΩ cm) by using an ultrasonic bath (UT-105 S, Sharp, Japan) and a shaker (EYELA Multi Shaker, Rikakikoi Co., Tokyo, Japan) to measure various water soluble inorganic ions. The ultrasonic and mechanical agitations were operated for 45 min each. The extracted solution was filtered through a microporous membranes (pore size, 0.45 μm, diameter, 25 mm), and filtrate was stored in a refrigerator at 4°C until chemical analysis. An ion chromatograph (Dionex, DX-120, USA) was used to measure Na+, NH +4 , K+, Mg2+, Ca2+, Cl−, NO −3 and SO 2−4 ions in the aqueous extract.
The determination of ions Na+, NH +4 , K+, Mg2+, Ca2+, Cl−, NO −3 and SO 2−4 was performed using the following setup: Separation columns, Ionpac AS 12A and CS 16A were used, respectively for anion and cations. Eluents of 2.7 mM Na2CO3 (Wako, Japan) and 0.3 mM NaHCO3 (Wako, Japan) were prepared and used for the detection of anions with a pump flow rate of 1.5 ml min−1. A 2.6 mM methane sulphonic acid (CH3SO3H) (Wako, Japan) solution was used as an eluent for cation analysis with a pump flow rate of 1 ml min−1. Ions were identified based on their retention time. The analytical uncertainties, generating from the non-ideal chemical or physical behavior of analytical systems, were checked for working standard solutions prepared freshly by proper dilutions of the stock standard solutions of all ions analyzed. The variation in calculated area under the peaks obtained for these ions (n = 6, for each ion analysis) were between 2–5%. The field blanks, as a measure for the experimental uncertainties, were determined to be less than the method detection limits only for ions, namely, NH +4 , Mg2+ and NO −3 . Blank concentration was measured using field blank samples. Blanks filters were extracted and passed through similar procedures as followed for the extraction and preparation of the regular particulate sample to analyze with the ion chromatography. The description on the method detection limit (DL) calculated using 1 ppm laboratory prepared calibration standards, analytical accuracy in terms of relative error and precision in terms of relative standard deviation are listed in Table 2 for all cations and anions analyzed. The overall mean concentrations for field blank samples (n = 30), collected over a year, are also shown in Table 2. All the reported water-soluble ion concentrations and PM mass have been corrected using field blanks.
2.4 Meteorological data
Daily meteorological data, including temperature (T, °C), rainfall (RF, mm), relative humidity (RH, %), vapour pressure (VP, kPa), wind speed (WS, m/s) and wind direction (WD, degree) etc. were obtained from Department of Agrometeorology, Indira Gandhi Agricultural University, Raipur, India. The meteorological data during the study period are presented in Table 1.
3 Results and discussion
3.1 PM2.5 and PM1 mass concentrations and seasonal variation
A total of 120 samples of PM2.5 and PM1 aerosols were collected from July 2009 to June 2010 at sampling site School of Studies in Chemistry, Pt. Ravishankar Shukla University, Raipur, India. The concentrations of PM2.5 and PM1 ranged from 24.0 to 269.7 μg/m3 and from 4.4 to 135.3 μg/m3, respectively. Respective annual mean concentration of 150.9 ± 78.6 and 72.5 ± 39.0 μg/m3 were recorded for PM2.5 and PM1. Table 3 shows mean concentration with standard deviation values for each fraction of the particulate matter (PM2.5 and PM1) collected during the sampling period. The results indicated that the observed annual mean PM2.5 concentration exceeded 65 μg/m3 of the annual National Ambient Air Quality Standards (NAAQS) of India (http://www.scorecard.org/env-releases/def/capnaaqs.html).
The seasonal variation of PM2.5 and PM1 aerosols was shown in Fig. 2, where the error bars show the standard deviation of seasonal mean. Figure 2 showed numerous high concentration as well as low concentration of PM2.5 and PM1. Several months of rainfall might be responsible for low concentration months. As shown in Fig. 2, the concentration of PM2.5 and PM1 were higher in winter compared to those in spring, summer and fall. The high concentration in winter was likely due to the high burning of fossil fuels for heating purpose. The low concentration in fall was likely related to more precipitation in this season. The high concentrations of PM2.5 in the present study may be due to the contribution from heavy traffic and other human particle-generating activities in near by areas. The highest concentrations of PM2.5 were experienced during December 2009, whereas the highest concentrations of PM1 were found in October 2009 compared with other sampling period of the year. Temperature was found to be at the lowest level (21.5°C) on winter as shown in Table 1. Figure 3 shows the weekly variation of PM2.5 and PM1 concentrations versus corresponding temperature, wind speed and mixing height during the study period. The temperature, wind speed and mixing height were found to be low during the winter weeks and gradually increased during summer weeks. Frequent changes in temperature and wind speed increased the atmospheric turbulence during summer weeks, thereby increasing the dispersion of PM emission. Further, in winter weeks winds were relatively calm (~ 1.3 m/s, October to January). These prevailing calm conditions favored more stable atmospheric condition, consequently reducing the dispersion of particulate matter. Thus stable and cold meteorology in most of the days in winter weeks favored the prolonged life of PM2.5 in ambient air. In summer weeks increased wind speed and temperature brought down PM concentrations remarkably. Similar trends were also found for PM1. PM concentration measured in winter was higher than that in the summer and spring, because many agricultural activities (burning rice straws) were carried out in winter. Many farmers burned rice straw to fertilize the land for the coming year and also due to wood and oil burning for residential heating, and condensation of semi-volatile organic species. High PM concentrations in Raipur could be attributed to the uncontrolled anthropogenic activities which may include high rate of construction activities without any preventive measures, refuse dust, biomass combustion, mechanical erosion from paved and unpaved roads and soil dusts generate particles larger than 2.5 and 1.0 μm.
The PM2.5 and PM1 particles are relatively different in their physical and chemical compositions. They generally originate from different sources or by different activities within the same sources (Oanh et al. 2006). Relationship between the PM2.5 and PM1 mass concentrations were analyzed in terms of linear regression (Fig. 4) and the results revealed that the coefficient of determination (R2) was 0.958 during the study period. The relatively high coefficients especially imply that PM2.5 and PM1 have some similar sources, and they were being influenced by the same local conditions (Karaca et al. 2005). Fine particles are formed primarily by combustion and/or secondary chemical reactions in the atmosphere (Ravindra et al. 2008). In our study, the increase in PM2.5 concentrations during the heating seasons was mainly driven by an increase in the PM1 concentrations.
To understand the contribution of PM1 in PM2.5, the ratio of PM1/PM2.5 is calculated. Since different sizes of atmospheric particle probably originate from different sources, the mean PM1/PM2.5 ratios have been used for identifying the sources of PM2.5 and PM1 (Chan et al. 2005; Sun et al. 2004; Charron and Harrison 2005; Bogo et al. 2003; Gehrig and Buchmann 2003). Higher ratios (larger than 0.50) are generally ascribed to relatively high contribution from secondary particles and the combustion sources, while lower ratio indicate significant contributions from primary sources such as re-suspended soil/road dust and the mechanical activities (Perez et al. 2008; Querol et al. 2004). The ratios of PM1/PM2.5 were also shown in Fig. 2. The mean ratio of PM1/PM2.5 was found to be 0.47 ± 0.08. It could be seen that PM1 accounted for 48%, 48%, 46% and 48% of PM2.5 in fall, winter, spring and summer, respectively. However, the ratio of PM1/PM2.5 is higher during the fall, winter and summer seasons (mean PM1/PM2.5 = 0.48), clearly indicating larger submicron particle fractions in PM2.5 at this location. Paired t-tests for mean PM1/PM2.5 ratios were conducted to know if there is significant difference in the ratios between different seasons. The results of paired t-tests (fall-winter, t = −0.30, p = 0.75; fall-spring, t = 0.70, p = 0.48; fall-summer, t = −0.04, p = 0.96; winter-spring, t = 1.12, p = 0.28; winter-summer, t = 0.11, p = 0.90; spring-summer, t = −0.48, p = 0.63) showed that there is no significant difference in the ratios of PM1/PM2.5 between the seasons.
3.2 Concentrations of water soluble ions in PM2.5 and PM1
The analytical results of water soluble ionic species in PM2.5 and PM1 size fractions collected during the period of July 2009 to June 2010 in eastern central India are presented in Table 3. Water soluble inorganic ions comprise a large part of aerosol particles and play an important role in the atmosphere. The average water soluble ionic concentrations of PM2.5 were found in the following order: SO 2−4 > NO −3 > Cl− > NH +4 > Na+ > Ca2+ > K+ > Mg2+. Besides, the mean ionic concentrations of PM1 were in the following order: SO 2−4 > NO −3 > NH +4 > Cl− > Na+ > Ca2+ > K+ > Mg2+. The concentrations of total water soluble inorganic ions (TWSII) in PM2.5 accounted for 13.0% of the total mass. The concentrations of total water soluble inorganic ions varied from 1.7% to 76.9% for PM2.5 and 1.5% to 95.0% for PM1, respectively. The mean level of ions in PM1 accounted for 18.6% with the major ions being SO 2−4 , NO −3 and NH +4 . The annual average of SO 2−4 , NO −3 and NH +4 ions were found to be 52.7%, 11.8% and 12.6% of the total mass of the ionic species in PM1. The results indicated that the average water soluble ionic species concentration in PM2.5 were higher than those of PM1. The concentrations of SO 2−4 , NO −3 , NH +4 and Cl− were highest among all ions in PM2.5. The annual means of SO 2−4 , NO −3 , NH +4 and Cl− were observed as 45.8%, 13.1%, 11.4% and 9.8% of total mass of the ionic species in PM2.5. Among all chemical species, SO 2−4 has the highest concentration in both the aerosols, which varied from 1.14 to 23.75 μg/m3 in PM2.5 with a mean concentration 7.86 μg/m3. The SO 2−4 concentrations in PM1 varied between 0.48 and 14.79 μg/m3 with a mean value of 5.61 μg/m3. NO −3 is the second most prominent species in both PM2.5 and PM1 aerosols, which varied from 0.03 to 16.14 μg/m3 in PM2.5 with a mean value of 3.12 μg/m3. The NO −3 concentrations in PM1 varied from 0.01 to 8.42 μg/m3 with a mean value of 1.81 μg/m3. The high sulphate and nitrate concentrations suggest their contribution from a large component of secondary inorganic aerosols resulting from emissions of SO2 and NOx by a variety of combustion sources using sulfurous fuel, such as coal and oil (Seinfeld and Pandis 1998). These include power plants and heavy road transport. Ammonium (NH +4 ) concentrations varied from 0.22 to 6.83 μg/m3 for PM2.5 and 0.11 to 3.33 μg/m3 for PM1. The mean value of NH +4 was 1.94 μg/m3 for PM2.5 and 1.26 μg/m3 for PM1. In PM2.5, the concentration of chloride (Cl−) ion varied from 0.01 to 7.82 μg/m3 with a mean concentration of 2.10 μg/m3. However, Cl− ion concentration in PM1 varied between 0.01 to 4.89 μg/m3 with a mean concentration of 1.19 μg/m3. Calcium (Ca2+) concentrations varied from 0.01 to 3.56 μg/m3 and 0.05 to 1.39 μg/m3, respectively for PM2.5 and PM1. The mean value of Ca2+ was 0.91 μg/m3 and 0.40 μg/m3 for PM2.5 and PM1, respectively. Similarly, Magnesium (Mg2+) concentration varied between 0.03 to 1.92 μg/m3 for PM2.5 and from 0.01 to 0.33 μg/m3 for PM1 with a mean concentration of 0.26 μg/m3 for PM2.5 and 0.08 μg/m3 for PM1. The mean concentrations of potassium (K+) and sodium (Na+) ions were 0.52 μg/m3 and 0.92 μg/m3 for PM2.5 and 0.37 μg/m3 and 0.61 μg/m3 for PM1, respectively.
The percentage contribution of each ionic species in both in PM2.5 and PM1 was shown in Fig. 5. The measured total water soluble ionic fraction contributed to approximately 13% of the total PM2.5 of which anions and cations account for about 9% and 4%, respectively as shown in Fig. 5. On the other hand, approximately 19% of the total PM1 was found to contribute as total water soluble ionic fraction of which anions and cations accounted for about 16% and 6%, respectively. The unanalyzed portion of both PM2.5 and PM1 samples is deemed to consist of carbonaceous aerosols such as black carbon and organic carbons and other insoluble elements. The analyzed ionic species in PM2.5 mainly consist of secondary inorganic aerosols (SO 2−4 , NO −3 and NH +4 ) which on an annual basis, accounts for 12.92 μg/m3 (9.09% of the PM2.5 mass), salt aerosols (Na+ and Cl−) for 3.02 μg/m3 (2.34%) and mineral matter (K+, Mg2+ and Ca2+) for 1.69 μg/m3 (1.58%). Out of total PM2.5 fractions, 86.99% (~133.2 μg/m3) are unanalyzed fractions. The PM1 are mainly made up of undetermined fractions (61.33 μg/m3, 81.43%), secondary inorganic aerosols (8.68 μg/m3, 16.26%), salt aerosols (1.80 μg/m3, 3.95%) and mineral matters (0.85 μg/m3, 1.69%).
3.3 Seasonal variation of water soluble ionic species
The seasonal variations of water soluble ionic species in PM2.5 and PM1 aerosols collected from July 2009 to June 2010 at eastern central India were shown in Fig 6. The period of study was divided into four seasons as shown in Table 1 in order to conduct a more detailed analysis of the behavior of the variations of the ion concentrations in PM2.5 and PM1. The mass concentration of the total water soluble ionic species was higher in winter (30.98 μg/m3 and 20.01 μg/m3, respectively for PM2.5 and PM1) and spring (18.82 μg/m3 and 12.26 μg/m3, respectively for PM2.5 and PM1) than summer (12.15 μg/m3 and 7.74 μg/m3, respectively for PM2.5 and PM1) and fall (4.52 μg/m3 and 2.73 μg/m3, respectively for PM2.5 and PM1). The high concentration in winter might be related to the industrial emissions in this season and low inverse layer there. In fall, the low concentration at the study area is related to more precipitation (average rainfall, 65.4 mm) that would wash out more aerosols. The tracers of soil/dust, Ca2+ and Mg2+, were frequently observed in lower concentrations in fall (Ca2+ average: 0.42 and 0.10 μg/m3 for PM2.5 and PM1, respectively; Mg2+ average: 0.06 and 0.02 μg/m3 for PM2.5 and PM1, respectively) and winter (Ca2+ average: 0.94 and 0.42 μg/m3 for PM2.5 and PM1, respectively; Mg2+ average: 0.07 and 0.03 μg/m3 for PM2.5 and PM1, respectively) seasons, which could be due to the more precipitation in these seasons, while in spring (Ca2+ average: 1.39 and 0.67 μg/m3 for PM2.5 and PM1, respectively; Mg2+ average: 0.74 and 0.26 μg/m3 for PM2.5 and PM1, respectively) and summer (Ca2+ average: 1.03 and 0.48 μg/m3 for PM2.5 and PM1, respectively; Mg2+ average: 0.39 and 0.10 μg/m3, respectively for PM2.5 and PM1) they were in higher concentrations, as the dry weather in these seasons was favorable for the re-suspension of soil particles.
The concentration of Cl− in PM2.5 and PM1 samples was higher in winter (4.35 and 2.68 μg/m3 for PM2.5 and PM1, respectively) and spring (1.73 and 0.69 μg/m3 for PM2.5 and PM1, respectively) than those in summer (1.16 and 0.60 μg/m3 for PM2.5 and PM1, respectively) and fall (0.30 and 0.12 μg/m3 for PM2.5 and PM1, respectively). As the sampling site is far from the sea, it has been demonstrated that the contribution to the aerosols from the sea could be ignored here (Tiwari et al. 2009). The major source of Cl− in Raipur, India might be coal burning, which could reasonably explain the higher Cl− concentration in winter season. High K+ concentrations averaged of 0.89 and 0.65 μg/m3 for PM2.5 and PM1 were observed in winter, which was higher than that in fall of 0.25 and 0.14 μg/m3 for PM2.5 and PM1, respectively. The seasonal variation of K+ was similar to Cl−, indicating that both K+ and Cl− were primarily related to the burning activities, which were enhanced in the cold season (Wang et al. 2006).
The seasonal variations of secondary components SO 2−4 , NO −3 and NH +4 were similar. The concentration of SO 2−4 was higher in winter (13.96 and 9.59 μg/m3 for PM2.5 and PM1, respectively) and spring (8.11 and 6.28 μg/m3 for PM2.5 and PM1, respectively) than that in summer (5.09 and 3.74 μg/m3 for PM2.5 and PM1, respectively) and fall (2.32 and 1.71 μg/m3 for PM2.5 and PM1, respectively) seasons. Sulphate showed similar trend with K+ and Cl−. Therefore, the source of this compound can be the biomass burning as well in addition to industrial activities. The NO −3 concentration in PM2.5 and PM1 was higher in winter (7.10 and 4.39 μg/m3 for PM2.5 and PM1, respectively) and spring (1.83 and 0.76 μg/m3 for PM2.5 and PM1, respectively) than that in other seasons. NO −3 is generally formed from the oxidation of NOx. Thus the variation of NO −3 is related closely to NO2 and meteorological factors. NO2 is mainly from the traffic emissions in the city. Since traffic emissions are generally evenly distributed over the year round, the variation in NO −3 might be strongly related to the meteorological factors, such as temperature and relative humidity. The lower temperature and stable meteorology favor the formation of NO −3 aerosol reacting with NH +4 (Park et al. 2005; Mariani and Mello 2007). The lower temperature in winter and spring would favor the shift from the gas phase of nitric acid to the particle phase of nitrate, which could lead to the high concentration of NO −3 in winter and spring. NH +4 concentration in PM2.5 and PM1 was highest in winter season in this study as 3.23 and 2.07 μg/m3, respectively for PM2.5 and PM1. The magnitude of the seasonal NH +4 concentration in PM2.5 and PM1 was found to be winter > spring > summer > fall.
3.4 Relationship between PM, water soluble ionic species and meteorological parameters
Meteorology plays a crucial role in ambient distribution of air pollution. The importance of meteorological factors in the transport and diffusion stage of the air pollution cycle is well recognized. The entering of pollutants from the ground surface, their residence in the atmosphere, and the formation of secondary pollutants is controlled not only by the rate of emission of the reactants into the air from the source, but also by wind speed, air temperature and precipitation. Thus, it is often important to understand the physical process leading to an observed concentration of pollutants at a given point. Rainfall is one of the reasons for low particulate pollutants in the monsoon season as the pollutants are washed out by rain (Bhaskar and Mehta 2010).
The relationship between PM and meteorological parameters during the study periods were investigated by Spearman correlation analysis. The meteorological data, which were found to be significant in describing the variation in the 24-h averaged PM concentrations utilizing in the present work, are: temperature (°C), rainfall (mm), relative humidity (%), vapour pressure (kPa) and wind speed (m/s). The correlation coefficients (r) between weekly average (24-h) PM and meteorological parameters are shown in Table 4. The correlation coefficients were marked in the table to indicate the significant levels (P < 0.01 or P < 0.05). The results showed that the meteorological parameters were negatively correlated with PM2.5 and PM1. Correlation between PM variables and wind speed were investigated during the study period. It is obvious that the concentrations of pollutants decrease effectively with increasing wind speed. There is a significant negative relationship between particulates mass concentrations and wind speed during the study period. Correlations between PM variable and relative humidity are negative and remain weak during the study period. PM variables shows significant negative correlations with atmospheric temperature, whereas no correlation is observed between PM variable and rainfall. Particulate concentrations exhibit significant negative correlations with vapour pressure. Re-suspended soil dust under unstable atmospheric conditions (low pressure and high wind speed) during the study period might explain the cause of negative correlations observed between particulate concentrations and vapour pressure. Atmospheric pressure influences pollutant concentrations indirectly by affecting the atmospheric stability conditions. In general, high atmospheric pressure is often related to low wind speed and stable stratification, limiting dispersion of pollutants within the atmosphere (Tsai 2005; Rehwagen et al. 2005; Turalioglu et al. 2005; Chaloulakou et al. 2003). The stable atmospheric conditions with high pressure and low wind speeds are most unfavorable in terms of air pollution, whereas low pressure with high wind speed apparently leads to high coarse particle concentrations due to re-suspension processes (Harrison et al. 2001).
The correlation coefficients among major components revealed that PM2.5 and PM1 was well correlated with K+, Cl−, NO −3 and SO 2−4 . Na+ correlated well with NH +4 and Mg2+. NH +4 had a strong correlation with K+, NO −3 and SO 24 . In the fine and submicron fractions a higher correlation between ammonium and sulphate suggested that these two species are associated in ambient air. NO −3 shows strong correlation with SO 2−4 for PM2.5 and PM1 aerosols. The high correlation between nitrate and sulphate is due to the formation of secondary inorganic aerosols in the atmosphere (Rastogi and Sarin 2005). A poor correlation between Na+ and Cl− in the PM2.5 and PM1 samples confirms the origin of Na+ and Cl− from non sea-salt substances.
Table 1 showed the meteorological data, including temperature, relative humidity, and wind speed during the study period. The relatively low temperature and wind speed in winter season favor the formation of the permanent temperature inversion, stable atmosphere and low mixed boundary layers, which would then lead to the accumulation of pollutants. Additionally, the higher the relative humidity in winter would accelerate the formation of secondary species such as sulphate, nitrate and ammonium from their precursors, SO2 and NOx, and aggravate the pollution level of the atmosphere. The relative humidity and wind speed were two most important factors affecting the concentration of pollutants in the aerosols. The secondary species (NH +4 , SO 2−4 and NO −3 ) showed strong positive correlation with relative humidity and negative correlation with wind speed. Besides, all pollution species showed relatively weak negative correlations with temperature, indicating that higher temperature would be against the accumulation of pollutants.
3.5 Principal component analysis
A preliminary source identification study of the water soluble ionic species was carried out by principal component analysis (PCA, Statistica 5.0 program). PCA is a widely used statistical technique for data reduction and interpretation. In our application of PCA, a small number of latent factors (principal component or PCs) were extracted from the concentration data, and they were used to interpret the relationship among the measured variables (Han et al. 2006). It is important to underline that none of the data, analysed for ionic species, was an outlier that could have caused overlapping of data points in PC analysis. The correlation matrix was used for the present study because the communality of these eight water soluble ionic species was rather reasonable. Furthermore, in this study all factors extracted with eigenvalues > 1.0, were retained for interpretation as suggested by the Kaiser criterion (Kaiser 1960). When the VARIMAX normalized rotation is used in PCA, as was the case in our study, each PC score contains information on correlations among all of the variables combined into a single number. The individual loadings for each ion indicate its relative contribution each of the PC scores. Table 5 shows factor loading for ionic species in PM2.5 and PM1 aerosols. Only factor loading >0.25 were included in Table 5, smaller loading (<0.25) were considered to be insignificant (Karakas and Semra 2008). The PCA model gives two factors for the water soluble ionic species in PM2.5 and PM1 aerosol samples, which accounted for 74.5% and 69.2% of the total variance in the concentration data, respectively. Factor 1 accounts for 49.2% and 45.7% of the total variance, respectively for PM2.5 and PM1 aerosol samples, and it was strongly loaded with NH +4 , K+, Cl−, NO −3 and SO 2−4 , suggesting likely origins from anthropogenic sources, especially coal combustion, traffic emissions and biomass burning. Factor 2 accounts for the 25.3% and 23.5% of the total variance for PM2.5 and PM1 aerosol samples, respectively, which shows significant higher loadings of Na+, Mg2+ and Ca2+, suggesting their partial association with natural, such as soil derived particles.
4 Conclusion
To better understand the causes of the air pollution problem in eastern central India, atmospheric aerosols PM2.5 and PM1 size fractions were collected between July 2009 and June 2010. This study elucidate the characteristics of PM2.5 and PM1 in capital city Raipur, India and their annual mean concentrations were found as 150.9 and 72.5 μg/m3, respectively. The annual mean concentrations of PM2.5 exceeded annual National Ambient Air Quality Standards (NAAQS) guidelines of India. Very high elevated levels of PM2.5 and PM1 were observed in winter (PM2.5: 225.1 μg/m3; PM1: 108.4 μg/m3) and spring (PM2.5: 172.1 μg/m3; PM1: 81.4 μg/m3) seasons indicating critical pollution situation, whereas low levels in rainy season (PM2.5: 91.3 μg/m3; PM1: 42.7 μg/m3) indicates the influence of monsoon precipitation washing down the pollutants. The ratios of PM1/PM2.5 were calculated to understand the contribution of PM1 in PM2.5. It could be seen that PM1 accounted for 48, 48, 46 and 48% of the PM2.5 in fall, winter, spring and summer, respectively, clearly indicating larger PM1 fractions in PM2.5 at this area of eastern central state of India.
The concentrations of total water soluble inorganic ions varied from 13.7% to 76.9% with an average of 11.8% for PM2.5 and from 1.5% to 85.0% with an average of 21.9% for PM1, respectively. The concentrations of SO 2−4 , NO −3 and NH +4 were significantly high in the total water soluble inorganic fraction in PM2.5 and PM1. Among the detected ions SO 2−4 was the major chemical component of PM2.5 (7.68 μg/m3, 45.8% of the TWSII) and PM1 (5.61 μg/m3, 52.7% of the TWSII), while NO −3 (3.12 μg/m3, 13.0% of the TWSII for PM2.5 and 1.81 μg/m3, 11.8% of the TWSII for PM1) was second higher water soluble component followed by NH +4 (1.94 μg/m3, 11.4% of the TWSII for PM2.5 and 1.26 μg/m3, 12.6% of the TWSII for PM1). The higher concentrations of SO 2−4 , NH +4 , NO −3 , Cl− and K+ were observed during the winter due to the enhanced burning activities and industrial emissions in this season, while Na+, Mg2+ and Ca2+ exhibited higher concentrations during the spring and summer as the dry weather in these seasons was favorable for the resuspension of soil particles.
The correlation study revealed a number of strong correlations between PM and different ionic pairs, pointing towards the probable common origin in the atmosphere. PM2.5 and PM1 were negatively correlated with temperature and wind speed, indicating that the higher temperature and wind speed would be against the accumulation of pollutants. Among the ionic species, NH +4 , NO −3 and SO 2−4 were found to be positively correlated with the relative humidity and negatively with the wind speed. PM2.5 and PM1 were positively correlated with K+, Cl−, NO −3 , SO 2−4 and NH +4 . NH +4 had strong correlation with K+, NO −3 and SO 2−4 . A poor correlation between Na+ and Cl− in PM confirms that the origin of Na+ and Cl− is from non-sea salt substances.
The source identification by Principal Component Analysis demonstrated the major contribution of ionic species from anthropogenic and natural sources. SO 2−4 , NH +4 , NO −3 , Cl− and K+ are attributed to an origin from anthropogenic sources. Furthermore, Na+, Mg2+ and Ca2+ are originated mainly from natural sources.
References
Badrinath, K.V.S., Latha, M., Chand, T.R.K., Reddy, R.R., Gopal, K.R., Reddy, L.S., Narasimhulu, K.S., Kumar, K.R.: Black carbon aerosols and gaseous pollutants in an urban area in North India during fog period. Atmos. Res. 85, 209–216 (2007)
Bhaskar, B.V., Mehta, V.M.: Atmospheric particulate pollutants and their relationship with meteorology in Ahmedabad. Aerosol Air Qual. Res. 10, 301–315 (2010)
Bhaskar, B.V., Rajasekhar, R.V.J., Muthusubramanian, P., Kesarkar, A.P.: Measurement and modeling of respirable particulate (PM10) and lead pollution over Madurai, India. Air Qual. Atmos. Health 1, 45–55 (2008)
Bogo, H., Otero, M., Castro, P., Ozafran, M.J., Kreiner, A., Calvo, E.J., Negri, R.M.: Study of atmospheric particulate matter in Buenos Aires city. Atmos. Environ. 37, 1135–1147 (2003)
Cabada, J.C., Rees, S., Takahama, S., Khlystov, A., Pandis, S.N., Davidson, C.I., Robinson, A.L.: Mass size distribution and size resolved chemical composition of fine particulate matter at the Pittsburgh Supersite. Atmos. Environ. 38, 3127–3141 (2004)
Chaloulakou, A., Kassomenos, P., Spyrellis, N., Demokritou, P., Koutrakis, P.: Measurements of PM10 and PM2.5 particle concentrations in Athens, Greece. Atmos. Environ. 37, 649–660 (2003)
Chan, C.Y., Xu, W.D., Li, Y.S., Wong, K.H., Ding, G.A., Chan, L.Y., Cheng, X.H.: Characteristics of vertical profile and sources of PM2.5, PM10 and carbonaceous species in Beijing. Atmos. Environ. 39, 5113–5124 (2005)
Charron, A., Harrison, R.M.: Fine (PM2.5) and coarse (PM2.5–10) particulate matter on a heavily trafficked London Highway: sources and processes. Environ. Sci. Technol. 39, 7768–7776 (2005)
Chen, J., Tan, M., Li, Y., Zheng, J., Zhang, Y., Shan, Z., Zhang, G., Li, Y.: Characteristics of trace elements and lead isotopes ratios in PM2.5 from four sites in Shanghai. J. Hazard. Mater. 156, 36–43 (2008)
Chowdhury, Z., Zheng, M., Schauer, J.J., Sheesley, R.J., Salmon, L.G., Cass, G.R., Russell, A.G.: Speciation of ambient fine organic carbon particles and source apportionment of PM2.5 in Indian cities. J. Geophys. Res. (2007). doi:10.1029/2007JD008386
Dan, M., Zhuang, G., Li, X., Tao, X., Zhuang, Y.: The characteristics of carbonaceous species and their sources in PM2.5 in Beijing. Atmos. Environ. 38, 3443–3452 (2004)
Deshmukh, D.K., Deb, M.K., Verma, S.K.: Distribution patterns of coarse, fine and ultrafine atmospheric aerosol particulate matters in major cities of Chhattisgarh. Indian J. Environ. Protect 30, 184–197 (2010)
Fang, G.C., Wu, Y.S., Lee, W.J., Chou, T.Y., Lin, I.C.: Study of ambient air particulates pollutants near Taichung airport sampling site in central Taiwan. J. Hazard. Mater. 144, 492–498 (2007)
Gadgil, A.S., Jadhav, R.S.: Street level concentrations of suspended particulate matter (SPM), nitrogen dioxide (NO2) and sulphur dioxide (SO2) in Pune city. J. Environ. Sci. Eng. 46, 143–150 (2004)
Ganguly, D., Jayaraman, A., Rajesh, T.A., Gadhavi, H.: Wintertime aerosol properties during foggy and nonfoggy days over urban center Delhi and their implication for shortwave radiative forcing. J. Geophys. Res. (2006). doi:10.1029/2005JD007029
Gehrig, R., Buchmann, B.: Characterizing seasonal variations and spatial distribution of ambient PM10 and PM2.5 concentrations based on long-term Swiss monitoring data. Atmos. Environ. 37, 2571–2580 (2003)
Grantz, D.A., Garner, J.H.B., Johnson, D.E.: Ecological effects of particulate matter. Environ. Int. 29, 213–239 (2003)
Han, Y.M., Du, P.X., Cao, J.J., Eric, S.P.: Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ. 355, 176–186 (2006)
Harrison, R.M., Yin, D., Mark, D., Stedman, J., Appleby, R.S., Booker, S.: Moorcroft studies of the coarse particle (2.5-10 μm) component in UK urban atmosphere. Atmos. Environ. 35, 3667–3679 (2001)
He, K., Yang, F., Ma, Y., Zhang, Q., Yao, X., Chan, C.K., Cadle, S., Chan, T., Mulawa, P.: The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 35, 4959–4970 (2001)
Hellebust, S., Allanic, A., Jourdan, C., Healy, D., Sodeau, J.R.: Sources of ambient concentrations and chemical composition of PM2.5–10 in Cork Harbour, Ireland. Atmos. Res 95, 136–149 (2010)
Johnson, K.S., Zuberi, B., Molina, L.T., Molina, M.J., Xie, Y.: Aerosol composition and source apportionment in the Mexico City metropolitan area with PIXE/PESA/STIM and multivariate analysis. Atmos. Chem. Phys. 6, 4591–4600 (2006)
Jung, C.H., Kim, Y.P.: Numerical estimation of the effects of condensation and coagulation on visibility using the moment method. J. Aerosol Sci. 37, 143–161 (2006)
Kaiser, H.F.: The application of electronic computers to factor analysis. Educ. Psychol. Meas. 20, 141–51 (1960)
Karaca, F., Alagha, O., Erturk, F.: Statistical characterization of atmospheric PM10 and PM2.5 concentrations at non-impacted suburban site in Istanbul, Turkey. Chemos 59, 1183–1190 (2005)
Karakas, S.Y., Semra, G.: Source apportionment of atmospheric trace element deposition. Environ. Eng. Sci. 25, 1263–1272 (2008)
Karar, K., Gupta, A.K.: Seasonal variations and chemical characterization of ambient PM10 at residential and industrial site of an urban region of Kolkata (Calcutta), India. Atmos. Res. 81, 36–53 (2006)
Katiyar, S.C., Khathing, D.T., Dwivedi, K.K.: Assessment of breathing level ambient air quality of Shilong: Part 1-total suspended particulate loadings. Indian J. Environ. Protect 22, 115–122 (2002)
Khan, M.F., Shirasuna, Y., Hirano, K., Masunaga, S.: Characterization of PM2.5, PM2.5–10 and PM>10 in ambient air, Yokohama, Japan. Atmos. Res. 96, 159–172 (2010)
Kulshrestha, A., Bisht, D.S., Masih, J., Massey, D., Tiwari, S., Taneja, A.: Chemical characterization of water-soluble aerosols in different residential environments of semi arid region of India. J. Atmos. Chem. 62, 121–138 (2009)
Kyotani, T., Iwatsuki, M.: Characterization of soluble and insoluble components in PM2.5 and PM10 fractions of airborne particulate matter in Kofu city, Japan. Atmos. Environ. 36, 639–649 (2002)
Lee, J.H., Kim, Y.P., Moon, K.C., Kim, H.K., Lee, C.B.: Fine particle measurements at two background sites in Korea between 1996 and 1997. Atmos. Environ. 35, 635–643 (2001)
Lee, J.H., Hopke, P.K., Turner, J.R.: Source identification of airborne PM2.5 at the St. Louis-Midwest Supersite. J. Geophys. Res. (2006). doi:10.1029/2005JD006329
Mariani, L.R., Mello, W.: PM2.5–10, PM2.5 and associated water-soluble inorganic species at a coastal urban site in the metropolitan region of Rio de Janeiro. Atmos. Environ. 41, 2887–2893 (2007)
Mena, L.H., Norena, H.S., Romero, P.C., Ramirez, R.C., Hernandez, B.E.: Ionic species associated with PM2.5 in the city of Guadalajara, Mexico during 2007. Environ. Monit. Assess. 161, 281–293 (2010)
Mkoma, S.L., Wang, W., Maenhaut, W.: Seasonal variation of water-soluble inorganic species in the coarse and fine atmospheric aerosols at Dar es Salaam, Tanzania. Nucl Inst. Meth Phys. Res. B 267, 2897–2902 (2009)
Nicolas, J.F., Galindo, N., Yubero, E., Pastor, C., Esclapez, R., Crespo, J.: Aerosol inorganic ions in a semiarid region on the southeastern Spanish Mediterranean Coast. Water Air Soil Pollut. 201, 149–159 (2009)
Niemi, J.V., Tervahattu, H., Vehkamaki, H., Martikainen, J., Laakso, L., Kulmala, M.: Characterization and source identification of a fine particle episode in Finland caused by wildfires in Eastern Europe. Atmos. Chem. Phys. 5, 2299–2310 (2005)
Oanh, N.T.K., Upadhyay, N., Zhuang, Y.H., Hao, Z.P., Murthy, D.V.S., Lesstari, P., Villarin, J.T., Chengchua, H.X., Co, H.X., Dung, N.T., Lindgren, E.S.: Particulate pollution in six Asian cities: spatial and temporal distributions, and associated sources. Atmos. Environ. 40, 3367–3380 (2006)
Park, S.S., Ondov, J.M., Harrison, D., Nair, N.P.: Seasonal and shorter term variations in particulate atmospheric nitrate in Baltimore. Atmos. Environ. 39, 2011–2020 (2005)
Pateraki, S., Asimakopoulos, D.N., Maggos, T., Vasilakos, C.: Particulate matter levels in a suburban Mediterranean area: Analysis of a 58-month long experienced campaign. J. Hazard. Mater. 182, 801–811 (2010)
Perez, N., Pey, J., Querol, X., Alastuey, A., Lopez, J.M., Viana, M.: Partitioning of major and trace components in PM10, PM2.5, PM1 at an urban site in Southern Europe. Atmos. Environ. 42, 1677–1691 (2008)
Querol, X., Alastuey, A., Ruiz, C.R., Artinano, B., Hansson, H.C., Harrisson, R.M., Buringh, E., Brink, H.M., Lutz, M., Bruckmann, P., Straehl, P., Schneider, J.: Speciation and origin of PM10 and PM2.5 in selected European cities. Atmos. Environ. 38, 6547–6555 (2004)
Ramachandran, S.: PM2.5 mass concentrations in comparison with aerosol optical depth over the Arabian Sea and India Ocean during winter monsoon. Atmos. Environ. 39, 1879–1890 (2005)
Raman, R.S., Hopke, P.K., Holsen, T.M.: Characterization of fine aerosol and its inorganic components at two rural locations in New York State. Environ. Monit. Assess. 144, 351–366 (2008)
Rastogi, N., Sarin, M.M.: Long-term characterization of ionic species in aerosols from urban and high-altitude sites in western India: Role of mineral dust and anthropogenic sources. Atmos. Environ. 39, 5541–5554 (2005)
Ravindra, K., Stranger, M., Grieken, R.V.: Chemical characterization and multivariate analysis of atmospheric PM2.5 particles. J. Atmos. Chem. 59, 199–218 (2008)
Rehwagen, M., Muller, A., Massolo, L., Herbarth, O., Ronco, A.: Polycyclic aromatic hydrocarbons associated with particle in ambient air from urban and industrial area. Sci. Total Environ. 348, 199–210 (2005)
Samburova, V., Zenobi, R., Kalberer, M.: Characterization of high molecular weight compounds in urban atmospheric particles. Atmos. Chem. Phys. 5, 2163–2170 (2005)
Sastry, M.S., Suneela, M., Kumar, N.P.S., Hussain, S.K.: Air quality status at selected locations in Hyderabad city. J. Environ. Sci. Eng. 46, 86–91 (2004)
Seinfeld, J., Pandis, S.: Atmospheric chemistry and physics: from air pollution to climate change. Willey, New York (1998)
Shen, Z., Cao, J., Tong, Z., Liu, S., Reddy, L.S.S., Han, Y., Zhang, T., Zhou, J.: Chemical characteristics of submicron particles in winter in Xi’an. Aerosol Air Qual. Res. 9, 80–93 (2009)
Shi, Z., Shao, L., Jones, T.P., Whittaker, A.G., Lu, S., Berube, K.A.: Characterization of airborne individual particles collected in an urban area, a satellite city and a clean air area in Beijing, 2001. Atmos. Environ. 37, 4097–4108 (2003)
Singh, R., Sharma, B.S., Chalka, S.N.: Seasonal air quality profile of inorganic ionic composition of PM10 near Taj Mahal in Agra, India. Environ. Monit. Assess. 168, 195–203 (2010)
Srivastava, A., Jain, V.K.: Seasonal trends in coarse and fine particles sources in Delhi by the chemical mass balance receptor model. J. Hazard. Mater. 144, 283–291 (2007)
Srivastava, A., Gupta, S., Jain, V.K.: Source apportionment of total suspended particulate matter in coarse and fine size ranges over Delhi. Aerosol Air Qual. Res. 8, 188–200 (2008)
Sun, Y., Zhuang, G., Wang, Y., Han, L., Guo, J., Dan, M., Zhang, W., Wang, Z., Hao, Z.: The air-born particulate pollution in Beijing-concentration, composition, distribution and sources. Atmos. Environ. 38, 5991–6004 (2004)
Tai, A.P.K., Mickley, L.J., Jacob, J.D.: Correlations between fine particulate matters (PM2.5) and meteorological variables in the United States: Implication for the sensitivity of PM2.5 to climate change. Atmos. Environ. 44, 3976–3984 (2010)
Taneja, A., Saini, R., Masih, A.: Indoor air quality of house located in the urban environment of Agra, India. Ann. N.Y. Acad. Sci., 1–18 (2008)
Tiwari, S., Srivastava, A.K., Bisht, D.S., Bano, T., Singh, S., Behura, S., Srivastava, M.K., Chate, D.M., Padmanabhamurty, B.: Black carbon and chemical characteristics of PM10 and PM2.5 at an urban site of north India. J. Atmos. Chem. 62, 193–209 (2009)
Tsai, Y.I.: Atmospheric visibility trends in an urban area in Taiwan 1961–2003. Atmos. Environ. 39, 5555–5567 (2005)
Tsang, H., Kwok, R., Miguel, A.H.: Pedestrian exposure to ultrafine particles in Hong Kong under heavy traffic conditions. Aerosol Air Qual. Res. 8, 19–27 (2008)
Turalioglu, F.S., Nuhoglu, A., Bayraktar, H.: Impacts of some meteorological parameters on SO2 and TSP concentration in Erzurum, Turkey. Chemos. 59, 1633–1642 (2005)
Wang, Y., Zhuang, G.S., Tang, A., Yuan, H., Sun, Y., Chen, S., Zheng, A.H.: The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 39, 3771–3784 (2005)
Wang, Y., Zhuange, G.S., Sun, Y.L., An, Z.S.: The variation of characteristics and formation mechanisms of aerosols in dust, Haze and clear days in Beijing. Atmos. Environ. 40, 6579–6591 (2006)
Zhang, R.J., Shen, Z.X., Zou, H., Wang, W., Han, Y., Zhou, J.: Study of elemental mass size distributions of aerosols in Lijiang, a background site in Southwest China. Aerosol Air Qual. Res. 8, 339–347 (2008)
Acknowledgements
The authors would like to thank Head, School of Studies in Chemistry, Pt. Ravishankar Shukla University, Raipur, India for providing laboratory support. The authors also express their gratitude to Prof. Yukio Suzuki, Public Health and Research Institute of Kobe City, Japan for the analysis of aerosol samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deshmukh, D.K., Deb, M.K., Tsai, Y.I. et al. Atmospheric ionic species in PM2.5 and PM1 aerosols in the ambient air of eastern central India. J Atmos Chem 66, 81–100 (2010). https://doi.org/10.1007/s10874-011-9194-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-011-9194-1