Abstract
The effect of ocean acidification, caused by the increase in pCO2 in seawater, on phytoplankton population and on related organic nitrogen production was experimentally examined by use of a natural coastal microbial population. pCO2 and pH were controlled by aeration with air in which pCO2 was at the current level (control), for which ambient air was used, and with air in which pCO2 was “800” and “1200” ppm, in 500-L culture vessels. The experiment was continued for 15 days after addition of the inorganic nutrients such as nitrate, phosphate, and silicate. During most of the experimental period, a minor increase in phytoplankton biomass was noted, probably because of low irradiance, an increase in phytoplankton biomass was observed at the end of the experiment. Flow cytometric and microscopic observations revealed that this increase was because of Chrysochromulina sp. (Haptophyceae). The growth of Chrysochromulina sp. was most obvious in the control vessel, and tended to be obscured by increasing pCO2 (decrease in pH), indicating the possibility that ocean acidification inhibits the growth of specific phytoplankton groups, for example Chrysochromulina sp. Production of particulate organic nitrogen (PON), determined by the 15N tracer method, also diminished under acidified conditions compared with that at the current level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The ocean is the earth’s most significant sink of anthropogenic carbon dioxide, absorbing approximately 2.2 GtC year−1 from the atmosphere, and accounts for approximately one third of the carbon dioxide emitted by the combustion of fossil fuels (Denman et al. 2007). It is believed that uptake of atmospheric CO2 by the ocean has delayed the progress of climate change, because the atmospheric partial pressure of CO2 (pCO2) would currently be 450 ppm without ocean absorption (Doney et al. 2009). However, uptake of CO2 by the ocean poses a new problem, i.e., “ocean acidification,” a phenomenon caused by release of hydrogen ions when carbonic acid dissociates first to bicarbonate then to carbonate ions. The pH of ocean surface water is believed to have decreased by 0.1 U since the beginning of the industrial revolution (Riebesell et al. 2000; Doney et al. 2009). Considering that atmospheric CO2 is predicted to increase further to 700 ppm by the end of this century (IS92a scenario, Meehl et al. 2007), the pH of the surface ocean will drop further by 0.3–0.4 U (Meehl et al. 2007). In the last decade, concern about the effect of acidification on marine organisms has increased rapidly.
The most frequently predicated effect of ocean acidification on marine organisms is that on the formation of the calcareous skeleton. The decrease in pH accompanies a concomitant decrease in the concentration of carbonate ions, causing, in turn, a drop in the saturation state of calcium carbonate (Feely et al. 2004; Fabry et al. 2008). This might inhibit healthy production of calcareous skeletons of the kind observed in the pteropod Clio pyramidata (Orr et al. 2005; Fabry et al. 2008) and the coccolithophorids Emiliania huxleyi and Gephyrocapsa oceanica (Riebesell et al. 2000; Engel et al. 2005).
A second possible effect is that of the increase in CO2 in seawater on the rate of photosynthesis. Most photosynthesizing microorganisms in oceanic environments fix CO2 via the carboxylating enzyme, ribulose-1,5-bisphosphate carboxylase/oxygenase (RUBISCO). Although this enzyme requires 25–35 μM CO2 to become saturated (Raven 1991), free CO2 is only ~10 μM in ambient seawater. To compensate for this difference, some phytoplankton groups activate a carbon concentration mechanism (CCM) to maintain a sufficient intracellular concentration of CO2 (Giordano et al. 2005). Such an increase in the concentration of free CO2 in seawater caused by the ocean’s uptake of atmospheric CO2 is expected to have little effect on the rate of photosynthesis of phytoplankton such as diatoms, which have an advanced CCM (Tortell et al. 1997; Beardall and Raven 2004). On the other hand, phytoplankton groups such as coccolithophorids, with a low affinity for inorganic carbon, are able to increase their rate of photosynthesis in response to an increase in CO2 (Rost et al. 2008). However, experiments using natural phytoplankton populations have sometimes noted an increase in the growth of diatoms at higher CO2 concentrations (Chen and Durbin 1994; Tortell et al. 2002). This implies the possibility that factor(s) other than CCM may be involved in the effect of CO2 concentration on phytoplankton growth. Recent experiments under high-CO2 conditions indicate that other factors, for example iron availability, irradiance, and water temperature, are inversely related to the rate of photosynthesis (Feng et al. 2008, 2010; Hurd et al. 2009; Shi et al. 2010). Although experimental studies using natural microbial populations under different CO2 conditions have greatly increased during this decade (Tortell et al. 2002; Kim et al. 2006; Riebesell et al. 2007; Hendriks et al. 2010; Yoshimura et al. 2010), further accumulations of data concerning the response of natural microbial populations will be needed to accurately analyze changes in phytoplankton populations and subsequent biogeochemical cycles.
In this study we conducted an incubation experiment at 3 different concentrations of CO2 (×1, ×2, and ×3 of the current level), in the winter season, using large (500-L) outdoor culture vessels to assess changes in the composition of phytoplankton populations and changes in nitrogen cycles. Flow cytometry (FCM) and the 15N tracer method were used to achieve these objectives.
2 Materials and methods
2.1 Experimental system
Our culture experiment under three pCO2 conditions was carried out at Shimoda Marine Station, University of Tsukuba, Shimoda, Shizuoka, Japan. Three cylindrical acrylic vessels (800 mm ϕ, 1000 mm in height, ca. 500 L in volume) were used for the experiment. The vessels were set in an outdoor pool 1 m deep with the water temperature controlled by the flow of coastal water through the pool; water temperature was measured by use of a temperature logger (MDS-MkV/T; JFE Advantech). On 22 January 2009, 400 L of sample sea water was slowly pumped up from a nearby coastal site and used to fill the vessel while being filtered through a 100-μm mesh to remove macrozooplankton. The culture vessel was kept under an acrylic cover except during water sampling. Light intensity was reduced to 50% by covering the vessels with black mesh throughout the experiment, and photon flux was measured every 3 h during the experiment (LI-250; LI-COR).
Air with three levels of pCO2, prepared by mixing ambient air supplied by an oil-less air compressor and 3% CO2 with pure air (N2 and O2), was introduced into the cultured water from the bottom of the vessel through two airstones (300 mm in length and 20 μm in pore size). We calculated the mixing ratio of the ambient air and 3% CO2 on the assumption that the pCO2 in the ambient air was 400 ppm. We prepared control (ambient air only), 800, and 1200 ppm air and introduced them to vessels (these are henceforth referred to as control, “800” and “1200” ppm vessels, respectively). The air sample supplied to each vessel was corrected from the outlet of the gas mixer, and pCO2 was measured by a CO2 analyzer (LI-6262; LI-COR). Air was released from the vessel through an outlet (1/4 inch ϕ) in the upper part of the vessel during aeration. The preparative aeration was started at 1400 hours on 22 January 2009 local time and continued until 0900 hours on 24 January at a flow rate of 10 L min−1 in each tank; a total of 25,800 L of air was introduced into each vessel. During the experiment, aeration was conducted for 6 h from 1000 to 1600 hours every day to maintain the pCO2 in each vessel.
Inorganic nutrients comprising KNO3 (12.6 μmol L−1), KH2PO4 (0.77 μmol L−1), and Na2SiO3 (12.4 μmol L−1) were added at 0900 hours on 24 Jan (day 1) to increase their concentrations, and mixed by aeration for 1 h (actual nutrient concentrations were measured as described below).
2.2 Sample collection
Water samples on day 1 were collected from each culture vessel 1 h after addition of the nutrients. Samples for analysis of pH and dissolved inorganic carbon (DIC) were collected in 500 ml glass bottles by use of an air-tight sampling system, and HgCl2 was added. Polycarbonate tubing 52 mm ϕ and 1000 mm in length was inserted into the culture solution and the tube was filled with water by submerging it to the bottom. The upper end of the tubing was then stoppered. The tube was then raised and the lower end was stoppered. In this way the water was sampled. This was repeated 5 times, and a total of approximately 8 L of the water was sampled from each vessel. Fractions of the raw water samples were divided for measurement of phytoplankton composition by both FCM and light microscopy. Water samples for FCM were filtered through a 59-μm mesh to remove large particles that might obstruct the sampling nozzle. The concentrations of chlorophyll a (Chl. a) for the non-filtered (all size) and filtered (<59 μm) samples was no different throughout the experiment, indicating that pretreatment with the 59-μm mesh caused only a minor compositional and quantitative bias in the phytoplankton population. Other water samples were filtered through precombusted (450°C, 4 h) glass fiber filters (Whatman GF/F). The filters and filtrates were stored at −20°C until analysis.
2.3 Sample analysis
The concentrations of nitrate, nitrite, and phosphate were determined by use of an autoanalyzer (AACSII, BRAN + LUEBBE) whereas those of ammonium and silicate were determined colorimetrically by the methods described in Parsons et al. (1984).
We measured total inorganic carbon and pH to monitor the CO2 system in seawater. Total inorganic carbon (TCO2) was analyzed coulometrically (Johnson et al. 1985) using an automated CO2 extraction unit equipped with a carbon coulometer (Nippon ANS). It was calibrated with a series of sodium carbonate solutions prepared from primary standard-grade anhydrous sodium carbonate (99.98%; Asahi Glass), dried at 600°C for 1 h, and with purified water provided by Milli-Q SP TOC (Ishii et al. 2000). Analysis of pH was conducted spectrophotometrically on a total hydrogen ion concentration scale using m-cresol purple as indicator dye at 25°C (Clayton and Byrne 1993) with an automated flow-cell system that we have developed for discrete bottle samples (Saito et al. 2008). Batches of certified reference material (CRM) provided by Dr A.G. Dickson (http://www-mpl.ucsd.edu/people/adickson/CO2QC/index.html) were also used for quality control of these analyses. Partial pressure of CO2 in seawater and pH at the in-situ temperature were computed from TCO2, pH at 25°C, temperature, and salinity, by use of the dissociation constants of carbonic acid given by Lueker et al. (2000).
The concentration of Chl. a was measured by fluorometry (10-005; Turner Design) after extraction with N,N-dimethylformamide (Suzuki and Ishimaru 1990). Concentrations of particulate organic carbon (POC) and nitrogen (PON) were determined by use of an elemental analyzer (EA1108; Carlo Erba). The 15N atom% of PON was measured by an isotope-ratio mass spectrometer (Delta Plus; Finnigan MAT) combined with EA.
The production of PON was calculated by use of Eq. 1 (Hama et al. 1983, 1993):
where, a is is 15N atom% of the incubated sample, a ns 15N atom% of the non-incubated sample, a in 15N atom% of the dissolved inorganic nitrogen, and [ON] concentration of PON (μM N).
In the experimental design of this study, tracer material (K15NO3) was added on day 1, and the organic nitrogen containing 15N was followed throughout the experiment. Thus, the calculated value corresponds to the concentration of photosynthetically produced PON from the start of the experiment to a given date (Hama et al. 2004), rather than to the rate at a date with a unit of μM N day−1. The values will be referred as the concentrations of photosynthetically produced PON (P-PON) with units of μM N. Because dissolved inorganic nitrogen was mostly composed of 15N-KNO3 at the start of the experiment, due to the low concentration of nitrate and ammonium, we used 99.5 atom%, which is the value of 15N-KNO3 added, as a in in this calculation.
The single filter sample was used for determination of POC, PON including 15N atom%, and P-PON; the coefficients of variation of the concentrations of these variables by use of our system are usually less than 5% (Hama and Yanagi 2001). Determination of Chl. a was performed by use of two filters.
Flow-cytometric analysis was performed with a FACSCalibur (4-color type; Becton–Dickinson) equipped with an argon ion laser (488 nm) and a red semi-conductor laser (635 nm). Emitted fluorescence was monitored at FL1 (bandpass 530 ± 15 nm: green), FL2 (585 ± 21 nm: orange), FL3 (>670 nm: long-pass red), and FL4 (661 ± 8 nm: narrow red), and at a forward-light scatter (SFC) and a side-light scatter (SSC). FACSFlow sheath fluid (Becton–Dickinson) was forced by a pressure of 4.5 psi through a 100-μm nozzle, with 60 μL of sample. Fluoresbrite microspheres (6 and 10 μm; Polysciences) were used to adjust the instrumental conditions. The measurements and data analysis were controlled by CELLQuest software. The FCM analysis was complete within 12 h of collection of the sample. In addition to samples collected from culture experiments using the natural population, a cultured strain of Chrysochromulina sp. (Haptophyceae) (isolated by micropipetting from Uno Port, Okayama Prefecture, Japan, on 15 October 2008 and cultured by Dr Nakayama) was also measured by FCM under the same analytical conditions. Phytoplankton was identified by optical microscopy, but detailed quantitative determination was not carried out.
3 Results
3.1 Physicochemical data
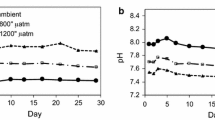
The temperature of the water flowing through the outdoor pool ranged from 11.7 to 13.8°C and the photon flux on the culture vessel varied with the range 50–129, 133–235, and 13–99 μmol m−2 s−1 at 0900, 1200, and 1500 hours, respectively, during the experiment. Figure 1 shows changes in the concentrations of DIC, pCO2, and pH in the three vessels. The concentrations of DIC changed within the ranges of 2063–2103, 2168–2205, and 2222–2260 μmol kg−1 in control, “800”, and “1200” ppm vessels, respectively (Fig. 1a). The DIC concentrations were higher in the 800 and 1200 ppm-treated vessels, reflecting the higher concentration of CO2. The pCO2 of the aeration gas collected just after the gas mixer was measured as 444–514 (mean 473; σ 38.4, n 4), 803–909 (mean 865; σ 47.4, n 4), and 1285–1300 ppm (mean 1290; σ 11.2, n 4) in the control, “800”, and “1200” ppm gas. The variation of actual pCO2 in the treatment air was likely to be because of fluctuation of pCO2 in ambient air. The pCO2 in water vessels treated with control, “800”, and “1200” ppm air varied within the ranges 345–431, 666–839, and 977–1221 ppm, respectively (Fig. 1b). pCO2 tended to gradually increase from days 1 to 4 in all vessels, but was subsequently relatively constant until the end of the experiment. The pH in the culture vessels reflected the different pCO2 of the treatment air (Fig. 1c). The highest values were observed in the control, ranging from 8.02 to 8.10, followed by the “800” ppm vessel, from 7.76 to 7.85. The most acidic values were observed in the “1200” ppm vessel, in which the pH changed from 7.61 to 7.70. pH tended to decrease in the first 4 days concomitant with the increase in pCO2. Differences in pH among the treatments were obvious throughout the experiment.
The concentrations of inorganic nutrients of the original sea water on 11 January were 0.93, 0.18 and 15.2 μM for nitrate, phosphorus, and silicate, respectively, and they increased to 13.3, 1.4 and 28–30 μM after addition of nutrients on day 1. The concentration of nutrients showed only slight variations throughout the experiment (Fig. 2). However, it is worth noting that a more than 1 μM N decrease in nitrate was found from days 11 to 15 in the control vessel. The decreases in the “800” and “1200” ppm-treated vessels were not obvious, although minor decreases of less than 0.4 μM were found. Changes in the concentrations of phosphate and silicate were almost the same as those of nitrate.
3.2 Chl. a, phytoplankton and flow cytometry
The concentration of Chl. a of the original sea water collected on 22 January was 0.06 μg L−1 and varied from 0.07 to 0.3 μg L−1 in every vessel from days 1 to 8 (Fig. 3); little increase was found even though inorganic nutrients had been added on day 1. This finding agrees with the fact that only a slight decrease in nutrient concentrations was found until day 8 in every vessel. Although no difference in the Chl. a concentration among 3 vessels was obvious on day 11, the concentration rapidly rose to 1.98 μg L−1 in the control vessel on day 15. The increases in Chl. a concentrations were also found in the "800" ppm vessel although it became less obvious as the pCO2 increased (decrease in pH); the concentrations of Chl. a in the “800” and “1200” ppm vessels on day 15 were 0.17 and 0.05, respectively, compared to that in the control.
Bacillariophyceae (total number of genera identified: 12), including Chaetoceros spp., Skeletonema sp., and Thalassiosira spp., and Cryptophyceae (1), Haptophyceae (1), Dinophyceae (4), and Prasinophyceae (3) were found to be constituents of the phytoplankton population throughout the experiment (Table 1). Bacillariophyceae and Dinophyceae were mainly observed in the early stage of the experiments, but Chrysochromulina sp. (Haptophyceae) and Tetraselmis sp. (Prasinophyceae) were found mainly in the latter experimental stage from days 8 to 15. Although detailed quantitative microscopic analysis was not conducted in this study, the rapid increase in Chrysochromulina sp. (Fig. 4) on day 15 was quite obvious. This strongly suggested that the increase in Chl. a on day 15 was because of the growth of this species.
The development of particular phytoplankton groups from days 11 to 15 was clearly revealed by FCM analysis. Figure 5 shows the results from FCM for the samples collected from the three vessels on days 1, 8, 11, and 15; the fluorescent intensities of FL4 and FL2 are shown. Although the gross distribution patterns of particles in the 3 culture vessels were similar through days 1 to 8, the particles with intensities of 102–103 of FL4 and 1–5 × 10 of FL2 (group 1) became evident in the control and “800” ppm vessels on day 11. A further increase in these particles was noticed from days 11 to 15, especially in the control vessel. The results of FCM analysis strongly suggested that the increase in Chl. a was caused by the growth of the phytoplankton included in group 1.
Results from flow cytometry of samples collected from control, “800”, and “1200” ppm vessels on days 1, 8, 11, and 15. Circles depict phytoplankton groups 1–3 (see text). The relationship between fluorescence of FL4 (band pass 661 ± 8 nm) and FL2 (band pass 585 ± 21 nm). a–d control; e–h “800” ppm; i–l “1200” ppm
To confirm the FCM analysis, we measured the cultured Chrysochromulina sp. isolated from Uno Bay, Okayama Prefecture, whose diagnostic features were found to be almost comparable with those in this study. The result of the FCM analysis (Fig. 6) clearly showed that the position of Chrysochromulina sp. cultured in the laboratory was identical with that in group 1, confirming that group 1 was composed of Chrysochromulina sp. Thus, our results concerning the composition of phytoplankton population suggested that growth of Chrysochromulina sp., a noncalcifying haptophyte, was inhibited by the increase in pCO2 (decrease in pH). Other groups with intensities of 5 × 101 to 2 × 102 of FL4 and 2 × 100 to 1 × 101 of FL2 (group 2) also showed the highest counts in the control vessel, though differences from the “800” and “1200” ppm vessel were much less than for group 1. We compared the position of this group with those of isolated species of various phytoplankton and it superimposed on that of haptophyte other than Chrysochromulina sp.. However, microscopic analysis observed Chrysochromulina sp. as the sole constituent of Haptophyceae on day 15. Thus, a phytoplankton genus corresponding to group 2 was not identified in this study. The same is true for that in group 3 (1 × 101 to 5 × 101 of FL4 and 1 × 100 to 7 × 101 of FL2) which was most abundant in the “1200” ppm vessel on day 15 (Fig. 5).
3.3 POC and PON
The measured concentration of POC was within the range 7.4–9.8 μM C in every vessel up to day 11 with no systematic variation (Fig. 7a). A rapid increase in that concentration was observed from days 11 to 15 in the control vessel, though the values in the other vessels remained constant. The concentrations of PON were 0.88–0.96 μM N in the three vessels at the start of the experiment (Fig. 7b). It tended to increase from day 11 in the control and “800” ppm vessels and a distinctly higher value (2.3 μM N), as well as those of Chl. a and POC, was observed on day 15 in the control vessel. Although high C/N ratios (9–11 molar ratio) were observed for POM at the start of the experiment, values around the Redfield ratio (6.6) were obtained on the other days.
3.4 Concentration of P-PON
The 15N tracer method was used to measure the concentration of P-PON in this study. Because 15N-KNO3 was added at the start of the experiment, the concentration of P-PON is comparable with the accumulation of PON produced by phytoplankton throughout the experiment; note that the value does not represent the daily production rate. The concentration of P-PON was low (less than 0.01 μM N up to day 6) with a little increase in every vessel (Fig. 7c). The concentration tended to increase from day 8 in every vessel, and a rapid increase was observed from days 11 to 15 in the control vessel. Although such increases were also measurable in the “800” and “1200” ppm vessels, they were far less obvious than those in the control vessel (17 and 12% of that of control vessel, respectively).
4 Discussion
In this decade, many studies have been conducted on the effect of ocean acidification on phytoplankton composition (Hendriks et al. 2010). The culture volume used for experiments on natural phytoplankton populations, with the carbonate system controlled by manipulation of pCO2, varies from 4 L (Tortell et al. 2002) to 27 m3 (Riebesell et al. 2007). It is relatively easy to maintain the carbonate system in a small-volume culture, but difficult to establish and maintain specific pCO2 in a large-volume culture. In an experiment using an extra-large culture vessel, aeration of the water column with air of specific pCO2 has been applied only before the start of the experiments (Engel et al. 2005; Kim et al. 2006; Riebesell et al. 2007), after which the upper air portion only was maintained by special pCO2. In our study, aeration for 43 h before addition of the nutrients was used, and it is conceivable that the carbonate system of the water samples in each vessel was approximately equilibrated with the air introduced. The total air volume used to aerate each vessel before day 1 (25,800 L) amounted to 65 times the water sample volume. Although a slight increase in pCO2 was noticed from days 1 to 4 in each vessel, this result indicates that an aeration volume of more than 65 times the water volume would almost suffice to equilibrate the carbonate system of seawater.
In this study, the pH decreased from ca. 8.05 in the control vessel to 7.65 in the “1200” ppm vessel. A decrease of 0.4 U is well within the range expected to occur until the end of this century (Meehl et al. 2007). Thus, the conditions established in this study would occur not in the far distant future but over the next 50–100 years. The variability of pH in each vessel changed little throughout the experiment (0.08–0.09 U), while the differences between the treatments (approx. 0.15 U) were kept constant. Thus, it can be concluded that control of the carbonate system proceeded successfully.
In this study, we clearly observed that an increase in pCO2 (decrease in pH) prevented the growth of Haptophyceae Chrysochromulina sp. The increase in free CO2 concentration accompanied by ocean acidification has been generally believed to increase the rate of photosynthesis of phytoplankton, though to what extent varies depending on the efficiency of CCM (Riebesell 2004; Rost et al. 2008). Experimental analysis under higher-CO2 conditions, in fact, generally produced an increase in phytoplankton productivity (Kim et al. 2006; Ramos et al. 2007; Tortell et al. 2008). An inhibitory effect had been reported for formation of the calcareous plankton skeleton, for example that of coccolithophorids (Riebesell et al. 2000; Delille et al. 2005; Hopkins et al. 2010). Recently, however, a negative effect on non-calcified phytoplankton has been reported (Feng et al. 2010; Hopkins et al. 2010; Yoshimura et al. 2010).
Feng et al. (2010) examined the response of the phytoplankton population in Ross Sea under conditions with different iron concentrations, irradiance, and pCO2. Under low irradiance they found that the concentration of 19′-hexanoyloxyfucoxanthin, the index pigment for haptophytes, was lower under high-pCO2 (736–776 ppm) conditions than under low conditions (363–385 ppm). This result suggests the dominant haptophyte, Phaeocystis antarctica, was negatively affected by the increase in pCO2. A comparable result has been obtained for the Equatorial Pacific phytoplankton assemblage (Tortell et al. 2002)—Phaeocystis sp. decreased by 60% under high-pCO2 conditions (750 ppm) but not under low pCO2 conditions(150 ppm). Although the species in these experiments were different from that of our experiment, it is noteworthy that the growth of haptophyte decreased under high-pCO2 and low-pH conditions. However, experimental results obtained so far indicate that the responses of phytoplankton to the increase in pCO2 (decrease in pH) did not show the same tendency despite being the same species (Hendriks et al. 2010; Paulino et al. 2008; Yoshimura et al. 2010). Factors other than pCO2 and pH, for example irradiance, macro nutrients, and iron are likely to affect the physiological response of phytoplankton (Feng et al. 2008; Hurd et al. 2009; Yoshimura et al. 2010). Thus, additional experiments using isolated Chrysochromulina sp. under different condition are desirable to elucidate whether the negative effect on growth under high pCO2 environments can be generalized.
The genus Chrysochromulina comprises diverse species of noncalcifying haptophytes, and is known to be widely distributed from coastal waters (Kaas et al. 1991; Hajdu et al. 2007) to the open ocean (Not et al. 2008; Liu et al. 2009). Recently, Liu et al. (2009) using genetic pigment and microscopy data, examined the distribution and abundance of photosynthetic picoplanktonic protists belonging to the Haptophyta in subtropical and subpolar oceanic waters. They discovered that most of the hundreds of haptophyte taxa belong to the same clade comprising the genus Chrysochromulina. Furthermore, they reported the novel finding that these haptophytes are responsible for 30–50% of the total standing stock of haptophytes, diatoms, and prokaryotes throughout the world’s oceans. The standing stock of haptophytes (2.5 × 109 kg as Chl. a) was found to be higher than those of either diatoms or prokaryotes, i.e., 1.3 × 109 and 1.1 × 109 kg as Chl. a, respectively (Liu et al. 2009). Although it is possible that the physiological response to ocean acidification is variable even in this genus, the high susceptibility to acidification of Chrysochromulina sp. observed in our study implies that ocean acidification caused substantial damage to significant components of photosynthetic eukaryotes.
The 15N tracer method clearly demonstrated the production processes of organic nitrogen. The selective growth of Chrysochromulina sp. in the control vessel was accompanied by an increase in the concentration of P-PON. The concentration of P-PON in acidified vessels accounted only for 17 and 11% of that under current pH conditions in the “800” and “1200” ppm vessels, respectively. This result suggests the possibility that ocean acidification reduces primary productivity by inhibiting the growth of specific phytoplankton groups. This finding does not agree with most previous experimental studies, which generally observed an increase in the rate of photosynthesis under increased CO2 conditions (Riebesell et al. 2000; Hein and Sand-Jensen, 1997; Czerny et al. 2009) assuming that ocean acidification enhances primary production (Hein and Sand-Jensen, 1997; Rost et al. 2008). The decrease in pH examined in this study (0.2–0.4 U) is most likely to be realized until the end of this century. The major contribution of noncalcified haptophytes composed mainly of the genus Chrysochromulina (Liu et al. 2009) implies the possibility of a substantial decline in oceanic primary production of these algal groups.
Indirect effects, for example grazing by zooplankton or infection by viruses, are comparable with the different biomass of Chrysochromulina sp. caused by different pH. Although water samples were prefiltered through 98-μm mesh before filling of the culture vessels, to remove macrozooplankton, some microzooplankton were possibly included in the experimental system. When microzooplankton is sensitive to changes in pCO2 or pH, the grazing pressure was possibly different among the treatments, resulting in change in the biomass of Chrysochromulina sp. However, any effect of grazing on our results is difficult to evaluate, because the effect of ocean acidification on non-calcifying zooplankton has not yet been elucidated (Fabry et al. 2008). On the other hand, it is known that Chrysochromulina species can be infected by viruses (Suttle and Chan 1995; Sandaa et al. 2001). Larsen et al. (2008) observed that the activity of some virus groups changes, depending on pH. Their result suggests the possibility that the difference in the Chrysochromulina sp. biomass among the vessels was caused by differences between viral populations. These “indirect” effects have not yet been assessed, but measurements of zooplankton or virioplankton during the experiments will afford significant information about the effect of ocean acidification on the food web and the flow of energy in microbial ecosystems.
Apart from the effect on the growth of haptophytes, addition of inorganic nutrients including silicate usually resulted in increased phytoplankton biomass mainly composed of diatoms in mesocosm experiments (Hama et al. 1988; Engel et al. 2002). However, a little increase in phytoplankton biomass was found in this experiment. Measured macronutrients, including silicate, were at high concentrations throughout the experiment, implying that macronutrients did not limit the growth of diatoms. Low light intensity is the most possible reason why dense phytoplankton bloom did not occur in this experiment. The photon flux at the culture vessel varied with the ranges 50–129, 133–235 and 13–99 μmol m−2 s−1 at 0900, 1200 and 1500 hours, respectively during the experiment. Referring to the reported saturation photon flux for phytoplankton photosynthesis including diatoms (100–500 μmol m−2 s−1; Sarthou et al. 2005; Lefebvre et al. 2007; Grangeré et al. 2009), the values observed in this study strongly suggest that light intensity was not enough to enable rapid accumulation of diatoms.
It is possible that the aeration to maintain the corresponding pCO2 and pH throughout the experiment resulted in a slight increase in phytoplankton bloom because of the turbulence (Thomas and Gibson 1990; Hurd et al. 2009). Although it is difficult to evaluate the effect quantitatively, we observed a dense bloom of phytoplankton mainly composed of diatoms in same experimental system in other experiments conducted from spring to autumn (unpublished data). This indicates that the slight increase in phytoplankton biomass in this study was not because of aeration.
In this study, one vessel was arranged for each CO2 treatment because of the difficulty of handling large vessels. More than two vessels for each experimental condition, certainly, are desirable to increase the reliability of the results. In this study, the stepwise decreasing trend in the standing stock of Chrysochromulina sp. with pCO2 is obvious though control, “800”, and “1200”ppm. From these results, we believe that the inhibitory effect of the increase in pCO2 on the growth of Chrysochromulina sp. is a dependable result, but that it is necessary to conduct the additional mesocosm experiments with more than duplicate vessels to confirm the inhibitory effect of acidification to Chrysochromulina sp. Further, culture experiments using isolated Chrysochromulina sp. at different pH will furnish valuable information enabling elucidation of how reduction of pH inhibits the growth of this genus.
References
Beardall J, Raven JA (2004) The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 43:26–40
Chen CY, Durbin EG (1994) Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar Ecol Prog Ser 109:83–94
Clayton TD, Byrne RH (1993) Spectrophotometric seawater pH measurements: total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep-Sea Res 40:2115–2129
Czerny J, Ramos JB, Riebesell U (2009) Influence of elevated CO2 concentration on cell division and nitrogen fixation rates in the bloom-forming cyanobacterium Nodularia spumigena. Biogeosciences 6:1865–1875
Delille B, Harley J, Zondervan I, Jacquet S, Chou L, Wollast R, Bellerby RGJ, Frankignoulle M, Borges AV, Riebesell U, Gattuso JP (2005) Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorids Emiliania huxleyi. Glob Biogeochem Cycles 19:GB2023. doi:10.1029/2004GB002318
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, Dias PLS, Wofsy SC, Zhang X (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Marquis M, Averyt K, Tignor MMB, Miller HL Jr, Chen Z (eds) Contribution of Working Group I to the forth assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York, pp 499–587
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192
Engel A, Goldthwait S, Passow U, Alldredge A (2002) Temporal decoupling of carbon and nitrogen dynamics in a mesocosm diatom bloom. Limnol Oceanogr 47:753–761
Engel A, Zondervan I, Aerts K, Beaufort L, Benthien A, Chou L, Delille B, Gattuso JP, Harlay J, Heemann C, Hoffmann L, Jacquet S, Nejstgaard J, Pizay MD, Rochelle-Newall E, Schneider U, Terbrueggen A, Riebesell U (2005) Testing the direct effect of CO2 concentration on a bloom of the coccolithophorid Emiliania huxleyi in mesocosm experiments. Limnol Oceanogr 50:493–507
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305:362–366
Feng Y, Warner ME, Zhang Y, Sun J, Fu F-X, Rose JM, Hutchins DA (2008) Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae). Eur J Phycol 43:87–98
Feng Y, Hare CE, Rose JM, Handy SM, DiTullio GR, Lee PA, Smith WO Jr, Peloquin J, Tozzi S, Sun J, Zhang Y, Dunbar RB, Long MC, Sohst B, Lohan M, Hutchins DA (2010) Interactive effects of iron, irradiance and CO2 on Ross Sea phytoplankton. Deep Sea Res I 57:368–383
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanism in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Grangeré K, Lefebvre S, Ménesguen A, Jouenne F (2009) On the interest of using field primary production data to calibrate phytoplankton rate processes in ecosystem models. Est Coast Shelf Sci 81:169–178
Hajdu S, Hoglander H, Larsson U (2007) Phytoplankton vertical distributions and composition in Baltic Sea cyanobacterial blooms. Harmful Algae 6:189–205
Hama T, Yanagi K (2001) Production and neutral aldose composition of dissolved carbohydrates excreted by natural phytoplankton populations. Limnol Oceanogr 46:1945–1955
Hama T, Miyazaki T, Ogawa Y, Iwakuma T, Takahashi M, Otsuki A, Ichimura S (1983) Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar Biol 73:31–36
Hama T, Handa N, Takahashi M, Whitney F, Wong CS (1988) Change in distribution patterns of photosynthetically incorporated C during phytoplankton bloom in controlled experimental ecosystem. J Exp Mar Biol Ecol 120:39–56
Hama T, Hama J, Handa N (1993) 13C tracer methodology in microbial ecology with special reference to primary production processes in aquatic environments. Adv Microbial Ecol 13:39–83
Hama T, Yanagi K, Hama J (2004) Decrease in molecular weight of photosynthetic products of marine phytoplankton during early diagenesis. Limnol Oceanogr 49:181–471
Hein M, Sand-Jensen K (1997) CO2 increases oceanic primary production. Nature 388:526–527
Hendriks IE, Duarte CM, Alvarez M (2010) Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Est Coast Shelf Sci 86:157–164
Hopkins FE, Turner SM, Nightingale PD, Steinke M, Bakker D, Liss PS (2010) Ocean acidification and marine trace gas emissions. PNAS 107:760–765
Hurd CL, Hepburn CD, Currie KI, Raven JA, Hunter KA (2009) Testing the effects of ocean acidification on algal metabolism: considerations for experimental designs. J Phycol 45:1236–1251
Ishii M, Inoue HY, Matsueda H (2000) Coulometric precise analysis of total inorganic carbon in seawater and measurements of radiocarbon for the carbon dioxide in the atmosphere and for the total inorganic carbon in seawater. Tech Rep Met Res Inst 41:64
Johnson KM, King AE, Sieburth JMcN (1985) Coulometric TCO2 analyses for marine studies; an introduction. Mar Chem 16:61–82
Kaas H, Larsen J, Mohlenberg F, Richardson K (1991) The Chrysochromulina polylepis bloom in the Kattegat (Scandinavia) May–June 1988. Distribution, primary production and nutrient dynamics in the late stage of the bloom. Mar Ecol Prog Ser 79:151–161
Kim JM, Lee K, Shin K, Kang JM, Lee HW, Kim M, Jang PG, Jang MC (2006) The effect of seawater CO2 concentration on growth of a natural phytoplankton assemblage in a controlled mesocosm experiment. Limnol Oceanogr 51:1629–1636
Langer G, Geisen M, Baumann KH, Klaes J, Riebesell U, Thomas S, Young JR (2006) Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem Geophys Geosyst 7:Q09006. doi:10.1029/2005GC001227
Larsen JB, Larsen A, Thyrhaug R, Bratbak G, Sandaa RA (2008) Response of marine vial populations to a nutrient induced phytoplankton bloom at different pCO2 levels. Biogeosciences 5:523–533
Lefebvre S, Mouget JL, Loret P, Rosa P, Tremblin G (2007) Comparison between fluorometry and oxymetry techniques to measure photosynthesis in the diatom Skeletonema costatum cultivated under simulated seasonal conditions. J Photochem Photobiol B: Biology 86:131–139
Liu H, Probert I, Uitz J, Claustre H, Aris-Brosou S, Frada M, Not F, Vargas C (2009) Extreme diversity in noncalcifying haptophytes explains a major pigment paradox in open oceans. PANS 106:12803–12808
Lueker TJ, Dickson AG, Keeling CD (2000) Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K 1 and K 2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar Chem 70:105–119
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, Raper SCB, Watterson IG, Weaver AJ, Zhao ZC (2007) Global climate projections. In: Solomon S, Qin D, Manning M, Marquis M, Averyt K, Tignor MMB, Miller HL Jr, Chen Z (eds) Contribution of Working Group I to the forth assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York, pp 747–845
Not F, Latasa M, Scharek R, Viprey M, Karleskind P, Balague V, Ontoria-Ovideo I, Cumino A, Goetze E, Vaulot D, Massana R (2008) Protistan assemblages across the Indian Ocean, with a specific emphasis on the picoeukaryotes. Deep-Sea Res I 55:1456–1473
Orr JA, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner GJ, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig MF, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, p 346
Paulino AI, Egge JK, Larsen A (2008) Effects of increased atmospheric CO2 on small and intermediate sized osmotrophs during a nutrient induced phytoplankton bloom. Biogeosciences 5:739–748
Ramos JBE, Biswas H, Schulz KG, LaRoche J, Riebesell U (2007) Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Global Biogeochem Cycles 21:GB2028. doi:10.1029/2006GB002898
Raven JA (1991) Physiology of inorganic carbon acquisition and implications for resource use efficiency by marine phytoplankton: relation to increased CO2 and temperature. Plant Cell Environ 14:779–794
Riebesell U (2004) Effects of CO2 enrichment on marine phytoplankton. J Oceanogr 60:719–729
Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FMM (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407:364–367
Riebesell U, Schulz KG, Bellerby RGJ, Botros M, Fritsche P, Meyerhofer M, Neill C, Nondal G, Oschlies A, Wohlers J, Zollner E (2007) Enhanced biological carbon consumption in a high CO2 ocean. Nature 450:545–548
Rost B, Zondervan I, Wolf-Gladrow D (2008) Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar Ecol Prog Ser 373:227–237
Saito S, Ishii M, Midorikawa T, Inoue HY (2008) Precise spectrophotometric measurement of seawater pHT with an automated apparatus using a flow cell in a closed circuit. Tech Rep Met Res Inst 57:31
Sandaa R-A, Heldal M, Castberg T, Thyrhaug R, Bratbak G (2001) Isolation and characterization of two viruses with large genome size infecting Chrysochromulina ericina (Prymnesiophyceae) and Pyramimonas orientalis (Prasinophyceae). Virology 290:271–280
Sarthou G, Timmermans KR, Blain S, Tréguer P (2005) Growth physiology and fate of diatoms in the ocean: a review. J Sea Res 53:25–42
Shi D, Xu Y, Hopkinson BM, Morel FMM (2010) Effect of ocean acidification on iron availability to marine phytoplankton. Science 327:676–679
Suttle CA, Chan A (1995) Viruses infecting the marine Prymnesiophyte Chrysochromulina spp.: isolation, preliminary characterization and natural abundance. Mar Ecol Prog Ser 118:275–282
Suzuki R, Ishimaru T (1990) An improved method for the determination of phytoplankton chlorophyll using N,N-Dimethylformamide. J Oceanogr Soc Jpn 46:190–194
Thomas WH, Gibson GH (1990) Effects of small-scale turbulence on microalgae. J Appl Phycol 2:11–71
Tortell PD, Morel FMM (2002) Sources of inorganic carbon for phytoplankton in the eastern Subtropical and Equatorial Pacific Ocean. Limnol Oceanogr 47:1012–1022
Tortell PD, Reinfelder JR, Morel FMM (1997) Active uptake of bicarbonate by diatoms. Nature 390:243–244
Tortell PD, DiTullio GR, Sigman DM, Morel FMM (2002) CO2 effects on taxonomic composition and nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Mar Ecol Prog Ser 236:37–43
Tortell PD, Payne CD, Li Y, Trimborn S, Rost B, Smith WO, Riesselman C, Dunbar RB, Sedwick P, DiTullio GR (2008) CO2 sensitivity of Southern Ocean phytoplankton. Geophys Res Lett 35:L04605
Yoshimura T, Nishioka J, Suzuki K, Hattori H, Kiyosawa H, Watanabe YW (2010) Impacts of elevated CO2 on organic carbon dynamics in nutrient depleted Okhotsk Sea surface waters. J Exp Mar Biol Ecol 395:191–198
Acknowledgments
We wish to thank Associate Professor Y. Kyodo of the University of Tsukuba for fruitful discussions concerning the culture system. We are also indebted to staff members of the Shimoda Marine Research Center, University of Tsukuba, for their valuable assistance during the culture experiment. This work was financially supported by a grant from Global Environmental Research Fund of the Ministry of the Environment, Japan. This report is contribution no. 753 from the Shimoda Marine Research Center, University of Tsukuba.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hama, T., Kawashima, S., Shimotori, K. et al. Effect of ocean acidification on coastal phytoplankton composition and accompanying organic nitrogen production. J Oceanogr 68, 183–194 (2012). https://doi.org/10.1007/s10872-011-0084-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-011-0084-6