Abstract
Herein we report on the synthesis, single crystal X-ray structure and spectroscopic properties of [{Cu2(tidf)(µ-ppz)}(ClO4)2]n (tidf = a Robson-type macrocyclic ligand obtained by condensation of 2,6-diformyl-4-methylphenol and 1,3–diaminopropane). The two copper(II) centers in the dicopper(II,II) chromophore share identical coordination environment. The geometry around the copper(II) is square pyramidal and has [Cu2(tidf)]2+ units bridged by piperazine and forming a 1D–coordination polymer. The structure is also supported by non-classic hydrogen bonding such as C–H···Operchlorate. Electronic spectroscopy agrees with the C4v micro-symmetry around the metal center as ligand-field transitions are splitted when compared to the spectrum of the parent complex [Cu2(tidf)(ClO4)2(H2O)2].
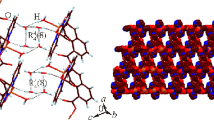
Graphical Abstract
The molecular structure and spectroscopic properties of [{Cu2(tidf)(µ-ppz)}(ClO4)2]n

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A considerable part of the coordination chemistry has been centered on the use of metal ions as template of cyclization reactions to produce macrocycle ligands [1–5]. Robson-type ligands, obtained from condensation of 2,6-diformyl-4-methylphenol and diamines, have been investigated over the years as their coordination compounds show interesting magnetic, redox and structural properties [6–20]. This category of macrocycle is appealing because it can bind two metal ions simultaneously allowing the preparation of homometallic and heterometallic compounds. In addition, there is always the possibility to occupy the axial positions of the related complexes with ambidentate ligands and obtain high nuclearity systems exhibiting unusual molecular architectures [21–23].
Earlier, we report three heteropolynuclear metal complexes of the Robson family. Results show a very interesting case of self-assembly of building molecules into extended structures in the solid state [7]. In this work, continuing our general interest in the synthesis of metal complexes of the tetraminodiphenolate macrocycle ligand (Scheme 1), we report the preparation and characterization of a new coordination polymer based on a dicopper macrocycle complex and piperazine as building blocks.
Experimental Section
Reagent grade chemicals were used in this work. [Cu2(tidf)(ClO4)2(H2O)2] was prepared as described elsewhere [24].
The macrocyclic ligand tidf was prepared by the condensation of 2,6-diformyl-4-methylphenol and 1,3-diaminopropane by a template reaction with magnesium acetate and magnesium nitrate according to the procedure of Nag and co-workers [25]. The magnesium complex [Mg2(tidf)](NO3)2·4H2O was used to prepare the binuclear compound [Cu2(tidf)(ClO4)2(H2O)2] through a transmetallation reaction as described by Mandal et al. [24]. Reaction with piperazine in a 1:1 molar ratio produced the polymeric complex [{Cu2(tidf)(µ-ppz)}(ClO4)2]n, which was characterized structurally and spectroscopically as described below.
Synthesis
[{Cu 2 (tidf)(µ-ppz)}(ClO 4 ) 2 ] n
Piperazine (56 mg, 0.65 mmol) was dissolved in 10 mL of distilled water and then 5 mL of isopropanol was added very slowly to form a new layer. After, a solution containing 0.5 g (0.65 mmol) of [Cu2(tidf)(ClO4)2(H2O)2] in 15 mL of acetonitrile was slowly poured to the bi-layered water/isopropanol mixture and the system was keep still for the diffusion of the solvents. Small dark-green crystals, suitable for X ray crystallography were then isolated, washed with methanol, acetonitrile, diethylether and dried under vacuum. The yield was 200 mg (38 %). C28H36Cl2Cu2N6O10: C, 41.28; H, 4.45; N, 10.32 %. Found: C, 40.82; H, 4.25; N, 10.07 %.
Physical Measurements
UV–visible spectra in the range 190–900 nm were obtained on a VARIAN Cary 100 spectrophotometer in the solid state by diffuse reflectance with a Labsphere® integration sphere. Infrared spectra were obtained with a FTS3500GX Bio-Rad Excalibur series spectrophotometer in the region 4,000–400 cm−1 in KBr pellets. Elemental analyses were performed on a Perkin–Elmer CHN 2400 analyzer.
Structural Determination and Refinement
A Bruker D8 VENTURE PHOTON diffractometer operated using graphite monochromator and Mo-Kα radiation (λ = 0.71073 Å) was used for the X-ray structure analyses. The crystal structure was solved by direct methods with SHELXS2013 [26]. The final structure was refined with SHELXL2013 [26] with anisotropic displacement parameters for all non-hydrogen atoms; hydrogen atoms were refined isotropically as riding atoms at their theoretical ideal positions [27]. Drawings were made with Olex2 [28]. More detailed information about the structure determinations is given in Table 1.
Results and Discussion
Structural Description
The complex [{Cu2(tidf)(µ-ppz)}(ClO4)2]n crystallizes in the monoclinic space group P21/c. Figure 1 shows the projection of the molecular structure where the copper(II) ions experiences a square pyramidal geometry. Each macrocyclic ligand (tidf) binds two metal centers in the equatorial plane through four imines and two phenolate groups, while the axial site is occupied by a piperazine ligand that bridges two units of [Cu2(tidf)]2+. The copper(II) ions are out of the equatorial plane, as indicated by the mean deviations from the least-square planes N1N2O1O1i (i = −x, −y + 1, −z−1) at 0.2040(12) Å. The shortest Cu–Cui distance is 3.1521(11) Å. The Cu-piperazine bond distance, Cu1–N3 at 2.312(3) Å, is significantly longer than the equatorial bond lengths (Cu1–N1 1.969(3) Å, Cu1–N2 1.987(3) Å and Cu1–O1 2.007(19) Å), which is a clear evidence of the tetragonal distortion expected for a d9 ion. Some main angles are N1-Cu1–N2 96.73(12)°, N1–Cu1–O1 92.10(10)°, O1–Cu1–O1i 75.75(9)°, N1–Cu1–N3 97.49(11)° and O1i–Cu1–N2 93.04(10)°. Other bond distances and angles are listed in Table 2.
Mandal and co-workers [24] reported the crystal structure of the dicopper complex [Cu2(tidf)(ClO4)2(H2O)2. It crystallizes in the monoclinic space group P21/a and the structure has two different molecules in the unit cell, one pseudooctahedral and the other square-pyramidal. Bond distances reported for the hexa-coordinated copper are Cu1–O(H2O) at 2.451 (9) Å, Cu1–O(ClO4) at 2.589 (10), Cu1–O(phenolate) at 1.981(6) Å and Cu1–N at 1.956(8). In the second molecule, the copper(II) is axially bound to a water molecule (Cu2–O(H2O) = 2.451(6) Å) and displaced by 0.083(6) Å from the planar equatorial donor atoms N2O2 toward H2O. The mode of coordination of Cu2 to the square plane N2O2 atoms is similar to Cu1 in the hexa-coordinated molecule. Typical equatorial bond lengths and angles are: Cu2–O = 1.970(6) Å, Cu2–N = 1.943(9) Å, cis O–Cu2–N = 93.9(3)o and tran O–Cu2–N 168.1(3)o. All equatorial bond lengths are shorter and the apical distances are longer than the ones found in our report, consistent with a stronger Jahn–Teller effect in the presence of weaker ligands, H2O and perchlorate ion when compared to piperazine.
More recently we described the crystal structure of the coordination polymer [{Cu2(tidf)(H2O)}2(μ-CN)2Fe(CN)4]·6H2O. In this case, the pentacoordinated copper(II) units [Cu2(tidf)(H2O)]2+ are linked by a hexacyanoferrate(II) ion through two cyano–bridges in a trans configuration and the unit cell revealed a complex supramolecular structure sustained by hydrogen-bond interactions between the metallo–complex and the crystallization water molecules [7].
An extended 1D architecture along the crystallographic axis b for complex [{Cu2(tidf)(µ-ppz)}(ClO4)2]n is shown in the packing, Fig. 2. The complex also expands in the ac crystallographic plane in a supramolecular tridimensional assembly through weak hydrogen bonds with the perchlorate ion, like C10–H10B···O12iv and C8-H8A···O13vi (iv = x, y−1, z; vi = x + 1, −y + 3/2, z + 1/2) at 3.274(6) Å and 3.331(6) Å for D…A distance. The N3 atom of piperazine is participating as donor in two hydrogen bonds with oxygen atoms of perchlorate. The corresponding geometrical parameters are given in Table 3.
Infrared Spectra
The infrared spectrum of [{Cu2(tidf)(µ-ppz)}(ClO4)2]n showed bands also observed in the related complex [Cu2(tidf)(ClO4)2(H2O)2], but at different wavenumbers. Main bands are at 1641 ν(C = N), 1,568 ν(C = C) and 1,326 ν(C–O) cm-1, characteristic of the tetraiminodiphenolate macrocycle. Piperazine exhibited the typical ν(N–H) mode at 3,284 cm−1, while bands at 561 and 524 cm−1 were accounted to the vibrational modes νCu–N and νCu–O, respectively. Values seen for the parent complex, [Cu2(tidf)(ClO4)2(H2O)2], for νCu–N and νCu–O were 594 and 519 cm−1 and consistent with the effect of the axial coordination of the piperazine to the copper(II) ion. Perchlorate ion presented typical bands at 1,095 and 622 cm−1.
UV–vis Spectra
Figure 3 shows the UV–visible spectrum of a solid sample of complex [{Cu2(tidf)(µ-ppz)}(ClO4)2]n (green line) along with the spectra of the related complex [Cu2(tidf)(ClO4)2(H2O)2]. The bands in the 200–400 nm range are mainly intra-ligand π(tidf) → π*(tidf) transitions, which are also observed in the spectrum of the free macrocyclic ligand. A metal-to-ligand charge transfer dπ(CuII) → pπ tidf transition was assigned to the band at 450 nm.
Complex [Cu2(tidf)(ClO4)2(H2O)2] shows a broad structure-less band centered at 605 nm, typical of copper(II) in a D4h ligand field symmetry and has contributions from three d–d transitions, z2 → x2−y2, xy → x2−y2 and (xz, yz) → x2−y2 [29]. This band splits in the spectrum of [{Cu2(tidf)(µ-ppz)}(ClO4)2]n (green line) at 540 and 680 nm, reflecting the different geometries around the copper(II) ions in both compounds. The lower energy component of the d–d transition for [{Cu2(tidf)(µ-ppz)}(ClO4)2]n can be explained using crystal-field arguments. Here, copper(II) is in a C4v symmetry and it is equivalent of an infinite elongation of one of the axial ligands, reducing the repulsions along the z direction. As a result, a new set of energies is established for the metal orbitals and the d–d bands are shifted, particularly the higher energy (xz, yz) → x2−y2 transition as represented in Scheme 2. The other two transitions z2 → x2−y2 and xy → x2−y2 are overlaid [7].
Conclusion
The coordination polymer [{Cu2(tidf)(µ-ppz)}(ClO4)2]n was prepared from the reaction between [Cu2(tidf)(ClO4)2(H2O)2] and piperazine (1:1 molar ratio) in a water/isopropanol/acetonitrile mixture. Structural analysis showed square pyramidal copper(II) ions covalently bridged by piperazine. Some intermolecular interactions by hydrogen bonding complete the molecular arrangement at the solid state. The electronic structure of the compound is sensitive to the geometry around the copper and the spectra can be correlated with the degree of tetragonal distortion.
Supporting Information Available
CCDC-1008584 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html [or from the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44(0)1223-336033; email: deposit@ccdc.cam.ac.uk].
References
Hakimi M, Moeini K, Mardani Z, Mohr F (2014) Polyhedron 70:92–100
Vaddypally S, Xu C, Zhao S, Fan Y, Schafmeister C, Zdilla MJ (2013) Inorg Chem 52:6457–6463
Kim E, Lee H (2013) Inorg Chim Acta 399:62–66
James L, Kose M, Metcalfe T, McKee V (2011) J Chem Crystal 41:577–581
Nunes FS, Murta PDL, da Cunha CJ (1999) J Coord Chem 47:251–267
Robson R, Pilkington NH (1970) Aust J Chem 23:2225–2236
Samulewski RB, Rocha JC, Stieler R, Lang ES, Evans DJ, Poneti G, Nascimento OR, Ribeiro RR, Nunes FS (2011) Polyhedron 30:1997–2006
Samulewski RB, Rocha JC, Fuganti O, Stieler R, Lang ES, Vaz MGF, Nunes FS (2010) J Mol Struct 984:354–358
Raimondi AR, Evans DJ, Nunes FS (2008) Spectrochim Acta A 70:651–654
Raimondi AR, Evans DJ, Drechsel SM, Hasegawa T, Nunes FS (2007) Spectrochim Acta A 67:145–149
Raimondi AC, Hasegawa T, Evans DJ (2005) Spectrochim. Acta A 61:1929–1932
Raimondi AC, Hitchcock PB, Leigh GJ, Nunes FS (2004) J Chem Crystal 34:83–87
Raimondi AC, Mangrich AS, Franco VS, Toma HE, Nunes FS (2004) Polyhedron 23:2069–2074
Raimondi AC, Hitchcock PB, Leigh GJ, Nunes FS (2002) J Chem Crystal 32:363–367
Thompson LK, Mandal SK, Tandon SS, Bridson JN, Park MK (1996) Inorg Chem 35:3117–3125
Gagné RR, Spiro CL, Smith TJ, Hamann CA, Thies WR, Shiemke AK (1981) J Am Chem Soc 103:4073–4081
Spiro CL, Lambert LL, Smith TJ, Duesler EN, Gagné RR, Hendrickson DN (1981) Inorg Chem 20:1229–1237
Gagné RR, Henling LM, Kistenmacher TJ (1980) Inorg Chem 19:1226–1231
Lambert SL, Hendrickson DN (1979) Inorg Chem 18:2683–2686
Okawa H, Kida S (1972) Bull Chem Soc Jap 45:1759–1764
Ishiruji FHO, Evans DJ, Benedito FL, Nunes FS (2008) Spectrochim Acta A 70:1029–1033
Atanasov M, Comba P, Hausberg S, Martin B (2009) Coord Chem Rev 253:2306–2314
Paraschiv C, Andruh M, Journaux Y, Zak Z, Kyritsakas N, Ricard L (2006) J Mater Chem 16:2660–2668
Mandal SK, Thompson LK, Newlands MJ, Gabe EJ (1989) Inorg Chem 28:3707–3713
Mohanta S, Nanda KK, Ghosh S, Mukherjee M, Heliwell M, Nag K (1996) J Chem Dalton Trans 22:4233–4238
Sheldrick GM (2008) Acta Cryst. A64:112–122
Johnson CK (1970b) Crystallographic Computing, Ahmed FR, Hall SR, Huber CP (eds) pp. 207–219. Copenhagen: Munksgaard
Dolomano OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement, analysis program. J Appl Cryst 42:339–341
Protasiewyck G, Nunes FS (2006) Spectrochim Acta A 65:549–552
Acknowledgments
F.S.N. and M.A.R. thank CNPq and CAPES for research fellowships. Diffractometer was supported by the CAPES – Pró-Equipamentos 024/2012 and visiting professor program A0099/2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Jesus, R.N., Ribeiro, M.A., Inoue, M.H. et al. Synthesis, Structural and Spectroscopic Characterization of a New Coordination Polymer Based on a Tetraiminodiphenolate Macrocycle and Piperazine: [{Cu2(tidf)(µ-ppz)}(ClO4)2]n . J Chem Crystallogr 44, 506–511 (2014). https://doi.org/10.1007/s10870-014-0541-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-014-0541-3