Abstract
The imidazolyl derived complex N,N′-butylenebis(imidazole):(oxalic acid)0.5 was prepared and structurally characterized by X-ray crystallography. The title compound crystallizes in the triclinic, space group P-1, with a = 4.4373(9) Å, b = 12.882(3) Å, c = 15.319(3) Å, α = 99.91(3)°, β = 94.53(3)°, γ = 98.72(3)°, V = 847.7(3) Å3, Z = 2. Two N,N′-butylenebis(imidazole) and two oxalic acid molecules form an annulus via intermolecular hydrogen bonds, with internal dimensions of about 7.1 × 11.1 Å. Neighboring annuluses were connected by N–H···O and C–H···O interactions to form 1D double chain structure. Adjacent double chains stacked just above each other along the a-axis direction, this arrangement of the double chains leads the extended supramolecular architecture to show a three-dimensional porous network.
Graphical Abstract
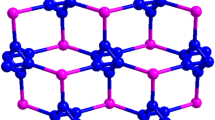
Two N,N′-butylenebis(imidazole) and two oxalic acid molecules form an annulus via intermolecular hydrogen bonds, with internal dimensions of about 7.1 × 11.1 Å. Neighboring annuluses were connected by N–H···O and C–H···O interactions to form 1D double chain structure. Adjacent double chains stacked just above each other along the a-axis, this arrangement of the double chains leads the extended supramolecular architecture to show a three-dimensional porous network.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, hydrogen bonding has been widely developed in the area of crystal engineering, supramolecular chemistry, material science, and biological recognition [1–6]. Especially, the application of intermolecular hydrogen bonds is a well-known and efficient tool in the field of organic crystal designing because of its strength and directional properties [7, 8].
Through hydrogen bonds we can form organic salts. In pharmaceuticals, salt formation is often used in order to modify the properties of the compounds [9], such as increasing or decreasing solubility, improving stability and reducing hygroscopicity of a drug product. The carboxylic acid contains the important hydrogen bonding functional group for crystal engineering [10]. Carboxylic acids aggregate in the solid state as dimer, catemer, and bridged motifs [11–13]. Thus, it is interesting to carry out premeditated crystal design not only with carboxylic acids [11–13], but, as recent trends indicate, by exploiting the robust and directionary recognition of carboxylic acids with N-heterocyclic moieties [14, 15]. In this context, it is also conceivable that great efforts have been directed to the development of organic molecular crystals containing a variety of imidazole architectures [16–21]. Among these supramolecular architectures, however, only a very few reports describing the crystals composed of hydrogen-bonding donors and diimidazole or triimidazole compounds [22–26].
For we are interested in the crystal engineering assembling through weak interactions [27, 28], here in we report the synthesis and crystal structure of one organic salt assembled through hydrogen bonding interactions. In this study, we get one organic complex composed of oxalic acid and N,N′-butylenebis(imidazole) (1) (Scheme 1).
Experimental Section
Materials and Methods
All reagents were commercially available and used as received. N,N′-butylenebis(imidazole) was prepared as described previously [29]. The C, H, and N microanalysis were carried out with a Carlo Erba 1106 elemental analyzer. The FT-IR spectra were recorded from KBr pellets in range 4000–400 cm−1 on a Mattson Alpha-Centauri spectrometer.
Preparation of N,N′-Butylenebis(imidazole):(oxalic acid)0.5 (1)
N,N′-butylenebis(imidazole) (38 mg, 0.2 mmol) was dissolved in 1 mL of methanol. To this solution was added oxalic acid (12.7 mg, 0.1 mmol) in 3 mL methanol. Colorless prisms were afforded after 1 week of slow evaporation of the solvent, yield 14.1 mg, 60%. Elemental analysis performed on crystals exposed to the atmosphere: Calc. for C11H15N4O2: C 56.11, H 6.37, N 23.80. Found: C 56.01, H 6.32, N 23.83. Infrared spectrum (KBr disc, cm−1): 3431s, 3117s, 1642s, 1597s, 1568m, 1513s, 1401s, 1288s, 1238s, 1099s, 1035w, 952m, 856m, 785s, 713s, 657m, 615m, 545m, 478m.
X-ray Crystallography
Suitable crystals were mounted on a glass fiber on a Bruker SMART 1000 CCD diffractometer operating at 50 kV and 40 mA using Mo Kα radiation (0.71073 Å). Data collection and reduction were performed using the SMART and SAINT software [30, 31]. The structures were solved by direct methods, and the non-hydrogen atoms were subjected to anisotropic refinement by full-matrix least squares on F 2 using SHELXTL package [32]. Hydrogen atom positions for the structure were located in a difference map and refined independently. Further details of the structural analysis are summarized in Table 1. Selected bond lengths and angles for complex 1 are listed in Table 2, the relevant hydrogen bond parameters are provided in Table 3.
Results and Discussion
Syntheses and General Characterization
N,N′-butylenebis(imidazole) has good solubility in common organic solvents, such as CH3OH, C2H5OH, CH3CN, CHCl3, and CH2Cl2. The crystals were grown by slow evaporation of the corresponding solution at room temperature. Crystallization of N,N′-butylenebis(imidazole) and oxalic acid was carried out in a 2:1 ratio. The complex is not hygroscopicity, it crystallized with no solvent molecules accompanied. The molecular structure and its atom labelling scheme for the structure are illustrated in Fig. 1.
In the preparation of 1, the acid is mixed directly with the base in methanol solution, which was allowed to evaporate at ambient conditions to give the final crystalline products. The elemental analysis data for the compound is in good agreement with its composition. The infrared spectra of 1 is consistent with its chemical formula determined by elemental analysis and further confirmed by X-ray diffraction analysis. The very strong and broad feature at 3431 cm−1 in the spectra of the compound arises from N–H stretching frequencies. Imidazolyl ring stretching and bending are attributed to the medium intensity bands in the regions of 1500–1630 cm−1 and 600–750 cm−1, respectively. The intense peak at 1642 cm−1 was derived from the existence of the C=O stretches, and the band at 1288 cm−1 exhibited the presence of the C–O stretches of the dicarboxylate. The absence of bands at ca. 2500 cm−1 and 1900 cm−1 in compound 1, was interpreted as a lack of co-crystal formation [33].
IR spectroscopy has also proven to be useful for the recognition of proton transfer compounds [34, 35]. The most distinct feature in the IR spectrum of proton transfer compounds is the presence of strong asymmetrical and symmetrical carboxylate stretching frequencies at 1550–1610 and 1300–1420 cm−1, respectively [36].
X-ray Structure of 1
The asymmetric unit of 1 consists of one cation of N,N′-butylenebis(imidazole) and half a dianion of oxalic acid, which is shown in Fig. 1. The N atoms of N,N′-butylenebis(imidazole) are protonated by oxalic acids, furnishing a pair of ionic N–H···O hydrogen bond. In the compound, there are two pairs of ion pair with no included solvent molecules, which is well agreement with the micro-analysis results.
The N(4)–O(1)#3(Symmetry code 3: −x, −y + 1, −z + 1) distance in these contacts is 2.671(9) Å, which is considerably less than the sum of the van der Waals radii for N and O (3.07 Å) [37]. And in three of the four ionic N–H···O hydrogen bonds in compound 1 there are shorter N–O distances than those formed between the perchlorate ion and the NH+ of N,N′-butylenebis(imidazolium) [38]. So in the solid state, there is consistently ionic hydrogen bond formed between the imidazolium NH+ and the oxalate ion, which is to be expected [39].
Every oxygen atom of the oxalate forms two ionic N–H···O hydrogen bonds with two different imidazolium cations. Each imidazolium NH+ forms double hydrogen bonds with two oxygen atoms of the oxalate ions. It was found that protonation of the imidazole ring nitrogen atoms causes no significant change in conformation of the title compound in comparison to the corresponding neutral molecule N,N′-butylenebis(imidazole) [40]. The imidazole rings in the title compound have trans-(ap) position in respect to C8–C9 bond (angle C7–C8–C9–C10, 174°), which is different from the corresponding salt N,N′-butylenebis(imidazolium) dihydrochloride [40], this is due to the fact that in the latter compound the hydrogen bond strength is more strong than that in the compound reported here. But the conformation of the salt 1 is similar to the corresponding salts N,N′-butylenebis(imidazolium) bis(perchlorate), N,N′-butylenebis(imidazolium) bis(triiodide), and N,N′-butylenebis(imidazolium) dinitrate in which the torsion angles of the butane spacer are all ca. 180° [38, 41, 42].
The r.m.s deviations of the imidazole ring atoms in the title compound from the mean planes of the rings containing pairs of atoms N(1) and N(2), and N3 and N4 are 0.0145 Å, and 0.0094 Å, respectively. The dihedral angle between two imidazole rings in the same cation is 3.5° indicating the coplanarity of both rings. The two imidazolium rings form dihedral angles of 88.4° and 91.8° with the plane defined by the C atoms of the –(CH2)4– aliphatic linker, respectively, which are different from our previously published result [42]. The anions form dihedral angles of 174.7°, and 175.4° respectively with both imidazole rings in the cation, so the anions are coplanar with the cationic rings. While the planes defined by the C atoms of the –(CH2)4– aliphatic linker are almost perpendicular with the anions (90.2°).
In the absence of hydrogen bonding and other electronic perturbations, the C–O bond lengths should be equal because of resonance. The formation of single or multiple hydrogen bonds at one oxygen atom should cause the associated C–O bond to lengthen. It is clear that the difference in bond lengths of C–O within the carboxylic acid group (0.11 Å) [16] is much greater than the one found in the oxalate anions (0.025 Å). Also the average distance for C–O (1.256 Å) in oxalate is less than the single bond C–O (1.306 Å) and greater than the double bond C=O (1.196 Å) in carboxylic acid group of oxalic acid [16]. This supports our assignment of the oxalate dianions.
In the structure, there are chains of imidazolium moieties linked via N–H···O hydrogen bonds in which the two carboxylate O atoms form three hydrogen bonds in tridentate bridging fashion with the imidazolium cation. One dimensional double chain is formed through imidazolium moieties forming hydrogen bonds to the di-ionic carboxylate groups of oxalate, which is shown in Fig. 2. In the same double chain, C–H···O contact (C4–H4···O2 with C4–O2 distance of 3.091 Å) also accompanies the N+–H···O− hydrogen bonds, which utilizes H4 on imidazole ring and carboxylate oxygen atom O2 on the CO2 −. Here the C–O distance of the C–H···O interaction is shorter than those presented in N,N′-butylenebis(imidazolium) bis(perchlorate) (the corresponding C–O distance is 3.228 Å) [38]. The existence of weak C–H···O hydrogen bonds in the superamolecule is propitious to stabilize the one-dimensional double-chain structure.
An alternative reading of this structure is possible when we emphasize the relative arrangement of the cations and the anions, i.e., two N,N′-butylenebis(imidazolium) cations and two oxalate anions form an annulus via intermolecular hydrogen bonds of N–H···O and C–H···O, with internal dimensions of about 7.1 × 11.1 Å. Neighboring annuluses were connected by N–H···O and C–H···O interactions to form 1D double chain structure. Adjacent double chains stacked just above each other along the a-axis, this arrangement of the double chains leads the extended supramolecular architecture to show a three-dimensional porous network. Furthermore, as far as we know, the samples describing self-assembled porous structures in this area are relatively rare so far.
Supplementary Material
Crystallographic data for the structural analysis has been deposited with the Cambridge Crystallographic data center, CCDC No. 681875 for 1. Copies of this information may be obtained free of charge from the +44(1223)336-033 or Email: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk.
References
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer-Verlag, Berlin
Aakeröy CB, Beatty AM (2001) Aust J Chem 54:409
Burrows AD (2004) Struct Bond 108:55
Braga D, Maini L, Polito M, Grepioni F (2004) Struct Bond 111:1
Etter MC (1990) Acc Chem Res 23:120
Holman KT, Pivovar AM, Swift JA, Ward MD (2001) Acc Chem Res 34:107
Aakeröy CB, Seddon KR (1993) Chem Soc Rev 22:397
Steed JW, Atwood JL (2000) Supramolecular chemistry. Wiley, Chichester
Gould PJ (1986) Int J Pharm 33:201
Desiraju GR (1989) Crystal engineering. The design of organic solids. Elsevier, Amsterdam
Leiserowitz L (1976) Acta Crystallogr B32:775
Kolotuchin SV, Fenlon EE, Wilson SR, Loweth CJ, Zimmerman SC (1995) Angew Chem Int Ed Engl 34:2654
Kuduva SS, Craig DC, Nangia A, Desiraju GR (1999) J Am Chem Soc 121:1936
Highfill ML, Chandrasekaran A, Lynch DE, Hamilton DG (2002) Cryst Growth Des 2:15
Vishweshwar P, Nangia A, Lynch VM (2002) J Org Chem 67:556
MacDonald JC, Dorrestein PC, Pilley MM (2001) Cryst Growth Des 1:29
Trivedi DR, Ballabh A, Dastidar P (2003) CrystEngCommun 5:358
Overgaard J, Schiøtt B, Larsen FK, Schultz AJ, MacDonald JC, Iversen BB (1999) Angew Chem Int Ed 38:1239
Moos WH, Humblet CC, Sircar I, Rithner C, Weishaar RE, Bristol JA, McPhail AT (1987) J Med Chem 30:1963
Karle IL, Ranganathan D, Haridas V (1996) J Am Chem Soc 118:7128
Akhriff Y, ServerCarrio J, Garcia-Lozano J, Folgado JV, Sancho A, Escriva E, Vitoria P, Soto L (2006) Cryst Growth Des 6:1124
Aakeröy CB, Salmon DJ, Leonard B, Urbina JF (2005) Cryst Growth Des 5:865
Aakeröy CB, Salmon DJ, Smith MM, Desper J (2006) Cryst Growth Des 6:1033
Cheruzel LE, Mashuta MS, Buchanan RM (2005) Chem Commun 2223
Roey PV, Bullion KA, Osawa Y, Bowman RM, Braun DG (1991) Acta Crystallogr C47:1015
Ma BQ, Coppens P (2004) Cryst Growth Des 4:1377
Jin SW, Chen WZ (2007) Chin J Struct Chem 3:287
Jin SW, Chen WZ, Wang DQ (2007) Chin J Inorg Chem 2:270
Schutze VW, Schubert H (1959) J Prakt Chem 8:307
Blessing RH (1995) Acta Crystallogr A51:33
Sheldrick GM (1996) SADABS “siemens area detector absorption correction”. University of Göttingen, Göttingen, Germany
SHELXTL-PC, version 5.03; Siemens Analytical Instruments. Madison, WI
Aakeröy CB, Desper J, Fasulo ME (2006) CrystEngCommun 8:586
Lynch DE, Thomas LC, Smith G, Byriel KA, Kennard CHL (1998) Aust J Chem 51:867
Smith G, White JM (2001) Aust J Chem 54:97
Williams DH, Fleming I (1995) Spectroscopic methods in organic chemistry, 5th edn. McGraw-Hill, London
Bondi A (1964) J Phys Chem 68:441
Yao JC, Zhang L, Qu L, Liu C, Wang W (2007) Acta Cryst E63:o3029
Felloni M, Blake AJ, Hubberstey P, Wilson C, Schröder M (2002) CrystEngComm 4:483
Królikowska M, Garbarczyk J (2005) Z Kristallogr NCS 220:103
Yao JC, Shi AE, Yu YH, Hou GF, Gao JS (2008) Acta Cryst E64:o1561
Jin SW, Wang DQ (2008) Acta Cryst E64:o169
Acknowledgement
The authors thank the Zhejiang Forestry University Science Foundation for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, S., Wang, D. One Porous Supramolecular Complex Assembled from Ionic and Neutral Hydrogen-bonds of N–H···O and C–H···O. J Chem Crystallogr 40, 914–918 (2010). https://doi.org/10.1007/s10870-010-9762-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9762-2