Abstract
Shikonin, a natural small agent, has shown inhibitory effect in many kinds of cells, which increases intracellular reactive oxygen species (ROS) level and causes mitochondrial injury. In this study, shikonin showed good inhibitory effect on nasopharyngeal carcinoma CNE-2Z cells in vivo and vitro. The results presented here revealed that ROS levels increased markly after shikonin treated. The electron microscopy displays the change in ultrastructure of CNE-2Z cells after treatment for shikonin, which indicated that shikonin induced necroptosis. Shikonin-induced cell death was inhibited by a necroptosis inhibitor, necrostatin-1 (Nec-1), while the activity was unaffected by the caspase inhibitor z-VAD-fmk. Furthermore, we have demonstrated that the activation of receptor-interacting kinase (RIP) led to necroptosis. Meanwhile, shikonin also significantly inhibited tumor growth in a CNE-2Z xenograft mouse model. Taken together, shikonin induced CNE-2Z cells death by producing ROS as a necroptosis inducer. It could serve as a new therapeutic agent for treating CNE-2Z cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor which has high incidence in the southeastern region of China (Hildesheim 1993). Although most patients with advanced NPC respond well to chemotherapy, recurrence of distant metastases is the major cause of treatment failure and has a poor prognosis (Chua et al. 2016; Peng et al. 2016). Therefore, we need a more effective therapy for NPC.
At present, there have been several moods of cell death including necrosis, apoptosis and autophagy (Majno and Joris 1995; Zhang et al. 2016). However, increasingly studies explain necroptosis as a brand new type of cell death (Koo et al. 2015; Linkermann 2014), which is different from apoptosis and necrosis. Necroptosis is a form of regulated necrosis that depends on the kinase of receptor-interacting kinase (RIP) (Humphries et al. 2015; Zhang and Liu 2013). Upon the induction of necroptosis, the activated RIP1 interacts motifs to form a protein complex named necrosome, in which RIP3 is activated by RIP1 or via auto-phosphorylation (Li et al. 2012). Necrostain-1 (Nec-1) is a highly specific inhibitor of kinase RIP1, necroptosis could be specific inhibited by Nec-1(Christofferson et al. 2012). On the contrary, in the presence of caspase inhibitor z-VAD-fmk, the apoptosis pathway was blocked and necroptosis became dominant (Vandenabeele et al. 2006). Shikonin is a compound purified from the Chinese medicinal herb Lithospermum erythrorhizon (Gong and Li 2011; Lu et al. 2011), was directly or indirectly to inhibit or modulate cellular targets associated with cancer (Liang et al. 2013). Shikonin has been reported to induce cell death in various tumor cell lines due to its wide spectrum of mechanisms of actions, such as multiple myeloma (Wada et al. 2015), breast cancer (Shahsavari et al. 2015; Wei et al. 2016), medullary thyroid carcinoma (Tang et al. 2016). Therefore shikonin seems to be a promising anti-cancer agent. However, there are no researches showing the efficacy of shikonin in NPC CNE-2Z cells. In recent reports, shikonin increases intracellular reactive oxygen species (ROS) levels and causes mitochondrial injury (Chang et al. 2010; Wiench et al. 2012). Mitochondria are indispensable part in ATP generation, cellular metabolism and production of ROS. Shikonin could induce accumulation of ROS by deregulating the mitochondrial membrane potential.
During our research, the efficacy of shikonin in CNE-2Z cells in vivo and in vitro were evaluated. And we initially explored the mechanisms underlying shikonin regulation of cell death in CNE-2Z cells. All of the results of shikonin on the CNE-2Z cell line are worthy of study.
Materials and methods
Regents
Roswell Park Memorial Institute (RPMI)-1640 (Gibco), shikonin (Sigma), 5-diphenyltetrazolium bromide (MTT), Necrostatin-1 (Nec-1) and N-acetyl-L-cysteine (NAC) were purchased from Sigma-Aldrich (St. Louis, MO, USA), z-VAD-fmk was got from Calbiochem. 2′, 7′-Dichlorofluorescin diacetate (DCFH-DA), 4′, 6-diamidino-2-phenylindole (DAPI) and propidium iodide (PI) assay kits were purchased from Beyotime Institute of Biotechnology (Wuhan, China). Dimethyl sulfoxide (DMSO) was from Biosharp (Hefei, China). Anti-RIP1 and anti-RIP3 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), anti-β-actin (BioSharp, Hefei, China).
Cell lines and cell culture
CNE-2Z cell line was obtained from the Shanghai Cell Bank (Shanghai, China). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum, penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37 °C in a 5% CO2 humidified atmosphere.
Cell proliferation assay
Viability of the cells was determined by MTT assay. The CNE-2Z cells were seeded at an aliquot of 7000 cells in each well of 96-well plate and cultured for 24 h. Then cells were stimulated with different concentrations of shikonin (0.4, 0.8, 1.6, 3.2, 6.4 and 12.8 μM) for 24, 48 and 72 h. After that, 15 μl MTT was added into each well to incubate for 4 h at 37 °C. At the end of the experiment, the supernatant was replaced with 150 μl of DMSO. 30 min later, absorbance was measured at a wavelength of 490 nm with a plate reader.
Colony formation assays
The cells were seeded in 6-well plate at a density of 1 × 104 per well for 24 h. The cells were exposed to various concentrations of shikonin (Control, 0.16, 0.32, 0.64 μM) for 5 days, washed with phosphate-buffered saline (PBS) twice, fixed with paraformaldehyde setting for 10 min at −20 °C and discarded the supernatant. Following stained with crystal violet and then washed with double-distilled water, then followed up with taking photos of them.
PI staining
The CNE-2Z cells (2 × 105 cells/ well) were allowed to grow in each well of 12-well plate for 24 h. Following treatment with shikonin (3.2, 6.4 and 12.8 μM) for 24 h and then stained with 600 μl propidium iodide (PI) /well for 2 h and analyzed by flow cytometry (BD Biosciences, State of New Jersey, U.S.A).
DAPI staining
This assay aimed to demonstrate the effect of shikonin on CNE-2Z cell death. We seeded 4 × 103 cells in each well of 6-well plate and treatment with shikonin for 24 h, and then fixed with paraformaldehyde setting for 10 min, washed three times with PBS and then added DAPI solution (2 μg/ ml) in each well for 5 min at 4 °C away from light. In the end, the cells in the plate washed with PBS and observed the cell nuclei under a laser confocal scanning microscope.
Detection of intracellular reactive oxygen species
DCFH-DA was used for the assessment of intracellular ROS formation in cultured CNE-2Z cells. Cells were plated at 2 × 105 cells/well in a 6-well plate for 24 h and treated with different concentrations of shikonin for 4 h. Dilute the DCFH-DA with serum-free medium to 10 μM, the supernatant was discarded and then added the DCFH-DA to each well. After incubation at 37 °C for 20 min, the substrate solution was removed and cells were washed with serum-free medium for three times. A laser confocal scanning microscope was used to observe the ROS levels.
Evaluation of cell death form by electron microscopy
Cultured cells were collected and fixed with 3% glutaraldehyde and 2% paraformaldehyde in 0.1 M PBS (pH 7.4) at 4 °C. Next, the CNE-2Z cells were post-fixed with 1% osmium tetroxide, and then treated with 3% aqueous uranyl acetone, before being dehydrated with a graded series of ethanol and acetone and embedded in Araldite. Using a Reichert ultramicrotome (Leica, Wetzlar, Germany) to cut the sections, post-stained with 0.3% lead citrate, and examined by TEM (Olympus JEOL, Peabody, MA, USA).
Western blot analysis
Cells were directly lysed in radioimmune precipitation buffer for 30 min on ice, the lysates were centrifuged at 12,000×g for 30 min at 4 °C. Proteins in the supernatant were subjected to SDS-PAGE and then blotted onto polyvinyl difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). After the membranes were blocked with 5% skim milk in PBS with 0.1% Tween20 for 4 h, the expression of various proteins was detected using primary and secondary antibodies. The membranes were imaged with gel imaging equipment (Bio-Rad, USA).
In vivo antitumor efficacy of shikonin
Four- to six-week-old female nude mice were purchased from the animal experimental center of Beijing vitalriver. CNE-2Z cells (3 × 106 cells per animal) were subaxillary injection into the nude mice. The mice were assigned to the control or experimental groups by random. Mice with tumors were intraperitoneally administrated shikonin (0.5 mg/kg, 1.0 mg/kg) and DDP (3.0 mg/kg) treatment every three days for 21 days. Tumor weight was measured before every injection. At the end of the experiment, tumors were removed and stored in 4% formalin solution, cut into small pieces that were stained with hematoxylin and eosin (H&E).
Statistical analysis
Data are showed as the mean ± SE. Statistical analyses were carried out using one-way analysis of variance. A p value < 0.05 was taken as the level of significance.
Results
Shikonin inhibited CNE-2Z cells proliferation
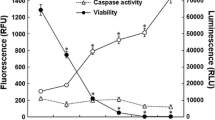
We used MTT assay to examine the effect of shikonin on cell viability of CNE-2Z cells. As shown in Fig. 1, shikonin obviously inhibited CNE-2Z cells proliferation in a time- and dose-dependent manner. PI staining was used to demonstrate the cell death and DAPI staining was aimed to detect death cells. The result of PI staining showed that the rate of cell death induced by shikonin was in a concentration-dependent manner (Fig. 1c). Shikonin-treated cells showed increasing number of death cell, and the cell nuclei exhibited condensed and fragmented nuclei when compared with the control cells (Fig. 1d).
Shikonin inhibited CNE-2Z cells proliferation. a CNE-2Z cells were treated with concentrations of shikonin for 24, 48 or 72 h. Cell viability was assessed by MTT assay. b Inhibition of colony formation in CNE-2Z cells by shikonin. CNE-2Z cells were treated with 0 (a), 0.16 (b), 0.32 (c), 0.64 (d) μM shikonin for 5 days. c Cells were treated with shikonin for 24 h and the morphology was examined by light microscopy. And flow cytometric analysis of cell death after treatment with shikonin for 24 h using PI staining. d CNE-2Z cells were treated with 0, 3.2, 6.4, 12.8 μM shikonin before being stained by DAPI (showing nucleus). The first row was the morphological change in the bright field. The white arrow denotes the nuclear fragmentation
Shikonin induced necroptosis in CNE-2Z cells
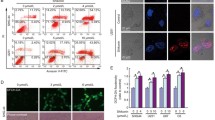
In order to research the mode of cell death induced by shikonin, we observed cells under an electron microscope. We could see that shikonin-treated CNE-2Z cells showed typical nuclear fragmentation, loss of plasma membrane integrity, and organelle (especially mitochondrial) swelling under the electron microscopy, which suggested necroptosis (Fig. 2a). Similarly, we treated cells with shikonin combined with either Nec-1 or z-VAD-fmk by MTT assay and PI staining. Through Fig. 2b, c we could see, cell death in CNE-2Z were significantly improved in the presence of Nec-1. RIP1 and RIP3 are the key proteins of necroptosis(Humphries et al. 2015), the results indicated that shikonin up-regulated the expression of RIP1 and RIP3 in a dose-dependent (Fig. 2d). Compared with using shikonin along, RIP1 and RIP3 proteins were decreased when combined with Nec-1 (Fig. 2e). Taken together, shikonin induces necroptosis in CNE-2Z cancer cells.
Shikonin induced necroptosis in CNE-2Z cells. a The mode of cell death induced by shikonin was observed by Electron microscopy. Scale bar, 500 nm. b CNE-2Z cells were treated with shikonin alone or combined with Nec-1 (20 μM), z-VAD-fmk (20 μM) for 24 h, and then the cell viability was detected. c CNE-2Z cells were treated with shikonin (6.4 μM), Nec-1 (20 μM), or shikonin + Nec-1 for 24 h before being analyzed using flow cytometry. Results are expressed as a percentage of control levels. d CNE-2Z cells treated with shikonin (0, 3.2, 6.4, 12.8 μM), RIP1 and RIP3 expression were examined by western blotting. e Cells were treated as in c, and examined RIP1 and RIP3 expression
Reactive oxygen species (ROS) production is involved in shikonin-induced necroptosis
After treatment with shikonin, we determined to examine intracellular ROS by the DCFH-DA probe. Observation by using fluorescence microscopy proved that the red fluorescence detected by DCFH-DA was obviously brighter in CNE-2Z cells. Following treatment of shikonin, ROS levels had a significant increase in a dose-dependent manner when compared with that of control cells (As shown in Fig. 3a). Clearly, the fluorescence intensity of the cells pre-treated with the antioxidant NAC (5 mM) was weaken than that of the cells treated with shikonin alone. Furthermore, the viability of the CNE-2Z cells treated with 3.2, 6.4, 12.8 μM shikonin was decreased significantly, and it was blocked by co-treated with NAC (Fig. 3b). These results indicated that shikonin induced overproduction of ROS. Considering that the increase of intracellular ROS is the result of disrupted balance between its production and clearance, we next detected shikonin-induced changes in mitochondrial superoxide. As revealed by fluorescence microscopy, the red fluorescence detected by Mitosox red was stronger in CNE-2Z cells treated with shikonin than that in the control group (Fig. 3c). Meanwhile, statistical analysis of the fluorescence intensity showed that shikonin induced increase of mitochondrial superoxide, but pre-treatment with the inhibitor Nec-1 markedly prevented the increase of them (Fig. 3d). In conclusion, shikonin induced increase of intracellular ROS during the necroptosis occurred.
CNE-2Z cells generated ROS after treatment with shikonin. a CNE-2Z cells were treated with 6.4 and 12.8 μM shikonin, with or without 5 mM NAC for 6 h. Cells were photographed using a fluorescence microscopy. b Increasing concentrations of shikonin were added to CNE-2Z cells after pre-treatment with 5 mM NAC for 1 h, and the cell viability was measured by MTT assay.* P < 0.05. c The representative fluorescence microscopic images of the CNE-2Z cells which were incubated with Mitosox red after treating with shikonin. d Statistical analysis of the red fluorescence density detected by Mitosox red
Effect of shikonin on tumor growth
To evaluate the antitumor effect of shikonin in vivo, CNE-2Z cells were xenografted into nude mice. The tumor-bearing mice were injected with shikonin (0.5 mg/kg/3d, 1.0 mg/kg/3d), and the negative control group were injected with PBS while the positive control group were treated with DDP (3.0 mg/kg/3d) for 21 days in all. We observed that shikonin reduced the growth to some extent, and the higher shikonin was more effective (Fig. 4a). Furthermore, shikonin-treated groups exhibited a significant decrease in the weight of nude mouse (Fig. 4b). The weight of tumors in shikonin treated group was lighter compared with negative control group significantly (Fig. 4c). H&E staining of the tumors demonstrate that the degree of tumor necrosis in shikonin group was higher compared with control group (Fig. 4d). All of the results told us that shikonin had significantly strong antitumor effects in vivo.
Discussion
It was recently showed that shikonin has a strong cytotoxic effect on a wide variety of cancer cell lines (Wiench et al. 2012). Nasopharyngeal carcinoma (NPC) is a special kind of malignant head and neck cancer with high incidence in Southeast Asia and Southern China (Ahmad and Ansari 2011). Therefore, in our study, shikonin presented potent antitumor activity for NPC CNE-2Z cells in vitro and in vivo.
Necroptosis is a mode of cell death different from apoptosis, which becomes the focus of morphologic research in recent years (Vanden Berghe et al. 2015). In the recent study, the pattern of cell death induced by shikonin was associated with two programmed cell death pathways, apoptosis and necroptosis (Shahsavari et al. 2015). So, in our research on CNE-2Z cells, we used Nec-1, an inhibitor of necroptosis, and z-VAD-fmk, an inhibitor of apoptosis, for the purpose of demonstrating the mode of cell death induced by shikonin. Respectively, shikonin induced cell death in CNE-2Z was obviously protected by Nec-1. And there was no significant change in the presence of z-VAD-fmk. These results were confirmed by electron microscopy. Although shikonin has induced both routs of cell death, indeed it is a potent necroptotic inducer (Shahsavari et al. 2015). RIP1 and RIP3 are regarded as crucial modulators of necroptosis (Shahsavari et al. 2016). As expect, we found that the protein levels of RIP1 and RIP3 were observably increased in CNE-2Z cell line after treatment with shikonin in a concentration dependent manner. These results indicated that cell death in CNE-2Z cell line induced by shikonin was via activation of RIP1 and RIP3.
Recent studies have shown that shikonin was accumulated in ROS level, shikonin is able to generate plenty of intracellular ROS (Huang et al. 2013; Wiench et al. 2012). While ROS production is not necessary in all cases of necroptosis, increasing mitochondrial ROS levels could serve as a second transmitter in the cell death signaling pathways (Yu et al. 2015). To evaluate if antitumor effects delivered by shikonin was associated with ROS, the ROS level was detected and the combination of shikonin and NAC was used. The ROS level in CNE-2Z cells induced by shikonin was not only dependent on shikonin concentration, but also reduced by adding ROS scavengers NAC clearly. Efficiency of shikonin on tumor growth was in accordance with the efficacy in cellular level.
Taken together, during our experiment, we draw a conclusion that shikonin could induce necroptosis and destroy the mitochondria through activating RIP1 and RIP3. Shikonin shows potential to be a new therapeutic agent for treating CNE-2Z cells. Further, in-depth testing is required to determine the efficacy.
Conclusion
We showed for the first time that shikonin induces necroptosis in NPC CNE-2Z cells occur through activation of reactive oxygen species (ROS). As an inducer of necroptosis in CNE-2Z cells, it up-regulated the expressions of RIP1 and RIP3, Nec-1 could block this effect. Since necroptosis has not been considered as a therapeutic strategy in treating NPC, this study might confirm it serve as a new modality leading to better control of CNE-2Z cells. In vivo experiments suggested shikonin slowed tumor growth in mice. These results support that shikonin may be a promising agents in treating NPC.
Abbreviations
- ROS:
-
reactive oxygen species
- Nec-1:
-
necrostatin-1
- RIP:
-
receptor-interacting kinase
- NPC:
-
nasopharyngeal carcinoma
- (RPMI)-1640:
-
Roswell Park Memorial Institute-1640
- MTT:
-
5-diphenyltetrazolium bromide
- NAC:
-
N-acetyl-L-cysteine
- DCFH-DA:
-
2′, 7′-Dichlorofluorescin diacetate
- DAPI:
-
4′, 6-diamidino-2-phenylindole
- PI:
-
propidium iodide
- DMSO:
-
Dimethyl sulfoxide
- PBS:
-
phosphate-buffered saline
- PVDF:
-
polyvinyl difluoride
- H&E:
-
hematoxylin and eosin
References
Ahmad S, Ansari AA (2011) Therapeutic roles of heparin anticoagulants in cancer and related disorders. Medicinal chemistry 7:504–517
Chang IC, Huang YJ, Chiang TI, Yeh CW, Hsu LS (2010) Shikonin induces apoptosis through reactive oxygen species/extracellular signal-regulated kinase pathway in osteosarcoma cells. Biological & pharmaceutical bulletin 33:816–824
Christofferson DE et al. (2012) A novel role for RIP1 kinase in mediating TNFalpha production cell death & disease 3:e320 doi:10.1038/cddis.2012.64
Chua ML, Wee JT, Hui EP (2016) Chan AT. Nasopharyngeal carcinoma Lancet 387:1012–1024. doi:10.1016/S0140-6736(15)00055-0
Gong K, Li W (2011) Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: a potential new treatment for hepatocellular carcinoma free radical biology & medicine 51:2259-2271 doi:10.1016/j.freeradbiomed.2011.09.018
Hildesheim A (1993) Levine PH. Etiology of nasopharyngeal carcinoma: a review Epidemiologic reviews 15:466–485
Huang C, Luo Y, Zhao J, Yang F, Zhao H, Fan W, Ge P (2013) Shikonin kills glioma cells through necroptosis mediated by RIP-1 PloS one 8:e66326 doi:10.1371/journal.pone.0066326
Humphries F, Yang S, Wang B (2015) Moynagh PN. RIP kinases: key decision makers in cell death and innate immunity Cell death and differentiation 22:225–236. doi:10.1038/cdd.2014.126
Koo MJ, Rooney KT, Choi ME, Ryter SW, Choi AM (2015) Moon JS. Impaired oxidative phosphorylation regulates necroptosis in human lung epithelial cells Biochemical and biophysical research communications 464:875–880. doi:10.1016/j.bbrc.2015.07.054
Li J et al. (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis cell 150:339-350 doi:10.1016/j.cell.2012.06.019
Liang D et al. (2013) Shikonin exerts anti-inflammatory effects in a murine model of lipopolysaccharide-induced acute lung injury by inhibiting the nuclear factor-kappaB signaling pathway International immunopharmacology 16:475-480 doi:10.1016/j.intimp.2013.04.020
Linkermann A (2014) Green DR. Necroptosis The New England journal of medicine 370:455–465. doi:10.1056/NEJMra1310050
Lu L et al. (2011) Shikonin extracted from medicinal Chinese herbs exerts anti-inflammatory effect via proteasome inhibition European journal of pharmacology 658:242-247 doi:10.1016/j.ejphar.2011.02.043
Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis. An overview of cell death. The American journal of pathology 146:3–15
Peng H et al (2016) The Tumour Response to Induction Chemotherapy has Prognostic Value for Long-Term Survival Outcomes after Intensity-Modulated Radiation Therapy in Nasopharyngeal Carcinoma. Scientific reports 6:24835. doi:10.1038/srep24835
Shahsavari Z, Karami-Tehrani F, Salami S (2015) Shikonin induced necroptosis via reactive oxygen species in the T-47D breast cancer cell line Asian Pacific journal of cancer prevention: APJCP 16:7261-7266
Shahsavari Z, Karami-Tehrani F, Salami S, Ghasemzadeh M (2016) RIP1K and RIP3K provoked by shikonin induce cell cycle arrest in the triple negative breast cancer cell line, MDA-MB-468: necroptosis as a desperate programmed suicide pathway tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 37:4479-4491 doi:10.1007/s13277-015-4258-5
Tang X, Zhang C, Wei J, Fang Y, Zhao R, Yu J (2016) Apoptosis is induced by shikonin through the mitochondrial signaling pathway. Molecular medicine reports 13:3668–3674. doi:10.3892/mmr.2016.4967
Vanden Berghe T, Kaiser WJ, Bertrand MJ, Vandenabeele P (2015) Molecular crosstalk between apoptosis, necroptosis, and survival signaling Molecular & cellular oncology 2:e975093 doi:10.4161/23723556.2014.975093
Vandenabeele P, Vanden Berghe T, Festjens N (2006) Caspase inhibitors promote alternative cell death pathways Science's STKE : signal transduction knowledge environment 2006:pe44 doi:10.1126/stke.3582006pe44
Wada N et al. (2015) Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells International journal of oncology 46:963-972 doi:10.3892/ijo.2014.2804
Wei Y, Li M, Cui S, Wang D, Zhang CY, Zen K, Li L (2016) Shikonin Inhibits the Proliferation of Human Breast Cancer Cells by Reducing Tumor-Derived Exosomes. Molecules:21. doi:10.3390/molecules21060777
Wiench B, Eichhorn T, Paulsen M, Efferth T (2012) Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. Evidence-based complementary and alternative medicine: eCAM 2012:726025. doi:10.1155/2012/726025
Yu X et al. (2015) Neoalbaconol induces cell death through necroptosis by regulating RIPK-dependent autocrine TNFalpha and ROS production Oncotarget 6:1995-2008 doi:10.18632/oncotarget.3038
Zhang YY, Liu H (2013) Connections between various trigger factors and the RIP1/ RIP3 signaling pathway involved in necroptosis Asian Pacific journal of cancer prevention: APJCP 14:7069-7074
Zhang Z et al (2016) Non-benzoquinone geldanamycin analogs trigger various forms of death in human breast cancer cells. Journal of experimental & clinical cancer research: CR 35:149. doi:10.1186/s13046-016-0428-6
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81372899, 81603155), Education Department of Anhui Natural Science Research Project China (KJ2016SD39), the International scientific and technological cooperation projects in Anhui Province (No.1503062024).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Z., Zhang, Z., Li, Q. et al. Shikonin induces necroptosis by reactive oxygen species activation in nasopharyngeal carcinoma cell line CNE-2Z. J Bioenerg Biomembr 49, 265–272 (2017). https://doi.org/10.1007/s10863-017-9714-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-017-9714-z