Abstract
Hypertension is one of the major risk factors of cardiac hypertrophy and magnesium deficiency is suggested to be a contributing factor in the progression of this complication. In this study, we aimed to investigate the relationship between intracellular free Mg2+ levels and electrophysiological changes developed in the myocardium of L-NAME induced hypertensive rats. Hypertension was induced by administration of 40 mg/kg of L-NAME for 6 weeks, while magnesium treated rats fed with a diet supplemented with 1 g/kg of MgO for the same period. L-NAME administration for 6 weeks elicited a significant increase in blood pressure which was corrected with MgO treatment; thereby cardiac hypertrophy developing secondary to hypertension was prevented. Cytosolic free magnesium levels of ventricular myocytes were significantly decreased with hypertension and magnesium administration restored these changes. Hypertension significantly decreased the fractional shortening with slowing of shortening kinetics in left ventricular myocytes whereas magnesium treatment was capable of restoring hypertension-induced contractile dysfunction. Long-term magnesium treatment significantly restored the hypertension-induced prolongation in action potentials of ventricular myocytes and suppressed Ito and Iss currents. In contrast, hypertension dependent decrement in intracellular Mg2+ level did not cause a significant change in L-type Ca2+ currents, SR Ca2+ content and NCX activity. Nevertheless, hypertension mediated increase in superoxide anion, hydrogen peroxide and protein oxidation mitigated with magnesium treatment. In conclusion, magnesium administration improves mechanical abnormalities observed in hypertensive rat ventricular myocytes due to reduced oxidative stress. It is likely that, changes in intracellular magnesium balance may contribute to the pathophysiology of chronic heart diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arterial systemic hypertension which is defined as afterload leads to ventricular hypertrophy in the heart (Chouabe et al. 2009). Though the underlying cellular mechanism still unresolved, altered ionic currents and prolongation in action potential (AP) as a consequence of left ventricular hypertrophy developed by hypertension is the main consensus (Li and Jiang 2000b). Significant alterations in the ionic currents that constitute AP configuration have been proposed as the main culprit of this prolongation, and indeed reduction of K+ and augmented L-type Ca2+ currents (ICaL) mediate prolongation of AP in hypertensive animals (Keung 1989; Chouabe et al. 2009). However, decreased or unchanged ICaL densities have also been reported in hypertension (Brooksby et al. 1993; Li and Jiang 2000).
Epidemiological and experimental studies point to the fact that magnesium (Mg2+) deficiency may be an important risk factor in the development of cardiovascular diseases and hypertension (Altura and Altura 1985; Liao et al. 1998; Sharikabad et al. 2001). Owing to strong relationship between Mg2+ malnutrition and its effects on blood pressure, it has been suggested that Mg2+ deficiency plays an important role in the pathogenesis of hypertension (Altura and Altura 1995; Sontia and Touyz 2007; Touyz 2008). Moreover, intracellular Mg2+ ions were shown to inhibit ICaL densities especially at positive potentials in rat ventricular myocytes (Wang and Berlin 2007). Griffiths et al. (Griffiths 2000) observed that Mg2+ deficiency does not change Ca2+ transient amplitudes, although it increases cell contraction significantly.
On the other hand reactive oxygen species (ROS) play an important role in the development of various cardiovascular diseases including hypertension. This situation originates from the decrease in antioxidant capacity of the cardiovascular system and/or excessive consumption of antioxidants. Superoxide anion (O2 −) and hydrogen peroxide (H2O2) are especially prominent in the production of oxidative stress in cardiovascular cells. Xanthine oxidase (XO), nitric oxide synthase (NOS) and NAD(P)H oxidase are enzymatic sources of ROS in hypertension and vascular diseases (Lacy et al. 1998; DeLano et al. 2006). Xanthine oxidase and ROS production are shown to increase in spontaneously hypertensive rat (SHR) and this alteration is congruent with increased arterial tonus (Paravicini and Touyz 2008). In addition, Mg2+ deficiency is culminated in increased oxidative stress injury and loss of function in cardiovascular diseases (Kramer et al. 1994; Kharb and Singh 2000). Superoxide dismutase (SOD) and catalase (CAT) activity decrease while H2O2 induced lipid peroxidation increases with Mg2+ deficiency (Prohaska 1991; Manju and Nair 2006). Besides H2O2 has been reported to directly modulate ICaL and thus induce early afterdepolarizations in rabbit cardiomyocytes (Xie et al. 2009).
It is shown that NO production in the body is disrupted in hypertensive patients, its bioavailability is decreased due to increased degradation. In addition, vasodilatory responses to NO stimulants is generally found to be defective in hypertensive patients (Morton et al. 1993; De Artinano and Gonzalez 1999). Findings supporting the view that NO deficiency contribute to the development of hypertension are set forth in animal studies (Gibbons 1997; Pollock 1999). “Hypertension Model due to NOS Blockade” is a model generated with L-arginine analogues and has been commonly used in previous studies (Zatz and Baylis 1998; Pollock 1999). In this study, it was aimed to determine electrophysiological changes in cardiomyocytes in L-NAME induced hypertension model and to reveal the relationships of these changes with intracellular Mg2+ levels, thereby unravel the impact of long-term Mg2+ treatment on the electrical and mechanical remodeling of hypertensive rat heart.
Material and methods
Preparation of animals
Healthy male Wistar albino rats, aged 8 weeks, were used in this study. All animals were provided by Akdeniz University Animal Care Unit. Three experimental groups, each consisting 20 rats were designed; control group (CON), L-NAME treated hypertensive group (HT) and L-NAME + MgO treated group (HT-Mg). Animals were housed in stainless steel cages at standard conditions (23 ± 1 °C) with a 12 h light–dark cycle and fed ad libitum with standard rat chow and tap water. All experimental protocols conducted on rats were performed in accordance with the standards established by the Institutional Animal Care and Use Committee at Akdeniz University.
L-NAME (40 mg/kg/day), a non-specific NOS inhibitor was added to drinking water of the animals to establish experimental hypertension model. MgO treated group was fed with food containing 1 g/kg of MgO. MgO and L-NAME were administred throughout 6 weeks of experimental period.
Blood pressure measurement
Blood pressure of the animals was measured from their tail arteries by non-invasive cuff method. Signals, collected with the loop shaped pressure probe attached to the tail were transferred to computer with MP 150 data collection system (BIOPAC Systems, CA-USA) and MAY-BPHR 9610-PC (Commat LTD., Ankara, Turkey) unit. After determination of basal values of all animals, blood pressure was monitored every 3 weeks. During periodical measurements at least five pressure traces were recorded for each rat and blood pressure was calculated from the average of that five measured values.
Isolation of cardiac myocytes
Isolation of cardiac myocytes performed enzymatically as described in previous studies (Aydemir et al. 2012; Ozturk et al. 2013). Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg body weight, ip) and heart removed quickly. Cannulation of aorta carried out with Langerdorrf apparatus and perfused retrogradely through the coronary arteries with Ca2+-free solution (in mM: 137 NaCl, 5.4 KCl, 1.2 MgSO4, 1.2 KH2PO4, 5.9 HEPES and 20 glucose at pH 7.2, bubbled with 100 % O2) for 5 min and then switched to same solution containing 0.8 mg/ml collagenase (Collagenase A Roche) and 0.07 mg/ml protease (Sigma type XIV) for 20 min. Ventricles were then removed and minced into small pieces and gently massaged through a nylon mesh. Subsequently, cell suspension was washed several times and Ca2+ was increased in a graded manner. Experiments were started 1 h after the isolation of the ventricular cells and performed at 36 ± 1 °C.
Measurement of Intracellular Free Mg2+ Concentration
After isolated cardiomyocytes were incubated with Mag-fura-2 AM (3 μM) at room temperature for 20 min, they were excited at 340 and 380 nm and then intracellular free Mg2+ changes were recorded by measuring florescence ratios focused at 510 nm. Florescence intensity obtained with Mag-Fura2-AM is converted to concentration with the following formula;
0.05 % TritonX was used to change the permeability of the cell. Maximum fluorescence (Rmax) and minimum fluorescence (Rmin) were obtained in the presence of 20 mM Mg2+ and 30 mM EGTA respectively. Fluorescence intensity at 380 nm wavelength with excessive amount of Mg2+ (Sb2) and of EGTA (Sf2) were calculated. Previously determined Kd value which is 1.5 mM was used for Mg2+ calculation (Raju et al. 1989). Records were made with IonOptix LLC system (Milton USA) and data analysis was performed with Ionwizard software (IonOptix, USA) program (Fatholahi et al. 2000).
Recording of action potential and potassium currents
AP recordings were performed at a frequency of 1 Hz with the help of electrodes with a resistance of 2–2.5 MΩ. Solution in the pipette (mM): 120 KCl; 6.8 MgCl2; 5 Na2ATP; 5; 0.4 Na2GTP; 10 EGTA; 4.7 CaCl2; 20 HEPES (pH = 7.4). For the record, small depolarizing pulses were injected to the cell within current-clamping configuration, the cell is excited and membrane potential changes were observed. 25, 50, 75, 90 % (APD25, 50, 75, 90) durations of repolarization phases of these obtained APs were evaluated.
Potassium currents were obtained with whole-cell configuration of voltage-clamping method. In order to obtain these currents, cardiomyocytes were evoked by 3 s test pulses from a holding potential of −70 mV and increasing in 10 mV steps between −60 mV and +60 mV.
Solutions used for these currents were prepared as follows: for the pipette (mM): 120 K-aspartate; 20 KCl; 10 NaCl; 5 MgATP; 10 Na-HEPES (pH = 7.2). In addition, CdCl2 (250 μM) was added to the intracapillary environment to block the Ca2+ currents. Transient outward potassium currents (Ito) were calculated by subtracting the current values at the end of the 3 s pulse which is defined as steady-state current (Iss) from the peak values. Then, measured current values were divided by cell capacity and presented as current density.
Contractile parameters
Isolated cells were placed into a cuvette having electrodes at both ends, through which Tyrode solution [(mM): 137 NaCI, 5.4 KCI, 0.5 MgCI2, 1.8 CaCI2, 11.8 Na-Hepes, 10 glucose, pH: 7.35)] is passed. Ventricular cells were stimulated with pulse of 0.5 Hz with 5–10 V amplitude and their shortening traces were recorded (IonOptix LLC, Milton USA). From these records, fractional shortening (L/L0), peak time (TP) and time to 50 % relaxation (RT50), 75 % to relaxation (RT75), and 90 % to relaxation (RT90) were calculated via Ionwizard software (IonOptix, USA) program.
Simultaneous Measurement of ICaL and triggered Ca2+ transients
In this part of the study ICaL and [Ca2+]i were measured simultaneously. These currents were recorded by using 1.5–2 MΩ electrodes in whole cell voltage clamping configuration. For measurements (mM): 120 L-aspartate, 20 CsCl, 10 NaCl2, 5 MgATP, 10 HEPES and 0.05 fura-2 potassium salt (pH = 7.2) were used as the pipette solution, and the bath solution contained (mM): 137 NaCl; 5.4 KCl; 1.5 CaCl2; 0.5 MgCl2; 10 Glucose; 11.8 HEPES (pH = 7.35). Following −45 mV prepulse by which sodium (Na+) currents were inactivated, 300 ms of depolarizing pulses from −50 mV to +80 mV with increments of 10 mV were applied. The associated Ca2+ transients were excited at Fura-2340 and 380 nm and recorded with the measurement of florescence ratios focused to 510 nm. Calcium currents filtered through filters of 3 kHz in patch-clamp amplificator (Axon 200B, Molecular Devices, USA) and were recorded with the 5 kHz sampling rate of Digidata 1200 with pClamp 10 software (Axon Instrument, Foster City CA, USA). Peak values were measured and then subtracted from the tail currents at the end of 300 ms. Current values were divided by cell capacitance and presented as current density.
For measurement of SR Ca2+ content 10 μM caffeine was applied on to myocyte for a period of 10 s via fast perfusion system. Inward INCX currents due to increase in intracellular Ca2+ content were recorded simultaneously with the caffeine response.
Biochemical parameters
Cardiomyocyte superoxide release and intracellular H2O2 concentrations
The release of superoxide (O2 •-) was quantified spectrophotometrically (lQuant; BioTek Instruments, Vermont, USA) by CuZn SOD reduction of cytochrome C at 550 nm, as previously described (Aslan and Canatan 2008). Briefly, the cardiomyocyote cell suspension was incubated with or without SOD (100 units/ml; Sigma-Aldrich Chemie, Steinheim, Germany) in the presence of cytochrome C (50 μM; Sigma-Aldrich Chemie, Steinheim, Germany) for 10 min at 37 °C.
Intracellular H2O2 concentrations were calculated from aminotriazole (AT; Sigma-Aldrich Chemie, Steinheim, Germany) -mediated inactivation of CAT activity. Cardiac cells were incubated with 10 mM AT at 37 °C, and intracellular CAT activity was determined spectrophotometrically before and after the 1-h incubation period.
Protein carbonyl content
Protein-bound carbonyls were measured via a protein carbonyl assay kit (Cayman Chemical, Ann Arbor, MI). The utilized method is based on the covalent reaction of the carbonylated protein side chain with 2.4-dinitrophenyl-hydrazine (DNPH) and detection of the produced protein hydrazone at an absorbance of 370 nm. The results are calculated using the extinction coefficient of 22 mM−1 cm−1 for aliphatic hydrazones and are expressed as nanomole per milligram of protein.
Statistical analysis
Statistical analysis of data was performed by using One-way ANOVA followed by Tukey Post Hoc test for comparison of the relevant groups. During comparison, values smaller than 0.05 (P < 0.05) was accepted significant for all results. Data are represented as mean ± SEM and “n” refers to the number of cells.
Results
Physiological properties of animals
To ensure that rats in the hypertensive groups receive L-NAME at the daily dose of 40 mg/kg for 6 weeks, daily drinking water amounts of the animals were monitored. No difference in respect to water consumption was detected among the groups. In addition, since groups receiving Mg treatment were fed with a diet containing 1 g/kg dose of MgO, daily food consumption of all groups were determined and MgO did not lead to a change in food consumption of the animals.
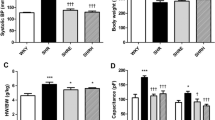
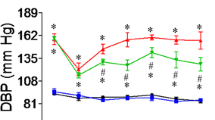
Blood pressure values measured in the beginning, 3rd and at the end of 6th week are given in Fig. 1. Although initial blood pressure values were not different among groups, there were significant increase in HT group values with respect to CON group at the end of experimental period (p < 0.001). This elevated blood pressure was restored in rats treated with MgO for 6 weeks (Fig. 1a).
Blood pressure and physiological parameters. The influence of 6 week magnesium (MgO) treatment on L-NAME induced hypertension. a Systolic blood pressure b Heart weight c Heart weight/Tibia length and d cell capacitance. Data are presented as means ± SEM, n = 25 rats per group. *p < 0.05 versus CON and #p < 0.05 versus HT
A significant increase was observed in heart weight and heart weight/tibia length ratio in HT group compared to CON group (Fig. 1b and c). Moreover L-NAME induced hypertension led to greater cell capacitances which implicate hypertrophic response at myocyte level as well. Mg2+ treatment was shown to correct cardiac hypertrophy at tissue level although it was not effective at cellular level (Fig. 1d).
Intracellular Mg2+ Concentration
Since Mag-fura-2-AM has been suggested to exert affinity to Ca2+ ion as well as Mg2+ to confirm the specifity of Mg2+ indicator, intracellular free Mg2+ level and Ca2+ florescence intensities were measured in different conditions. When electrical pulses of 0.5 Hz frequency were applied to cells loaded either with Mag-fura2-AM or Fura2-AM, transients were not triggered in myocytes loaded with Mg2+ indicator, despite apparent Ca2+ transients were observed in cells loaded with Ca2+ indicator (Fig. 2a). Secondly ATP, the cytosolic Mg2+ buffer, was used in cells loaded with Mag-fura2-AM and a decrease in florescence intensity was elicited with the application of 10 μM ATP, despite a remarkable increase in florescence intensity was observed in myocytes loaded with Fura2-AM (Fig. 2b). As a result, it was confirmed that Mag-fura-2-AM is ion specific and can be used in determination of intracellular Mg2+ changes.
The changes in intracellular Mg2+ in cells loaded with Mag-fura2-AM or Fura2-AM and stimulated by different stimulant. Data confirm that Mag-fura2-AM is specific to Mg2+ changes. a Responses that myocytes loaded with Mag-fura2-AM and fura2-AM give against electric stimuli at 0.5 Hz frequency, b Time-dependent fluorescence changes in myocytes loaded with Mag-fura2-AM and fura2-AM give agains10 μM ATP, c Intracellular free Mg2+ concentration
In order to understand the effect of hypertension on intracellular free Mg2+ levels in cardiomyocytes, intracellular basal Mg2+ concentration was measured in myocytes loaded with Mag-fura2-AM (3 μM). These values and basal Mg2+ concentrations obtained for groups are given in Fig. 2c. L-NAME induced hypertension led to significant decrease in basal Mg2+ concentrations when compared with values of the control group. This decreased Mg2+ concentrations was restored in rats treated with MgO for 6 weeks.
Action potential parameters
Sample records of AP obtained from groups are given in Fig. 3a. At the end of the experimental period, no difference was detected in resting membrane potentials (CON: −71.5 ± 0.7 mV; HT: −73.2 ± 0.8 mV; HT-Mg: −69.74 ± 1.4 mV) and maximum depolarization of myocytes isolated from rats of either group (data not shown).
The effect of magnesium (MgO) treatment on action potential characteristics of L-NAME induced hypertensive rats. a Current clamp recordings from cardiac myocyte cells demonstrated action potential duration in all groups, b Action potential duration (APD) at 25 %, 50 %, 75 %, 90 % of repolarization phase. Data are presented as means ± SEM, n = 18 to 20 cells. *p < 0.05 versus CON, #p < 0.05 versus HT
However repolarization duration of the hypertensive group (APD25, 50, 75, 90: 14.2 ± 3.2 ms; 39.4 ± 7.0 ms; 56.8 ± 8.3 ms; 69.8 ± 8.5 ms) was significantly longer than control values (5.5 ± 0.9 ms; 13.4 ± 1.9 ms; 27.9 ± 4.9 ms; 42.7 ± 3.7 ms) (Fig. 3). Whereas, MgO supplementation shortened the prolonged repolarization period of the hypertensive group (8.6 ± 1.5 ms; 20.9 ± 4.2 ms; 35.0 ± 5.6 ms; 49.3 ± 6.3 ms) and this change had statistical significance.
Effect of Hypertension and Mg2+ Treatment on K+ Currents
Potassium currents are major ionic currents that play role in repolarization phase of AP. Therefore Ito and Iss were measured in this study. Mean Ito values were shown to be significantly different in HT group compared to control group which means high blood pressure suppresses these currents (Fig. 4). In addition, significant improvement was detected in HT-Mg group (CON: 15.9 ± 1.04 pA/pF; HT: 10.2 ± 0.9 pA/pF; HT-Mg: 13.9 ± 1.3 pA/pF, for +60 mV).
Repolarizing K+ currents of experimental groups recorded by 3 s duration test pulses between −60 mV and +60 mV following 200 ms prepulse to inactivate Na+ currents. Current densities were plotted as a function of voltage. a. Original traces of CON, HT and HT-Mg are shown, b. Amplitude of Ito was calculated as the difference between peak and last part of the current which was defined as, c. Iss. Data are given as mean ± SEM; n = 20 to 25 cells. *p < 0.05 versus CON, #p < 0.05 versus HT
Hypertension led to significant decrease in Iss current densities with respect to control group values. However, despite a modest increase 6 weeks of MgO treatment could not reverse significantly the suppression of this current, (CON: 6.3 ± 0.5 pA/pF; HT: 4.7 ± 0.4 pA/pF; HT-Mg: 5.4 ± 0.4 pA/pF, values obtained for +60 mV).
Fractional shortening
In order to determine the relevance of changes in Mg2+ levels on functional parameters that hypertension leads to the contractility of cells were examined. High blood pressure was significantly decreased the amount of fractional shortening of ventricular myocytes and 6 weeks of MgO treatment improved these changes (Fig. 5b).
Contractile properties of cardiomyocytes isolated from hearts of CON, HT and HT-Mg groups. Myocyte contraction was measured by detecting the length of two edges with contractility at 0.5 Hz frequency of stimulation. a Sample traces of electrically stimulated ventricular myocytes, b Changes in fractional shortening, c Time to 50 % relaxation (RT50), d time to 75 % relaxation (RT75), e time to 90 % relaxation (RT90), f time to peak (TP). Data are means ±SEM, n = 40 to 45 cells. *p < 0.05 versus CON, #p < 0.05 versus HT
When peak time (TP), time to reach 50 %, 75 % and 90 % of relaxation (RT50, RT75, RT90, respectively) were measured, it was seen that TP, RT50, RT75, RT90 durations were significantly prolonged in HT group, compared to CON group. In HT-Mg group, MgO treatment elicited significant improvement in hypertension related abnormalities (Fig. 5c–f).
Effect of Hypertension and Mg2+ Treatment on Intracellular Ca2+ Handling
Ca2+ permeability increases with the opening of L-type Ca2+ channels (LTCCs) in plateau phase of the AP and Ca2+ enters into the cell from outside. LTCCs not only influence AP shape, but are also among the primary cellular mechanisms regulating excitation-contraction coupling that triggers Ca2+ release from SR through RyR.
Therefore, in order to examine the effect of hypertension on Ca2+ handling, Ca2+ currents and corresponding Ca2+ transients were simultaneously recorded in Fura-2 loaded myocytes. Current density of LTCCs as a function of membrane potential is given in Fig. 6. Neither hypertension nor MgO administration had significant effect on ICaL and associated Ca2+ transients’ amplitude.
Effects of L-NAME and magnesium on L-type Ca2+ currents and triggered Ca2+ transients of ventricular myocytes of experimental groups recorded under whole cell configuration of voltage clamp. Current and Ca2+ transients obtained during depolarizing pulses ranging between −50 and +60 mV. Data are represented as mean ± SEM. *P < 0.05 versus CON group and #P < 0.05 versus HT group
In order to confirm the effects of hypertension and Mg2+ administration on cytosolic Ca2+ regulation SR Ca2+ content and INCX currents were measured simultaneously during 10 mM caffeine application for 10 s. There was no difference in Ca2+ quantity stored in SR between the groups (Fig. 7b). Consistent with this, integrated INCX values were also similar (Fig. 7c).
SR Ca2+ content and INCX of ventricular myocytes isolated from CON, HT and HT-Mg groups. a Example of Ca2+ transients and membrane currents during a 10-s application of 10 mmol/L caffeine in experimental groups, b Average data for Ca2+ transients amplitude, c integrated inward INCX. Data are represented as mean ± SEM, n = 13 cells, *P < 0.05 versus CON group and #P < 0.05 versus HT group
Biochemical parameters
Superoxide release and H2O2 levels were also measured and high blood pressure caused more than two fold increase in superoxide release of the ventricular myocytes, while long-term MgO treatment resulted in a significant decrease in released superoxide level. Hypertension elicited a significant increase in cardiomyocyte H2O2 concentrations which were decreased after MgO treatment. Furthermore, protein carbonyl levels were also increased in HT group and MgO treatment significantly reduced the levels of protein carbonyl formation (Fig. 8).
Effect of MgO treatment on upregulated oxidative stress parameters of hypertensive rat heart. a Superoxide anion release, b H2O2 production and c Protein carbonyl content of hearts from CON, HT, and HT-Mg rats. Data are represented as mean ± SEM, *P < 0.05 versus CON group and #P < 0.05 versus HT group
Discussion
In this study, we observed a significant correlation between hypertension-induced electrophysiological alterations of myocardium and Mg2+ amount of rat ventricular myocytes in L-NAME-induced hypertension model. We further showed that modulation of oxidative stress levels is the likely mechanism of this beneficial effect of MgO treatment.
Arterial systemic hypertension is the most common cause of left ventricular pressure overload that can induce ventricular hypertrophy as part of cardiac remodeling (Chouabe et al. 2009). Blocking of NOS was achieved by 40 mg/kg L-NAME which elicited 36 % increase in blood pressure and resulted in hypertrophic remodeling in rat heart which was abolished via MgO treatment. Epidemiological and experimental studies have demonstrated a negative relationship between serum Mg2+ levels and blood pressure which implicate a role for Mg2+ in the pathogenesis of hypertension (Altura and Altura 1985, 1995; Liao et al. 1998; Sharikabad et al. 2001; Sontia and Touyz 2007; Touyz 2008). Consistently, in the present study MgO treatment reduced systolic blood pressure to the control level in hypertension group. This data imply that oral Mg2+ administration may be beneficial in preventing blood pressure elevation and thus cytosolic Mg2+ level can be one of the physiological mechanisms underlying blood pressure regulation.
Mg2+ is not only regulates contractile proteins and modulates transmembrane transport of ions but also acts as an essential cofactor in the activation of ATPase and controls various cellular and subcellular pathways (Grubbs and Maguire 1987). Although transport mechanisms responsible for the movement of Mg2+ across the cell membrane in cardiovascular cells is not well-defined, the presence of Na+/Mg2+ antiporters have been demonstrated in both smooth muscle and cardiac cells (Almulla et al. 2006). Recently TRP7/MIC channels were also suggested as the major physiological pathway for Mg2+ influx in rat ventricular myocytes (Tashiro et al. 2014). Studies performed with different techniques have presented incompatible cytosolic Mg2+ concentrations ranging between 0.4 and 3.5 mM (Gupta et al. 1978; Gupta and Moore 1980; Kirschenlohr et al. 1988) and small change in extracellular and/or intracellular Mg2+ levels have been suggested to cause alterations in cardiac stimulation, vascular tonus and contraction (Ishiguro et al. 1997). Accordingly, Mg2+ may have a physiological role in regulation of blood pressure and significant changes in Mg2+ level can contribute to pathophysiological processes of hypertension. Consistent with this, both tissue content and intracellular concentration of Mg2+ have been shown to decrease in various experimental hypertension models (Mahboob et al. 1996; Laurant et al. 1997). In our study, intracellular free Mg2+ levels in resting myocytes were measured by fluorescent dye Magfura2- AM and we found that the intracellular concentration of free Mg2+ is lower in cardiomyocytes isolated from L-NAME-induced hypertensive rats and long-term MgO treatment was capable of reversing this effect.
In our study myocyte contraction was decreased in hypertensive rats. Doggrell et al. 1999 showed that cardiac contractility is negatively regulated in spontaneous hypertensive rats in the presence of left ventricle hypertrophy. Contractile dysfunction was also confirmed by Mertens et al. 1992 and Li et al. 2005. Similarly, we measured prolonged relaxation time along with reduced Ca2+ removal rate. Correspondingly, our data infer that decreased Mg2+ level leads to contractile abnormalities in hypertensive rat heart and MgO treatment is capable of ameliorating contractile functions due likely to restoration of cellular Mg2+ level.
Atrial systemic hypertension causes ventricular hypertrophy and provokes cardiac electrical remodeling. Cardiac hypertrophy and associated AP prolongation may lead to ventricular arrhythmias and increase the risk of sudden cardiac death. Although left ventricular hypertrophy yield to contradictory results about ionic homeostasis, APD prolongation is well-documented in ventricular myocytes (Li et al. 2000). APD has been shown to prolong in SHR model (Brooksby et al. 1993; Sonoyama et al. 2005; Chen-Izu et al. 2007; Chan et al. 2011) and this slower repolarization was correlated with altered contraction in hypertension (Brooksby et al. 1993). In our experimental model, hypertension prolonged APD in ventricular myocytes and 6 week MgO treatment corrects this change in a considerable extent. Several mechanisms have been proposed for prolongation of APD in rodents; reduction of K+ currents is one of the likely culprits of these changes in AP repolarization time (Keung 1989; Brooksby et al. 1993; Chouabe et al. 2009). Brooksby et al. 1993 observed a significant decrease in inward rectifier potassium current although there was no difference in Ito and delayed rectifier potassium currents. However, in TGR 27 transgenic mouse model, Ito decreased in a considerable amount (Chouabe et al. 2009). Furthermore, Michailova et al. 2004 demonstrated a correlation between intracellular Mg2+ levels and AP repolarization time. Consistent with this in our study hypertension elicited significant decrease in Ito and Iss densities of ventricular myocytes and long-term Mg2+ treatment ameliorated these currents. Although the mechanism is not known explicitly, altered ion gradients may be responsible for altered K+ currents. It is likely that Mg2+ deficiency reduces myocyte Na+/K+ ATPase activity which can result in decreased intracellular K+ since Na+/K+ ATPase is the primary transport mechanism of K+ ions and requires Mg2+ (Altura and Altura 1985, 1995; Bara and Guiet-Bara 1984).
Another ion current that may lead to change in cardiac AP duration is ICaL. Contradictory results showing either unchanged or increased ICaL in hypertrophied heart has been presented in previous reports (Keung 1989; Brooksby et al. 1993; Li and Jiang 2000). Accordingly we did not observe a significant change in ICaL of hypertensive rat myocytes. These findings evidently implicate that the longer repolarization phase of AP was a result of diminished K+ currents but not LTCC currents. Cytosolic Mg2+ has been suggested to have regulatory effects on LTCC currents via modulation of channel kinetics or change of ion permeability rate (Wang et al. 2004). Although cytosolic Mg2+ levels under or over the physiological concentrations have been suggested to cause remarkable changes on Ca2+ currents (Kiyosue 2002; Wang and Berlin 2007), reduced cytosolic Mg2+ levels with respect to control myocytes did not affect Ca2+ currents in our study. This discrepancy may stem primarily from dissimilarities of experimental design. In fact, extreme concentrations of cytosolic Mg2+ have been tested in these cellular and in vitro studies.
SR Ca2+ content was also examined, and there wasn’t any change in the SR Ca2+ store of hypertensive rats’ ventricular myocytes which confirms the similarity of Ca2+ transients between groups. Of note, this result is in accordance with Milnes and MacLeod (2001) report. The two basic components that determine SR Ca2+ content in ventricular myocytes are SERCA2 and RyR. Although Mg2+ has been suggested as one of the biologic modulators for RyR and SERCA2 (Gusev and Niggli 2008), we did not observe significant change in SR Ca2+ content of either groups. Another important Ca2+ regulating mechanism in cardiomyocytes that may be also modulated by intracellular Mg2+ is NCX. Consistent with previous study that has been demonstrated unchanged INCX in SHRs ventricular myocytes (Chen-Izu et al. 2007) we did not measure significant difference between INCX values of experimental groups. These results clearly imply that altered contractile function of ventricular myocytes in this experimental model is not relevant to Ca2+ handling changes.
Alongside many clinically important heart diseases, cardiac hypertrophy has been also linked to upregulated ROS generation and resultant damage. Interestingly oxidative stress may cause cardiac insufficiency with ROS-dependent modification of sarcomeric proteins regardless of a significant change in Ca2+ homeostasis (Luo et al. 2006; Wang et al. 2008; Sumandea and Steinberg 2011). ROS prevents the formation of crossed-bridge by modulating Ca2+ binding areas of myofilaments and decreases the production of Ca2+-mediated force. Despite low intracellular Mg2+ levels did not result in a significant change in ICaL, SR Ca2+ content and NCX activity, prominent decrease in contractility along with prolonged relaxation were measured in hypertensive rat cardiomyocytes. These changes obtained in myocyte contraction are most likely stem from decrease in myofilament Ca2+ sensitivity due to increased ROS production. Superoxide release and H2O2 content of cardiomyocyte were also measured to indicate whether oxidative stress parameters are relevant to functional alterations indeed. L-NAME-induced hypertension caused increase in H2O2 levels and superoxide release of the cardiomyocytes, while both of them decreased significantly after long-term MgO treatment. Furthermore, protein carbonyl amount was also measured to assess protein damage and we observed decreased protein oxidation after MgO treatment. Similarly in SHRs Ren (Ren 2007) has shown remarkably higher protein oxidation levels which were resulted in decreased contraction rate and extension of relaxation period in cardiomyocytes. Consistent with previous reports our results imply that contractile function of myocardium is disrupted due probably to oxidative attack of proteins and improving effect of Mg2+ on contractility can be attributed to its antioxidant activity. In fact Mg2+ deficiency has been shown to reduce the activities of SOD and CAT enzymes and increase H2O2 induced lipid peroxidation in the heart (Manju and Nair 2006).
Decreased intracellular Mg2+ levels is a hallmark of hypertension in various experimental models and it is stated that this condition has prominent role in electrophysiological dysfunctions of hypertrophied heart that subjected to hypertension. Consequently, in this study; electrophysiological changes occurred in cardiac myocytes as well as the relationships of ROS and intracellular Mg2+ levels with these changes are presented in hypertension model. Also, the effects of long term Mg2+ treatment upon electrical, mechanical and biochemical changes of heart were examined and the presented findings led us to suggest that Mg2+ may exhibit significant antihypertensive and antioxidant effects in L-NAME-induced hypertension model.
The use of animal models in cardiovascular research can provide useful information for potential therapeutic interventions as well as understanding of the physiological mechanisms of several diseases including hypertension. However, due to the potential physiological disparities between species one should be cautious about extrapolating electrical and structural responses of rat myocardium to human heart. Another potential limitation of this study is the lack of sufficient knowledge about the causes of essential hypertension which is the biggest dilemma of experimental models.
References
Almulla HA, Bush PG, Steele MG, Flatman PW, Ellis D (2006) Sodium-dependent recovery of ionised magnesium concentration following magnesium load in rat heart myocytes. Pflugers Arch 451:657–667
Altura BM, Altura BT (1985) New perspectives on the role of magnesium in the pathophysiology of the cardiovascular system. I. Clinical aspects. Magnesium 4:226–244
Altura BM, Altura BT (1995) Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res 41:347–359
Aslan M, Canatan D (2008) Modulation of redox pathways in neutrophils from sickle cell disease patients. Exp Hematol 36:1535–1544
Aydemir M, Ozturk N, Dogan S, Aslan M, Olgar Y, Ozdemir S (2012) Sodium tungstate administration ameliorated diabetes-induced electrical and contractile remodeling of rat heart without normalization of hyperglycemia. Biol Trace Elem Res 148:216–223
Bara M, Guiet-Bara A (1984) Potassium, magnesium and membranes. Review of present status and new findings. Magnesium 3:215–225
Brooksby P, Levi AJ, Jones JV (1993) The electrophysiological characteristics of hypertrophied ventricular myocytes from the spontaneously hypertensive rat. J Hypertens 11:611–622
Chan V, Fenning A, Levick SP, Loch D, Chunduri P, Iyer A, Teo YL, Hoey A, Wilson K, Burstow D, Brown L (2011) Cardiovascular changes during maturation and ageing in male and female spontaneously hypertensive rats. J Cardiovasc Pharmacol 57:469–478
Chen-Izu Y, Chen L, Banyasz T, McCulle SL, Norton B, Scharf SM, Agarwal A, Patwardhan A, Izu LT, Balke CW (2007) Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. Am J Physiol Heart Circ Physiol 293:14
Chouabe C, Ricci E, Kurdi M, Legrand C, Bricca G, Bonvallet R (2009) Evaluation of remodeling in left and right ventricular myocytes from heterozygous (mRen2)27 transgenic rats. Gen Physiol Biophys 28:24–38
De Artinano AA, Gonzalez VL (1999) Endothelial dysfunction and hypertensive vasoconstriction. Pharmacol Res 40:113–124
DeLano FA, Parks DA, Ruedi JM, Babior BM, Schmid-Schonbein GW (2006) Microvascular display of xanthine oxidase and NADPH oxidase in the spontaneously hypertensive rat. Microcirculation 13:551–566
Doggrell SA, Nand V, Henderson CJ (1999) The effects of lignocaine and tetrodotoxin on the action potentials and contractions of left ventricles from normo- and hypertensive rats. Gen Pharmacol 32:429–437
Fatholahi M, LaNoue K, Romani A, Scarpa A (2000) Relationship between total and free cellular Mg(2+) during metabolic stimulation of rat cardiac myocytes and perfused hearts. Arch Biochem Biophys 374:395–401
Gibbons GH (1997) Endothelial function as a determinant of vascular function and structure: a new therapeutic target. Am J Cardiol 79:3–8
Griffiths EJ (2000) Calcium handling and cell contraction in rat cardiomyocytes depleted of intracellular magnesium. Cardiovasc Res 47:116–123
Grubbs RD, Maguire ME (1987) Magnesium as a regulatory cation: criteria and evaluation. Magnesium 6:113–127
Gupta RK, Moore RD (1980) 31P NMR studies of intracellular free Mg2+ in intact frog skeletal muscle. J Biol Chem 255:3987–3993
Gupta RK, Benovic JL, Rose ZB (1978) Magnetic resonance studies of the binding of ATP and cations to human hemoglobin. J Biol Chem 253:6165–6171
Gusev K, Niggli E (2008) Modulation of the local SR Ca2+ release by intracellular Mg2+ in cardiac myocytes. J Gen Physiol 132:721–730
Ishiguro S, Matsuyama T, Sakaguchi H, Nishio A (1997) Ex vivo study of the increased sensitivity to NO of endothelium-denuded thoracic aortas isolated from dietary magnesium-deficient rats. Magnes Res 10:21–31
Keung EC (1989) Calcium current is increased in isolated adult myocytes from hypertrophied rat myocardium. Circ Res 64:753–763
Kharb S, Singh V (2000) Magnesium deficiency potentiates free radical production associated with myocardial infarction. J Assoc Physicians India 48:484–485
Kirschenlohr HL, Metcalfe JC, Morris PG, Rodrigo GC, Smith GA (1988) Ca2+ transient, Mg2+, and pH measurements in the cardiac cycle by 19F NMR. Proc Natl Acad Sci U S A 85:9017–9021
Kiyosue T (2002) Removal of intracellular Mg(2+) activates cardiac Na(+)/Ca(2+) exchanger. Cardiovasc Res Feb 1;53(2):290–1.
Kramer JH, Misik V, Weglicki WB (1994) Magnesium-deficiency potentiates free radical production associated with postischemic injury to rat hearts: vitamin E affords protection. Free Radic Biol Med 16:713–723
Lacy F, Gough DA, Schmid-Schonbein GW (1998) Role of xanthine oxidase in hydrogen peroxide production. Free Radic Biol Med 25:720–727
Laurant P, Touyz RM, Schiffrin EL (1997) Effect of magnesium on vascular tone and reactivity in pressurized mesenteric resistance arteries from spontaneously hypertensive rats. Can J Physiol Pharmacol 75:293–300
Li X, Jiang W (2000) Electrical remodeling of membrane ionic channels of hypertrophied ventricular myocytes from spontaneously hypertensive rats. Chin Med J 113:584–587
Li L, Desantiago J, Chu G, Kranias EG, Bers DM (2000) Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol 278:H769–H779
Li SY, Golden KL, Jiang Y, Wang GJ, Privratsky JR, Zhang X, Eason AR, Culver B, Ren J (2005) Inhibition of sarco(endo)plasmic reticulum Ca2 + −ATPase differentially regulates contractile function in cardiac myocytes from normotensive and spontaneously hypertensive rats: role of Ca2+ regulatory proteins. Cell Biochem Biophys 42:1–12
Liao F, Folsom AR, Brancati FL (1998) Is low magnesium concentration a risk factor for coronary heart disease? The atherosclerosis risk in communities (ARIC) study. Am Heart J 136:480–490
Luo J, Xuan YT, Gu Y, Prabhu SD (2006) Prolonged oxidative stress inverts the cardiac force-frequency relation: role of altered calcium handling and myofilament calcium responsiveness. J Mol Cell Cardiol 40:64–75
Mahboob T, Mumtaz M, Haleem MA (1996) Electrolyte content of serum, erythrocyte, kidney and heart tissue in salt induced hypertensive rats. Life Sci 59:731–737
Manju L, Nair RR (2006) Magnesium deficiency augments myocardial response to reactive oxygen species. Can J Physiol Pharmacol 84:617–624
Mertens MJ, Mathy MJ, Pfaffendorf M, van Zwieten PA (1992) Depressed inotropic response to beta-adrenoceptor agonists in the presence of advanced cardiac hypertrophy in hearts from rats with induced aortic stenosis and from spontaneously hypertensive rats. J Hypertens 10:1361–1368
Michailova AP, Belik ME, McCulloch AD (2004) Effects of magnesium on cardiac excitation-contraction coupling. J Am Coll Nutr 23:514S–517S
Milnes JT, MacLeod KT (2001) Reduced ryanodine receptor to dihydropyridine receptor ratio may underlie slowed contraction in a rabbit model of left ventricular cardiac hypertrophy. J Mol Cell Cardiol 33:473–485
Morton JJ, Beattie EC, Speirs A, Gulliver F (1993) Persistent hypertension following inhibition of nitric oxide formation in the young Wistar rat: role of renin and vascular hypertrophy. J Hypertens 11:1083–1088
Ozturk N, Yaras N, Ozmen A, Ozdemir S (2013) Long-term administration of rosuvastatin prevents contractile and electrical remodelling of diabetic rat heart. J Bioenerg Biomembr 45:343–352
Paravicini TM, Touyz RM (2008) NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31: dc08–s247
Pollock DM (1999) Chronic studies on the interaction between nitric oxide and endothelin in cardiovascular and renal function. Clin Exp Pharmacol Physiol 26:258–261
Prohaska JR (1991) Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr 121:355–363
Raju B, Murphy E, Levy LA, Hall RD, London RE (1989) A fluorescent indicator for measuring cytosolic free magnesium. Am J Phys 256:C540–C548
Ren J (2007) Influence of gender on oxidative stress, lipid peroxidation, protein damage and apoptosis in hearts and brains from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 34:432–438
Sharikabad MN, Ostbye KM, Lyberg T, Brors O (2001) Effect of extracellular Mg(2+) on ROS and Ca(2+) accumulation during reoxygenation of rat cardiomyocytes. Am J Physiol Heart Circ Physiol 280:H344–H353
Sonoyama K, Igawa O, Miake J, Yamamoto Y, Sugihara S, Sasaki N, Shimoyama M, Hamada T, Taniguchi S, Yoshida A, Ogino K, Shigemasa C, Hoshikawa Y, Kurata Y, Shiota G, Narahashi T, Horiuchi M, Matsubara H, Ninomiya H, Hisatome I (2005) Effects of angiotensin II on the action potential durations of atrial myocytes in hypertensive rats. Hypertens Res 28:173–179
Sontia B, Touyz RM (2007) Role of magnesium in hypertension. Arch Biochem Biophys 458:33–39
Sumandea MP, Steinberg SF (2011) Redox signaling and cardiac sarcomeres. J Biol Chem 286:9921–9927
Tashiro M, Inoue H, Konishi M (2014) Physiological pathway of magnesium influx in rat ventricular myocytes. Biophys J 107:2049–2058
Touyz RM (2008) Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol 294:11
Wang M, Berlin JR (2007) Voltage-dependent modulation of L-type calcium currents by intracellular magnesium in rat ventricular myocytes. Arch Biochem Biophys 458:65–72
Wang M, Tashiro M, Berlin JR (2004) Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J Physiol 555:383–396
Wang L, Lopaschuk GD, Clanachan AS (2008) H(2)O(2)-induced left ventricular dysfunction in isolated working rat hearts is independent of calcium accumulation. J Mol Cell Cardiol 45:787–795
Xie LH, Chen F, Karagueuzian HS, Weiss JN (2009) Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104:79–86
Zatz R, Baylis C (1998) Chronic nitric oxide inhibition model 6 years on. Hypertension 32:958–964
Acknowledgments
This study was supported by a grant from Akdeniz University Scientific Research Coordination Unit, Turkey (2011.03.0122.006). This study was carried out as PhD thesis by N. Ozturk presented to Akdeniz University Health Sciences Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Rights and permissions
About this article
Cite this article
Ozturk, N., Olgar, Y., Aslan, M. et al. Effects of magnesium supplementation on electrophysiological remodeling of cardiac myocytes in L-NAME induced hypertensive rats. J Bioenerg Biomembr 48, 425–436 (2016). https://doi.org/10.1007/s10863-016-9666-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-016-9666-8