Abstract
Simulation and experiment have been used to establish that significant artifacts can be generated in X-pulse CPMG relaxation dispersion experiments recorded on heteronuclear ABX spin-systems, such as 13C i –13C j –1H, where 13C i and 13C j are strongly coupled. A qualitative explanation of the origin of these artifacts is presented along with a simple method to significantly reduce them. An application to the measurement of 1H CPMG relaxation dispersion profiles in an HIV-2 TAR RNA molecule where all ribose sugars are protonated at the 2′ position, deuterated at all other sugar positions and 13C labeled at all sugar carbons is presented to illustrate the problems that strong 13C–13C coupling introduces and a simple solution is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relaxation dispersion NMR spectroscopy has become a powerful tool for studying conformational dynamics of biomolecules on the μs–ms time-scale (Palmer 2004; Eisenmesser et al. 2005; Korzhnev et al. 2004; Ishima et al. 1998). For molecules undergoing ms dynamics, Carr–Purcell–Meiboom–Gill based experiments are particularly useful (Palmer 2004). Here the evolution of transverse magnetization under a series of refocusing 180° pulses is quantified to obtain information on the time-scale of the exchange process as well as on the population(s) and chemical shifts of the excited state(s). In addition, experiments can be repeated as a function of temperature or pressure to obtain insight into the energy landscape of the system (Bezsonova et al. 2006; Korzhnev et al. 2004). A variety of CPMG-based experiments have been developed for the study of exchange in proteins, including those that make use of 1H, 13C and 15N nuclei (Ishima and Torchia 2003; Orekhov et al. 2004; Loria et al. 1999; Tollinger et al. 2001; Hill et al. 2000; Skrynnikov et al. 2001) focusing on single-, zero-, double- and multiple-quantum coherences (Dittmer and Bodenhausen 2004; Loria et al. 1999; Tollinger et al. 2001; Korzhnev et al. 2005) as reporters of exchange.
Recently, our laboratories have initiated studies of dynamics in RNA. Here we have made use of the deuteron as a spin-spy probe of motion, using a labeling scheme where all of the ribose carbons are uniformly 13C labeled and all of the ribose hydrogens labeled with 2H, with the exception of the H2′ hydrogen (Vallurupalli et al. 2006; Scott et al. 2000), Fig. 1. A series of 1H→13C→2H out-and-back experiments have been developed for quantifying 2H spin relaxation (Vallurupalli et al. 2006). This labeling scheme produces an H2′ proton that is isolated from scalar (J) couplings to other ribose protons and our goal was to exploit the “apparent” 1H2′–13C2′ AX spin system on each of the ribose sugars to record 1H CPMG experiments measuring exchange in RNA. To our initial surprise, however, many more “dispersions” were observed than expected in the HIV-2 TAR RNA system studied here, and all of these were artifactual. At the root of the problem is the fact that the C2′ and the adjacent C3′ carbons can have very similar chemical shifts, with differences <1ppm for eight of the 30 nucleotides in HIV-2 TAR (Brodsky and Williamson 1997; Dayie et al. 2002), and since 1 J C2′C3′ ∼ 40 Hz the two carbons become strongly coupled in cases where the shift differences are small. The strong coupling introduces artifacts in X-pulse CPMG based experiments recorded on heteronuclear ABX spin systems that one might not initially anticipate, and in what follows we provide a theoretical description of the problem, simulations to illustrate how large the effects can be, followed by a simple pulse element that largely suppresses the artifacts.
Labeling scheme used to study both ps-ns (Vallurupalli et al. 2006; Scott et al. 2000) and ms dynamics in ribose units of RNA. All ribose carbons are 13C labeled and all the protons except H2′ are replaced by 2H. In the HIV-2 TAR RNA construct under study (Brodsky and Williamson 1997; Dayie et al. 2002) the C2′ and C3′ carbons can have very similar chemical shifts (74.8–76.8 ppm) and (71.9–77.6 ppm), respectively (Brodsky and Williamson 1997; Dayie et al. 2002), so that strong coupling between the carbons is a distinct possibility in many cases (1 J C1′C2′∼ 40 Hz; 8 C2′–C3′ pairs with 1 J C1′C2′/Δv > 0.27, where Δv is the difference in shifts between C2′ and C3′ at 600 MHz 1H frequency). The C1′ chemical shifts (89.2–94.5 ppm) are well separated from C2′ (Brodsky and Williamson 1997; Dayie et al. 2002)
Results and discussion
We first consider a 3-spin heteronuclear ABX spin-system and evaluate the response of transverse X magnetization to the application of an X-pulse CPMG train. This spin system is sufficient to describe the artifacts that are observed in 1H CPMG relaxation dispersion experiments recorded on RNA samples labeled as in Fig. 1 (Vallurupalli et al. 2006), where spins A and B are 13C2′ and 13C3′, respectively, and spin X is H2′. In what follows we first examine the spin echo pulse element, τ−π X −τ, that forms the basis of the X-pulse CPMG scheme. The Hamiltonian of interest during the evolution time τ, neglecting both the effects of relaxation and chemical exchange, is given by,
where ω A , ω B and ω X are the Larmor frequencies of spins A, B and X, respectively, C J j∈{X, Y, Z} is the J-component of the C∈{A, B, X} spin-angular momentum operator, J AX is the one bond heteronuclear scalar coupling constant between directly attached spins A and X, J AB is the homonuclear coupling constant connecting spins A and B and we assume that J BX = 0. In the case of a heteronuclear AMX spin system where homonuclear spins A and M are only weakly coupled and starting from X X (proportional to the x-component of X magnetization), the density matrix at the completion of the τ−π X −τ echo is given by
where \({U_{AMX}=\hbox{e}^{-iH_{AMX}\tau}\hbox{e}^{-i\pi X_X}\hbox{e}^{-iH_{AMX}\tau}}.\) Since all of the terms comprising the Hamiltonian commute U AMX can be replaced by \({\hbox{e}^{-i\pi X_X}}.\) Thus both chemical shift and scalar coupled evolution during τ are refocused and the starting magnetization is completely recovered. In the case of an ABX system, evolution of X magnetization during the spin echo leads to,
where
Equation (3.1) can be simplified by noting that [H o, H 1 ABX ] = 0 so that
where
Note that the unitary operator U eff ABX cannot be replaced by \({\hbox{e}^{-i\pi X_X}}\) in Eq. (3.6) because [A X B X + A Y B Y , 2A Z X Z ] ≠ 0. Thus, X spin magnetization is incompletely refocused after a spin echo in this case. More quantitatively, in the limiting case where δω = ω A −ω B = 0 and starting from X X , the fraction of transverse X magnetization at the completion of a single spin echo is given by
and the general case where δω ≠ 0 is illustrated in Fig. 2. In the limit that δω ≫ J AB an AMX spin system results and Fig. 2 shows that there is complete refocusing of magnetization, as expected.
Fraction of transverse X magnetization, X X , in a heteronuclear ABX spin system at the completion of a single spin echo, τ −π X −τ, as a function of τ and δω, the difference in chemical shifts between spins A and B (ppm). The initial magnetization is X X . A static magnetic field strength of 14.1T (600 MHz 1H frequency and 150 MHz 13C frequency) is assumed along with X = 1H2′, A = 13C2′, B = 13C3′, J AB = 40 Hz, J AX = 160 Hz.
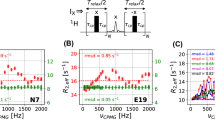
The loss of X spin magnetization in the ABX system after a single spin echo can lead to artifacts in CPMG experiments that are comprised of multiple echo trains. In the constant time (CT) variant of the CPMG experiment (Mulder et al. 2001), a series of spin echo elements, (τ−π X −τ) N , where N is even, is applied during a constant relaxation delay, T CPMG = N × 2τ. The magnitude of the x-component of transverse X magnetization (I) is measured in a series of experiments in which the number of π pulses is varied in the constant time interval. Subsequently, relaxation dispersion curves are generated from the variation of the effective relaxation rate \({R_{2, \rm eff} (\nu_{\rm CPMG})=\frac{-1}{T_{\rm CPMG}}\ln \frac{I(\nu_{\rm CPMG})}{I_o}}\) with νCPMG, where \({\nu_{\rm CPMG}=\frac{1}{4\tau}}\) and I 0 is measured from a reference spectrum with T CPMG = 0. Note that in the absence of chemical exchange and neglecting spin relaxation R 2,eff(νCPMG) profiles are expected to be flat since I(νCPMG) should be independent of νCPMG. However, for an isolated ABX spin system the loss of X-transverse magnetization that can result from A–B strong coupling effects during the T CPMG interval ultimately leads to “spikes” in CPMG dispersion profiles corresponding to elevated R 2,eff values that have nothing do with chemical exchange, Fig. 3A. It is noteworthy that artifacts are also produced using a scheme in which in-phase (X X ) and anti-phase (2A Z X Y ) transverse magnetization are interchanged in the middle of the CPMG element that eliminates contributions to dispersion profiles from differential relaxation of these two modes (Loria et al. 1999), Fig. 3B.
Constant time X-pulse CPMG schemes along with simulated relaxation dispersion profiles for a heteronuclear ABX spin system with X = 1H2′, A = 13C2′, B = 13C3′, J AB = 40 Hz, J AX = 160 Hz, as a function of δω = ω A −ω B . The strength of the CPMG field is given by \({\nu_{\rm CPMG} =\frac{1}{4\tau}},\) where 2τ is the time between the 180° pulses and RF inhomogeneity is not taken into account. The length of the constant time CPMG element, T CPMG = 40 ms, is 2Nτ in A and C and 4Nτ in B. The starting magnetization is X X in schemes A, C and 2A Z X Y in B. The element in the center of the CPMG interval of scheme B interchanges 2A Z X Y and X X with τ b set to 1/(4J AX ) (Loria et al. 1999). A CW spin lock field of 100 kHz, on resonance for spin A, has been assumed in C. The intrinsic relaxation rates of all spins are set to zero and chemical exchange effects are not included. Highlighted are dispersion curves for particular δω values for which large artifacts (very high R 2,eff values) were obtained
Certain aspects of the profiles of Fig. 3A can be understood by comparing the evolution of X-spin transverse magnetization during CPMG elements with very short delays, τ, (large νCPMG) relative to the evolution for longer values of τ. In the limit that H 1 ABX τ ≪ 1, H 2 ABX τ ≪ 1, the relevant propagator for the evolution of transverse X magnetization, \({\hbox{e}^{-iH_{ABX}^1\tau} \hbox{e}^{-iH_{ABX}^2 \tau} \hbox{e}^{-i\pi X_X}}\) (see above) can be approximated by \({\hbox{e}^{-i(H_{ABX}^1 +H_{ABX}^2)\tau} \hbox{e}^{-i \pi X_X}};\) since [H 1 ABX + H 2 ABX , X X ] = 0, X transverse magnetization is preserved. For longer τ values \({\hbox{e}^{-iH_{ABX}^1 \tau} \hbox{e}^{-iH_{ABX}^2\tau} \neq \hbox{e}^{-i(H_{ABX}^1 +H_{ABX}^2)\tau}}\) and the incomplete refocusing can lead to large R 2,eff rates for certain νCPMG values. This can be appreciated on an intuitive level by noting that transverse X magnetization, X X , evolves due to A–X scalar coupling (2A Z X Z ; Eq. (3.5)) to produce 2A Z X Y which in turn evolves under (2A X B X + 2A Y B Y , Eq. (3.5)) to generate other magnetization modes which are not refocused (see Eq. (3.7)). Thus, a simple solution to the problem becomes apparent: the strong coupling artifacts can be eliminated using the scheme of Fig. 3A if the buildup of anti-phase magnetization, 2A Z X Y , is prevented. Interestingly, in the case where the starting coherence is 2A Z X Y , Fig. 2B, evolution due to strong coupling occurs even in the limit of large νCPMG values, leading to loss of magnetization and thus non-zero R 2,eff(νCPMG = ∞) rates even though intrinsic relaxation times are assumed infinite in the simulations.
Figure 3C shows that by decoupling spins A and B, and hence preventing the scalar coupled evolution of X X , artifacts disappear (note the z-axis scale). Here a 100 kHz CW field was employed to illustrate the concept and to ensure complete decoupling; such field strengths are, of course, not of any practical interest. When more reasonable fields are used artifacts do result, however, as illustrated in Fig. 4A where a 3 kHz CW field is employed and dispersion profiles simulated as a function of offset from the resonance frequencies of spins A and B that are degenerate. This again can be understood intuitively by noting that a CW field of strength Z kHz can be thought of as a series of 180° pulses (one immediately following the other), each of duration 1/(2Z) ms. If the field strength is such that “an integer number of such pulses” can be applied for each τ interval in the CPMG train then A–X scalar coupled evolution is refocused. By contrast, if a half integer number of pulses is applied then the centers of the A/B and X 180° pulses coincide, leading to scalar evolution that for certain T CPMG values produces spikes. Thus, spikes in R 2,eff can be realized for τ values (ms) of {0.5, 1.5, 2.5, 3.5, …./(2Z), although the details do very much depend on T CPMG, and on the parameters of the spin system including couplings and chemical shift offsets, as well as on the offset of the CW field from the spins; indeed spikes can also be obtained for other τ values as well. For example, for a 3 kHz CW field and T CPMG = 30 ms considered in Fig. 4A, large artifacts are obtained for νCPMG values of 200, 333, 433 and 600 Hz, corresponding to τ values that “accommodate 7.5, 4.5, 3.5 and 2.5 pulses”, respectively. Similar artifacts are observed at νCPMG values of 200, 333, 429 and 600 Hz even when J AB = 0. Thus, it is important to choose the decoupling field strength with care. This can be done by simulating strong coupling effects prior to the experiment and subsequently avoiding νCPMG values that lead to artifacts. However, the approach that we favor is to vary the CW field strength, νCW, for each νCPMG value in such a way so as to ensure that an integral number of decoupling pulses is present in every τ element. This is accomplished by choosing νCW = 2kνCPMG where k is an integer, Fig. 4B. Flat dispersion profiles are obtained, with the exception of the first point (νCPMG = 33 Hz) in the case of large Δω values where the limited bandwidth of the CW field becomes an issue. Thus, dispersion curves can be faithfully recorded in this manner for values of νCPMG >50 Hz, using CW fields that vary between 2.1 kHz and 3 kHz (see legend to Fig. 4). Detailed simulations with T CPMG values that vary from 20 ms to 100 ms establish that, in general, for νCPMG >60 Hz, artifacts in R 2,eff are less than 1 s−1. Finally, it is straightforward to compensate for the differential heating that may result from this approach by including a short variable A/B spin decoupling element immediately prior to the start of sequence so that the average power dissipated in the sample is independent of νCPMG.
Relaxation dispersion curves obtained using the pulse sequence of Fig. 3C, with T CPMG = 30 ms, δω = 0 and Δω is the offset of C2′/C3′ resonance frequencies from the 13C carrier (see legend to Fig. 3 for additional parameters used in the simulations of the non-exchanging heteronuclear spin system). (A) A constant 3 kHz CW field is used for all νCPMG values, producing large artifacts at a number of frequencies, as described in the text. RF inhomogeneity is not taken into account. (B) CW fields are varied with each value of νCPMG, so as to satisfy νCW = 2kνCPMG, with k an integer, eliminating the artifacts in A (νCW values ranging between 2.1 kHz and 3 kHz are employed). Values of R 2,eff larger than 1 s−1 are only observed in the first point (νCPMG = 33.3 Hz) for large Δω due to the limited bandwidth of the CW field.
Figure 5 shows experimental dispersion profiles generated for A35 (A) and G34 (B) of HIV-2 TAR RNA using the schemes of Fig. 3B (green) and 3C (red). The dispersion profiles generated with a standard sequence (Fig. 3B) could easily (and erroneously) be interpreted in terms of exchange, yet clearly reflect the effects of strong coupling. Reasonably flat dispersions are obtained when a CW field satisfying νCW = 2kνCPMG is applied during the CPMG pulse train, as expected on the basis of the simulations discussed above. A measure of how “flat” such dispersions are can be obtained from the quantity \({\hbox{RMSD}=\sqrt{\frac{1}{N}\sum\limits_{i=1}^N{\left({\frac{R_{2,{\rm eff}, i}^{\rm Exp}-R_{2,{\rm eff}, i}^{\rm BestFit}}{\sigma_{R_{2,{\rm eff}, i}^{\rm Exp}}}}\right)^{2}}}},\) where N is the number of νCPMG points defining the dispersion, \({R_{2,{\rm eff}, i}^{\rm Exp}}\) is the measured relaxation rate for the ith νCPMG value, \({R_{2,{\rm eff}, i}^{\rm BestFit}}\) is the value of the horizontal line which best approximates the data and \({\sigma_{R_{2, {\rm eff}, i}^{\rm Exp}}}\) is the error in the measured \({R_{2,{\rm eff}, i}^{\rm Exp}}\) rate. Values of RMSD were 0.9 and 0.6 for A35 and G34, respectively, and varied between 0.5 and 0.9 for the range of strongly coupled residues that could be quantified in spectra.
Comparison of experimental relaxation dispersion curves recorded on a 30 nucleotide, 1 mM HIV-2 TAR RNA sample (Vallurupalli et al. 2006) described previously, 25°C, 600 MHz. The schemes of Fig. 3B (green; T CPMG = 40 ms) and 3C (red; T CPMG = 30 ms) have been used. The CW decoupling field (νCW) for each νCPMG value was chosen according to the relation νCW = 2kνCPMG (with k an integer, as described in the text) and varied between 2.8 kHz and 2.27 kHz. The 13C and 1H carriers were placed at 75.5 ppm and 4.5 ppm, respectively. (A) Residue A35 with |δω C2′C3′| = 0.1 ppm. (B) Residue G34, |δω C2′C3′| = 0.7 ppm
In summary we have presented an example of where strong coupling effects can lead to substantial artifacts in relaxation dispersion profiles. Dispersion curves generated from 1H CPMG experiments using 13C–13C–1H spin system probes, that are not undergoing chemical exchange but where the pair of 13C spins are strongly coupled, can be produced that are not dissimilar from those that might be obtained in cases with exchange. A simple solution involving the use of CW decoupling fields during the CPMG interval substantially reduces the artifacts so that robust measures of exchange can be obtained.
References
Bezsonova I, Korzhnev DM, Prosser RS, Forman-Kay JD, Kay LE (2006) Hydration and packing along the folding pathway of SH3 domains by pressure-dependent NMR. Biochemistry 45:4711–4719
Brodsky AS, Williamson JR (1997) Solution structure of the HIV-2 TAR-argininamide complex. J Mol Biol 267:624–639
Dayie KT, Brodsky AS, Williamson JR (2002) Base flexibility in HIV-2 TAR RNA mapped by solution 15N, 13C NMR relaxation. J Mol Biol 317:263–278
Dittmer J, Bodenhausen G (2004) Evidence for slow motion in proteins by multiple refocusing of heteronuclear nitrogen/proton multiple quantum coherences in NMR. J Am Chem Soc 126:1314–1315
Eisenmesser EZ, Millet O, Labeikovsky W, Korzhnev DM, Wolf-Watz M, Bosco DA, Skalicky JJ, Kay LE, Kern D (2005) Intrinsic dynamics of an enzyme underlies catalysis. Nature 438:117–121
Hill RB, Bracken C, Degrado WF, Palmer AG (2000) Molecular motions and protein folding: Characterization of the backbone dynamics and folding equilibrium of α2D using 13C NMR spin relaxation. J Am Chem Soc 122:11610–11619
Ishima R, Torchia DA (2003) Extending the range of amide proton relaxation dispersion experiments in proteins using a constant-time relaxation-compensated CPMG approach. J Biomol NMR 25:243–248
Ishima R, Wingfield PT, Stahl SJ, Kaufman JD, Torchia DA (1998) Using amide 1H and 15N transverse relaxation to detect millisecond time-scale motions in perdeuterated proteins: Application to HIV-1 protease. J Am Chem Soc 120:10534–10542
Korzhnev DM, Neudecker P, Mittermaier A, Orekhov VY, Kay LE (2005) Multiplesite exchange in proteins studied with a suite of six NMR relaxation dispersion experiments: an application to the folding of a Fyn SH3 domain mutant. J Am Chem Soc 127:15602–15611
Korzhnev DM, Salvatella X, Vendruscolo M, Di Nardo AA, Davidson AR, Dobson CM, Kay LE (2004) Low-populated folding intermediates of Fyn SH3 characterized by relaxation dispersion NMR. Nature 430:586–590
Loria JP, Rance M, Palmer AG (1999) A relaxation-compensated Carr-Purcell- Meiboom-Gill sequence for characterizing chemical exchange by NMR spectroscopy. J Am Chem Soc 121:2331–2332
Mulder FAA, Skrynnikov NR, Hon B, Dahlquist FW, Kay LE (2001) Measurement of slow (µs-ms) time scale dynamics in protein side chains by 15N relaxation dispersion NMR spectroscopy: Application to Asn and Gln residues in a cavity mutant of T4 lysozyme. J Am Chem Soc 123:967–975
Orekhov VY, Korzhnev DM, Kay LE (2004) Double- and zero-quantum NMR relaxation dispersion experiments sampling millisecond time scale dynamics in proteins. J Am Chem Soc 126:1886–1891
Palmer AG 3rd (2004) NMR characterization of the dynamics of biomacromolecules. Chem Rev 104:3623–3640
Scott LG, Tolbert TJ, Williamson JR (2000) Preparation of specifically 2H- and 13C-labeled ribonucleotides. Method Enzymol 317:18–38
Skrynnikov NR, Mulder FA, Hon B, Dahlquist FW, Kay LE (2001) Probing slow time scale dynamics at methyl-containing side chains in proteins by relaxation dispersion NMR measurements: application to methionine residues in a cavity mutant of T4 lysozyme. J Am Chem Soc 123:4556–4566
Tollinger M, Skrynnikov NR, Mulder FA, Forman-Kay JD, Kay LE (2001) Slow dynamics in folded and unfolded states of an SH3 domain. J Am Chem Soc 123:11341–11352
Vallurupalli P, Scott L, Hennig M, Williamson JR, Kay LE (2006) New RNA labeling methods offer dramatic sensitivity enhancements in 2H NMR relaxation spectra. J Am Chem Soc 128:9346–9347
Acknowledgments
PV acknowledges a postdoctoral fellowship from the Canadian Institutes of Health Research Training Grant in Protein Folding and Disease. This research was supported by a grant from the Natural Sciences and Engineering Research Council to LEK. LEK holds a Canada Research Chair in Biochemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vallurupalli, P., Scott, L., Williamson, J.R. et al. Strong coupling effects during X-pulse CPMG experiments recorded on heteronuclear ABX spin systems: artifacts and a simple solution. J Biomol NMR 38, 41–46 (2007). https://doi.org/10.1007/s10858-006-9139-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-006-9139-1