Abstract
Fast and stable repair of segmental bone defects remains a challenge for clinical orthopedic surgery. In recent years, porous tantalum has been widely applied in clinical orthopedics for low modulus of elasticity, with three-dimensional microstructures similar to cancellous bone and excellent biocompatibility. To further improve bone the repairing ability of porous tantalum, the cyclo(–RGDfK-) peptide was coated on the surface of porous tantalum scaffolds. A model of 15 mm segmental defect was made at the midshaft of right radius in New Zealand White rabbits. In the experimental group, defects were implanted (press-fit) using porous tantalum scaffolds modified with cyclo(-RGDfK-) peptide. Control animals were implanted with non-modified porous tantalum scaffolds or xenogeneic cancellous bone scaffolds, respectively. No implant was provided for the blank group. Bone repair was assessed by X-ray and histological observations at 4, 8, and 16 weeks post-operation, with biomechanical tests and micro-computed tomography performed at 16 weeks post-surgery. The results showed that bone formation was increased at the interface and inside the inner pores of modified porous tantalum scaffolds than those of non-modified porous tantalum scaffolds; biomechanical properties in the modified porous tantalum group were superior to those of the non-modified porous tantalum and xenogeneic cancellous bone groups, while new bone volume fractions using micro-computed tomography analysis were similar between the modified porous tantalum and xenogeneic cancellous bone groups. Our findings suggested that modified porous tantalum scaffolds had enhanced repairing ability in segmental bone defect in rabbit radius, and may serve as a potential material for repairing large bone defects.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Despite multiple available reports, repair of segmental bone defect remains a urgent challenge in clinic. Autologous bone graft, especially with blood supply, is currently considered the ideal method [1–3]. However, significant drawbacks, such as limited bone amount, second site surgery, prolonged surgical time, and postoperative complications at the donor site, limit its clinical application. Therefore, the development of ideal bone graft substitute materials represents a hot topic in the field of tissue engineering and biological materials science. Ideal substitute materials should have the following characteristics [4, 5]: (1) good biocompatibility; (2) sufficient source and moderate price; (3) sufficient mechanical strength; (4) three-dimensional porous structure similar to the normal bone; (5) good plasticity, osteoconduction and osteoinduction; (6) degradability. Although commonly degradable materials have three-dimensional porous microstructure similar to those of cancellous bone, and are suitable for new bone ingrowth and mineralization, insufficient mechanical strength limits their applications in load-bearing parts such as spine, joints, and limbs [6–8]. Porous metal materials are not degradable, but their high mechanical strength can provide good support for new tissue growth, which is especially suitable for load-bearing parts of bone repair. To date, the commonly used porous metal materials are mainly porous tantalum and titanium, and their respective alloys. Porous tantalum produced by Zimmer Corporation (Warsaw, IN, USA) has been widely used in orthopedic clinic, e.g., in arthroplasty, spinal interbody fusion surgery, and femoral head necrosis [9–12]. This porous material has many advantages, including good biocompatibility, high porosity (75–80%) with a structure similar to that of cancellous bone, low elastic modulus that best matches human bone, relatively high friction coefficient, excellent corrosion–erosion resistance, bone conductibility and low bacteria adhesion. However, it is expensive, and not suitable for clinical application in China and other less developed areas of the world. Therefore, cooperating with numerous Chinese research institutions, Chongqing Runze Pharmaceutical Co., Ltd. (Chongqing, China) successfully developed porous tantalum materials, which were prepared by slip-casting of powder using the teeming technology. Physical and mechanical indexes showed that porous tantalum possesses high porosity (65–80%), appropriate pore diameter size (400–600 µm) and good mechanical characteristics (Table 1) that are similar to those of human bone. Therefore, porous tantalum may be an excellent substitute material for bone graft in the future.
However, tantalum is an inert metal material, which cannot induce osteoblast adhesion and proliferation rapidly in vivo, and contact closely with host bone at early stage. In order to improve its biological activity, surface modification of the material is needed that can provide good biological interface for adhesion proliferation and differentiation of seed cells, promoting the generation and calcification of the extracellular matrix. In the end, a solid bone interface is established quickly, which meets the requirements of early weight-bearing activities. In the present study, we used the cyclo(-RGDfK-) peptide for surface modification; this peptide can bind specifically to the αvβ3 and αvβ5 integrin subunits on osteoblasts, and is commonly associated with fibronectin, with the ability to promote osseointegration of metal materials [13, 14]. In our previous study, the cyclo(-RGDfK-) peptide showed good bio-ability of promoting osteoblast adhesion and proliferation on the surface of Chinese porous tantalum in vitro.
The aim of this study was to assess whether cyclo(-RGDfK-) peptide modification enhances the osseointegration ability of porous tantalum in repairing segmental bone defects in rabbit radius, providing an experimental basis for the clinical application of the Chinese porous tantalum material.
2 Materials and methods
2.1 Scaffolds
The porous tantalum scaffolds were developed by Chongqing Runze Pharmaceutical Co., Ltd. (Chongqing, China), and prepared by slip-casting of powder through the teeming technology. The material included 250-mesh pure tantalum powder, a certain amount of additives, and sponge carrier to control the pore diameter, porosity and pore distribution. Then, porous tantalum, sintered at high temperature (1500–2100˚C), was fabricated by slip casting forming and post-treatment technology of necessary preparation (the preparation method has been submitted for patenting) [15]. Porous tantalum scaffolds were oval-shaped cylinders, 3.5 mm in diameter and 15–25 mm in height (Fig. 1).
The cyclo-(RGDfK)-Acp-Acp-Acp-mercaptopropanoic acid peptide provided by Shanghai top-Peptide Biotechnology co., Ltd. (Shanghai, China) was dissolved in phosphate-buffered saline (PBS) at 100 µmol/L. A 100 µmol/L solution of the RGD peptide was shown to enhance osteoblast-like cell adhesion to many kinds of RGD-coated materials in previous studies [14, 16–18]. According to the surface modification method of cyclo(-RGDfK-) peptide by Elmengaard [13] and Kroese-Deutman [19], porous tantalum scaffolds were immersed in the peptide solution overnight at room temperature, and subsequently washed three times in PBS, followed by air drying in a laminar airflow chamber. Both modified porous tantalum (MPT) and non-modified porous tantalum (NMPT) were sterilized using γ-ray radiation at 30 kGy for 16 h preoperatively.
Xenogeneic cancellous bones (XCB) were provided by Shanxi library of medical organization (Shanxi, Taiyuan, China). Following hygiene standards, the materials were taken from human cancellous bones, followed by freezing, drying, irradiation, and sterilization. The Xenogeneic bones not only preserve natural bone morphogenetic proteins and other active substances, but also have good three-dimensional porous structure, and are widely used in clinical repair of bone defect in China. The materials were rectangular cylinders, 3.5 mm in length and 25–45 mm in height (Fig. 2).
2.2 Experimental animals
Eighty-one adult male New Zealand white rabbits (weight, 2.5–3.0 kg) were provided by the Experimental Animal Center of North China University of Science and Technology (Hebei, Tangshan, China). The study was approved by the Institutional Animal Review Committee of Tangshan Orthopedic Hospital Affiliated to North China University of Science and Technology. The rabbits were randomly divided into four groups: (1) MPT (n = 24); (2) NMPT (n = 24); (3) XCB (n = 24); (4) blank (n = 9) groups. All rabbits were fed for adaptation in individual cages for one week before the operation. To reduce trauma and improve the postoperative survival rate, only the right radial of each rabbit was selected for surgery.
2.3 Surgical procedure
Prior to surgery, all surgical instruments were sterilized by γ-ray irradiation at 30 kGy. The rabbits were weighed and anesthetized by intraperitoneal injection of 10% chloral hydrate at a dose of 3 mL/kg body weight. The operations were performed under sterile conditions. After shaving and disinfection, the animal was fixed in the supine position, and a straight skin incision of 3.0 cm was made in the midsection of right radial diaphysis (Fig. 3a). A 15 mm long segmental defect was created using a wire saw at midshaft radius (Fig. 3b). The periosteum and interosseous membranes of the forearm, 0.5 cm from the proximal to distal sites of the broken ends, were removed completely. The ulna was left intact. After irrigation with saline, the animals were randomly implanted with the MPT, NMPT or XCB, respectively, in the created bone defect; no materials were implanted in the blank group (Figs. 3c–f). The wound was irrigated with saline, and the muscle and skin were closed in layers using an absorbable suture material (5–0 Vicryl) (Fig. 3g). After the operation, the animals were individually caged and normally fed. To prevent wound infection, the rabbits were administered penicillin (15 mg/kg body weight) for 3 days after operation.
2.4 General observations
Diet, daily activity, wounds, and weight change of the rabbits were observed after surgery.
2.5 Radiological assessment
At the day of surgery, and 4, 8, and 16 weeks post-surgery, each rabbit was examined by digital X-ray (Mobilett X-ray machine, Siemens, Munich, Germany) to evaluate the repair process. Irradiation parameters were as follows: 100 mA, 50 kV, 10 ms and 75 cm of target distance.
2.6 Histology
At 4, 8, and 16 weeks post-surgery, respectively, the animals were euthanized by air embolism. The specimens, including the implants and about 5-mm of adjacent host bones, were resected carefully, and fixed in 4% paraformaldehyde for 72 h, rinsed with water for 24 h, dehydrated in graded ethanol for 6 h, and embedded in methyl methacrylate for 1 week. Non-decalcified histological sections were cut serially along the longitudinal axis of porous tantalum using a Leica SP1600 hard tissue slicer (Leica Microsystems GmbH, Wetzlar, Germany), and stained with toluidine blue. New bone growth at the interface and inside the inner pores was observed under a light microscope (Olympus Bx60, Olympus Corporation, Tokyo, Japan).
2.7 Biomechanical tests
At 16 weeks post-surgery, six fresh radius specimens were randomly selected in the MPT, NMPT, and XCB groups, respectively. Meanwhile, six normal radius specimens were harvested randomly from the left forearms of experimental rabbits as the control group. Before the tests, all soft tissues around the specimens were cleared away, and each specimen was fixed on the biomechanical testing instrument (ElectroForce 3200, BOSE, Massachusetts, USA) with a span of 50 mm. A three-point bending test was performed with a displacement rate of 10 mm/min until the radius fractured. Maximal load force (Fmax) was recorded when fracture or breakage of the specimen occurred. Bending strength was calculated as follows: bending strength = Fmax × 3 × L/2bh2. Average values of Fmax and bending strength among the groups were compared.
2.8 Micro-CT analysis
Micro-CT was applied to evaluate new bone formation in the radial defect before histology at 16 weeks postoperatively. The specimens were scanned on an eXplore Locus Micro CT (eXplore Locus SP, GE Healthcare, London, Canada). Six specimens per group around the bubble fixed were placed vertically in the sample holder. Scanning parameters were as follows: 14 μm resolution, 270 min scanning time, 360° rotation, 0.4° rotation step, 3000 ms exposure time, 80 kV, 80 μA, average frame of 4, and pixel combination of 1 × 1. The newly formed bone volume fraction (BV/TV) in radial defect was calculated with the three-dimensional reconstruction and visualization software (MicroView ABA 2.1.2, GE Healthcare, USA). Mean values of each group were compared.
2.9 Statistical analysis
Data were analyzed with the SPSS 20.0 statistical software (IBM, Armonk, NY, USA). Data are mean and standard deviation (SD). In biomechanical and Micro-CT analyses, data among various groups were compared by one-way ANOVA. Multiple comparisons were performed with LSD (equal variance) or Tamhane’s T2 (unequal variance). P < 0.05 was considered statistically significant.
3 Results
3.1 General observations
All rabbits were revived within 2 h of surgery, and returned to normal diet, movement and body weight 2 week postoperatively. Wounds healed primarily without infection and other complications. All the animals survived uneventfully.
3.2 Radiological assessment
The implants were well positioned without displacement and fracture at the day of surgery. There was 15 cm defect neatly in length at the radius midsection in the blank group.
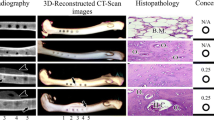
With time, the combination between the implant and host bone in each group became increasingly close, and the fracture line gradually disappeared. The amount of new callus formed in MPT animals was more than that in the NMPT group (Fig. 4a, b). The implant contour in the XCB group was fuzzy, the new bone well shaped, and the medullary cavity recanalized partially at 16 weeks postoperatively (Fig. 4c). There were always obvious radial defects in the blank group at 4, 8, and 16 weeks after surgery, and bone density of the stump increased with the marrow cavity closed at 16 weeks post-surgery (Fig. 4d).
3.3 Histological findings
At 4, 8, and 16 weeks after surgery, new bone mass and maturity gradually increased at the interface and inside the inner pores in the MPT and NMPT groups, the new bone gradually grew from the surrounding to the inner pore, and the new bone formed in the MPT group was better than that of the NMPT group (Fig. 5a, b). The xenograft bones were gradually replaced by new bone tissues in the XCB group (Fig. 5c).
3.4 Biomechanical findings
At 16 weeks post-surgery, the three point bending test showed Fmax in the normal radius, MPT, NMPT and XCB groups of 123.44 ± 10.69 N, 111.71 ± 4.36 N, 82.65 ± 5.65 N and 71.05 ± 3.82 N, respectively. Meanwhile, bending strength in these groups were 122.70 ± 3.15, 105.16 ± 2.93, 78.53 ± 1.16 and 67.13 ± 2.38 MPa, respectively. Fmax and bending strength in the normal radius group were overtly higher than in the MPT, NMPT and XCB groups, and the differences were statistically significant (P < 0.01). Among the MPT, NMPT, and XCB groups, mechanical properties in the MPT group were highest and lowest in the XCB group. The differences among the three groups were also statistically significant (P < 0.01 ) (Table 2, Fig. 6a, b), indicating a good repairing effect of porous tantalum on segmental radial defects in rabbits. Compared to the normal radius, Fmax and bending strength values in the MPT group reached 90.5 and 85.7%, respectively. These findings indicated that porous tantalum scaffolds modified with the RGD peptide had excellent biomechanical properties and the potential for repairing segmental bone defects in rabbit radius.
3.5 Micro-CT data
Cross-sectional and coronal scanning as well as three-dimensional reconstruction images at 16 weeks after surgery showed that there were newly formed bones in the surrounding and inner pores of the implants; the combination at the interface between the implant and host bone was firm without porous tantalum fracture, and only a small amount of materials were left in the XCB group (Fig. 7a–i). New bone volume fractions (BV/TV) in the MPT, NMPT and XCB groups were 38.57 ± 3.98, 25.07 ± 4.34 and 42.48 ± 4.66%, respectively. The amounts of newly formed bone in the MPT and XCB groups were significantly higher than those of the NMPT group (P < 0.05), but no statistically significant difference was obtained between the MPT and XCB groups (P < 0.05) (Table 3, Fig. 8).
Transverse a–c, coronal d–f and 3-D reconstructed g–i images of micro-CT in each group at 16 weeks after the operation. MPT a, d, g, NMPT b, e, h, XCB c, f, i. Tantalum (light blue) and new bone (yellow) in the MPT and NMPT groups. New bone (red) and xenogeneic cancellous bone (yellow) in the XCB group
4 Discussion
Because large-segmental bone defects cannot heal without treatment, the normal life and work of patients is affected seriously due to the loss of bone support function. Although autologous bone graft is currently the most effective treatment method, it sometimes does not meet the needs of a wide range of bone defects. Thus, many researchers began to develop bone tissue substitute materials, with considerable progress. Thanks to their three-dimensional structure similar to natural bones, porous materials have now become the main research direction for bone substitute materials. Many studies have demonstrated that porous structures can promote tissue growth into materials, accelerate bone conduction, and integration between the implant and host bone, and effectively improve early implant stability [20, 21]. The size of interconnected microporous structures between pores is the key factor allowing osteoblasts, osteotropic agents and vasculature to enter the inner pores of materials [22]. Lu et al. [23] reported in porous bioceramics on bone recolonization in vitro and in vivo that the size of interconnected micropores must be over 50 µm to favor new bone growth inside the pores. Furthermore, large interconnected micropores can provide greater space for vascular ingrowth and maturation, which can accelerate nutrient supply for the growth and development of new bone tissues [24]. However, larger pores, high porosity, and the degradation process of biodegradable materials can seriously reduce the mechanical strength of materials [25, 26]. Therefore, most biodegradable materials, such as porous hydroxyapatite and tri-calcium phosphate, could only be applied in unload-bearing parts of bone defects. Thanks to good mechanical strength and non-degradability, porous tantalum scaffolds provide mechanical support for segmental bone defect repair of load-bearing parts. In the present study, the porous tantalum scaffold used was developed by Chongqing Runze Medical Devices Co., Ltd. Under a scanning electron microscope, the material showed a three-dimensional porous structure with appropriate pore size (400–600 µm), interconnected pores (50–200 µm) and porosity (65–80%). Furthermore, the benefits of porous tantalum also include suitable elastic modulus similar to that of bone, higher surface friction, and corrosion resistance. Thus, porous tantalum may be a good alternative materials for application in clinic for bone defect repair of load-bearing parts.
We used rabbit radius segmental bone defects, a common model to assess the validity and security of bone graft materials, and widely applied in segmental bone defect repair in vivo [27–29]. Generally speaking, the length of bone defect needs to exceed self-repairing capability, which is defined as critical-sized bone defect [30]. In many defect models of previous studies, radial defect reaching 15 mm in length could not be repaired by the animal itself [31–33]. Therefore, we chose the 15 mm length of radial defect as the experimental model in the present study. Our results showed that no animals achieved repair by themselves in the blank group, further indicating that the defect length was suitable as experimental model of bone defect in rabbits.
To better assess bone repair ability of porous tantalum scaffold modified with the RGD peptide, we designed the XCB and NMPT groups as control groups. Due to wide source, good bone formation, no complications caused by secondary surgery, and short operation time, XCB is recognized a good bone graft substitute material [34], suitable as control in osteogenesis assessment of porous materials.
In this study, histological observation showed that new bone mass and maturity gradually increased at the interface and inside the inner pores with time in each group, and extended to the material center. The new bone mass and maturity in the MPT group were greatly improved compared with the NMPT group at the three time points assessed, which showed that the surface modification of porous tantalum with the RGD peptide could effectively promote new bone formation. These findings were consistent with previous research evaluating porous materials modified with the RGD peptide [16, 19].
Imaging is also an important evaluation means in the field of bone tissue engineering research. At present, common methods are X-ray and Micro-CT. X-ray examination is mainly used to assess osseointegration between the material and host bone; Micro-CT is employed to evaluate microstructure changes, and particularly applicable to assess small animal bone tissues [31, 35]. In this study, more and more bone callus was formed around the tantalum scaffolds and XCB was gradually replaced by new bone over time. New bone volume fraction analysis by three dimensional reconstruction of Micro-CT images at 16 weeks after surgery showed that there was no statistically significant difference between the MPT and XCB groups, indicating that porous tantalum scaffolds modified with the RGD peptide had similar ability to promote new bone formation compared to XCB scaffolds.
In addition to the above evaluation methods, biomechanical assays are also essential in bone tissue engineering research. In the current study, we used the three point bending test to evaluate mechanical performances of the bone interface between the implant and host bone. At 16 weeks postoperatively, Fmax and bending strength in the XCB group were lower than in the MPT and NMPT groups (P < 0.01), indicating that tantalum has better biomechanical properties and matches the host bone to effectively reduce intraosseous stress shield and bone resorption [36, 37]. Meanwhile, the MPT group showed higher values compared with the NMPT group (P < 0.01), which reached 90.5 and 85.7% of normal radius, respectively, further indicating that tantalum modified with the RGD peptide has an excellent potential for repairing bone defects in rabbit radius.
However, this study has shortcomings. First, the modification method and cyclo(-RGDfK-) peptide density used was based on other biological materials, and we did not optimize concentration and the modification method for the Chinese porous tantalum. Therefore, further improvements are needed in future experiments. Second, the animals were observed for only 16 weeks after surgery. For segmental bone defects, although bone modeling could be basically completed, more time may be still needed to observe changes in bone formation, which would be also included in next experiments.
5 Conclusions
In summary, segmental bone defect in a rabbit radius model was repaired using porous tantalum scaffolds modified with the cyclic (-RGDfK-) peptide in this study. By means of imaging, histologic and biomechanical evaluations, it was confirmed that Chinese porous tantalum scaffolds modified with the cyclic (-RGDfK-) peptide with appropriate pore size and high porosity, have excellent tissue compatibility, biomechanical properties, and osteoconductive potential to repair segmental bone defects, and might become a promising bone graft substitute material in clinical practice.
References
Han CS, Wood MB, Bishop AT, Cooney WP. Vascularized bone transfer. J Bone Joint Surg Am. 1992;74:1441–9.
Soucacos PN, Dailiana Z, Beris AE, Johnson EO. Vascularised bone grafts for the management of non-union. Injury. 2006;37:S41–50.
Fujioka M, Hayashida K, Murakami C. Vascularized bone graft is a better option for the reconstruction of maxillary defects. Eur Arch Otorhinolaryngol. 2013;270:2779–81.
Brekke JH, Toth JM. Principles of tissue engineering applied to programmable osteogenesis. J Biomed Mater Res. 1998;43:380–98.
Deschamps AA, Claase MB, Sleijster WJ, de Bruijn JD, Grijpma DW, Feijen J. Design of segmented poly (ether ester) materials and structures for the tissue engineering of bone. J Control Release. 2002;78:175–86.
Ryan G, Pandit A, Apatsidis DP. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials. 2006;27:2651–70.
Yang J, Chen HJ, Zhu XD, et al. Enhanced repair of a critical-sized segmental bone defect in rabbit femur by surface microstructured porous titanium. J Mater Sci Mater Med. 2014;25:1747–56.
Zhang M, Wang GL, Zhang HF, et al. Repair of segmental long bone defect in a rabbit radius nonunion model: comparison of cylindrical porous titanium and hydroxyapatite scaffolds. Artif Organs. 2014;38:493–502.
Kamath AF, Lewallen DG, Hanssen AD. Porous tantalum metaphyseal cones for severe tibial bone loss in revision knee arthroplasty: a five to nine-year follow-up. J Bone Joint Surg Am. 2015;97:216–23.
Wigfield C, Robertson J, Gill S, Nelson R. Clinical experience with porous tantalum cervical interbody implants in a prospective randomized controlled tria. Br J Neurosurg. 2003;17:418–25.
Zhang Y, Li L, Shi Z, Wang J, Li ZH. Porous tantalum rod implant is an effective and safe choice for early-stage femoral head necrosis: a meta-analysis of clinical trials. Eur J Orthop Surg Traumatol. 2013;23:211–7.
Pakos EE, Megas P, Paschos NK, et al. Modified porous tantalum rod technique for the treatment of femoral head osteonecrosis. World J Orthop. 2015;6:829–37.
Elmengaard B, Bechtold JE, Søballe K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 2005;26:3521–6.
Mas-Moruno C, Dorfner PM, Manzenrieder F, et al. Behavior of primary human osteoblasts on trimmed and sandblasted Ti6Al4V surfaces functionalized with integrin αvβ3-selective cyclic RGD peptides. J Biomed Mater Res A. 2013;101:87–97.
Wang Q, Zhang H, Li Q, et al. Biocompatibility and osteogenic properties of porous tantalum. Exp Ther Med. 2015;9:780–6.
Kantlehner M, Schaffner P, Finsinger D, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1:107–14.
Magdolen U, Auernheimer J, Dahmen C, et al. Growth promoting in vitro effect of synthetic cyclic RGD-peptides on human osteoblast-like cells attached to cancellous bone. Int J Mol Med. 2006;17:1017–21.
Mas-Moruno C, Garrido B, Rodriguez D, et al. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J Mater Sci Mater Med. 2015;26:1–12.
Kroese-Deutman HC, van den Dolder J, Spauwen PH, et al. Influence of RGD-loaded titanium implants on bone formation in vivo. Tissue Eng. 2005;11:1867–75.
Periasamy K, Watson WS, Mohammed A, et al. A randomised study of peri-prosthetic bone density after cemented versus trabecular fixation of a polyethylene acetabular component. J Bone Joint Surg Br. 2011;93:1033–44.
Sinclair SK, Konz GJ, Dawson JM, Epperson RT, Bloebaum RD. Host bone response to polyetheretherketone versus porous tantalum implants for cervical spinal fusion in a goat mode. Spine. 2012;37:E571–E80.
Yoshikawa H, Tamai N, Murase T, Myoui A. Interconnected porous hydroxyapatite ceramics for bone tissue engineering. J R Soc Interface. 2009;6:S341–8.
Lu JX, Flautre B, Anselme K, et al. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J Mater Sci Mater Med. 1999;10:111–20.
Bai F, Wang Z, Lu J, et al. The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: a quantitative study. Tissue Eng Part A. 2010;16:3791–803.
Barralet JE, Grover L, Gaunt T, Wright AJ, Gibson IR. Preparation of macroporous calcium phosphate cement tissue engineering scaffold. Biomaterials. 2002;23:3063–72.
Lin AS, Barrows TH, Cartmell SH, Guldberg RE. Microarchitectural and mechanical characterization of orientedporous polymer scaffolds. Biomaterials. 2003;24:481–9.
Kroese-Deutman HC, Vehof JW, Spauwen PH, Stoelinga PJ, Jansen JA. Orthotopic bone formation in titanium fiber mesh loaded with platelet-rich plasma and placed in segmental defects. Int J Oral Maxillofac Surg. 2008;37:542–9.
Roohani-Esfahani SI, Dunstan CR, Davies B, Pearce S, Williams R, Zreiqat H. Repairing a critical-sized bone defect with highly porous modified and unmodified baghdadite scaffolds. Acta biomater. 2012;8:4162–72.
Kim J, McBride S, Donovan A, Darr A, Magno MH, Hollinger JO. Tyrosine-derived polycarbonate scaffolds for bone regeneration in a rabbit radius critical-size defect model. Biomed Mater. 2015;10:035001
Schmitz JP, Hollinger JO. The critical size defect as an experimental model for cranion mandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308.
Hedberg EL, Kroese-Deutman HC, Shih CK, et al. Methods: a comparative analysis of radiography, microcomputed tomography, and histology for bone tissue engineering. Tissue Eng. 2005;11:1356–67.
Han D, Li J. Repair of bone defect by using vascular bundle implantation combined with Runx II gene-transfected adipose-derived stem cells and a biodegradable matrix. Cell Tissue Res. 2013;352:561–71.
Li X, Lin Z, Duan Y, et al. Repair of large segmental bone defects in rabbits using BMP and FGF composite xenogeneic bone. Genet Mol Res. 2015;14:6395–400.
Athanasiou VT, Papachristou DJ, Panagopoulos A, et al. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: an experimental study in rabbits. Med Sci Monit. 2010;16:BR24–31.
Wang G, Zhao S, Yu H, et al. Design, analysis and simulation for development of the first clinical micro-CT scanner. Acad Radiol. 2005;12:511–25.
Huiskes R, Weinans H, Van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res. 1992;274:124–34.
Hanzlik JA, Day JS, Rimnac CM, Kurtz SM. Is There A Difference in Bone Ingrowth in Modular Versus Monoblock Porous Tantalum Tibial Trays? J Arthroplasty. 2015;30:1073–8.
Acknowledgements
We thank Dr. Jun Wang and Pengzhen Cheng from Xijing Hosipital affiliated, Fourth Military Medical University of China, for technical help in Micro-CT and biomechanical tests. This study was supported by the National Key Technology Support Program of China (Contract Grant No. 2012BAE06B03).
Author contributions
Z.W. and Q.L. conceived and designed the study; H.W., Q.W., H.Z., W.S., H.G., and H.S. performed the experiments; H.W., Z.W., and Q.L. analyzed the data; H.W. drafted the article; Z.W., Q.L., and H.S. revised the article. All authors approved the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Wang, H., Li, Q., Wang, Q. et al. Enhanced repair of segmental bone defects in rabbit radius by porous tantalum scaffolds modified with the RGD peptide. J Mater Sci: Mater Med 28, 50 (2017). https://doi.org/10.1007/s10856-017-5860-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5860-4