Abstract
Craniofacial bone defects such as alveolar cleft affect the esthetics and functions that need bone reconstruction. The advanced techniques of biomaterials combined with stem cells have been a challenging role for maxillofacial surgeons and scientists. PCL-coated biphasic calcium phosphate (PCL-BCP) scaffolds were created with the modified melt stretching and multilayer deposition (mMSMD) technique and merged with human dental pulp stem cells (hDPSCs) to fulfill the component of tissue engineering for bone substitution. In the present study, the objective was to test the biocompatibility and biofunctionalities that included cell proliferation, cell viability, alkaline phosphatase activity, osteocalcin, alizarin red staining for mineralization, and histological analysis. The results showed that mMSMD PCL-BCP scaffolds were suitable for hDPSCs viability since the cells attached and spread onto the scaffold. Furthermore, the constructs of induced hDPSCs and scaffolds performed ALP activity and produced osteocalcin and mineralized nodules. The results indicated that mMSMD PCL-BCP scaffolds with hDPSCs showed promise in bone regeneration for treatment of osseous defects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Craniofacial bone defects from trauma, tumor or alveolar cleft affect a patient’s quality of life due to an abnormal facial appearance and malfunction. The reconstruction of bony destruction using autogenous bone from the iliac crest is the gold standard. However, there are weak points, for instance, donor site morbidity, pain, blood loss and also the limitation of donor volume [1]. Tissue engineering is a developing technology used to reconstruct the damaged area or lost tissue such as bone. Bone tissue engineering provides repair or a substitute for the bony defect by proper biomaterials, such as scaffold applications with osteoinducing molecules like growth factors and osteogenic cells to create normal form and function [2]. The requirements of biomimetic scaffolds need to include not only biocompatibility with the recipient tissues, but also the appropriate porosity for the osteogenic cells to permit attachment and proliferation and a proper degradability rate as well. The degradability of bioscaffolds is important due to natural tissue replacement later [3]. Specifically for dentoalveolar reconstruction, suitable scaffolds have to degrade between 5 and 6 months considering the ideal materials for new bone formation processes and also remodeling [4].
The numerous types of biomaterials developed for scaffolds can be classified into natural and synthetic polymers, ceramics and composites. For example, the natural polymers are collagen and chitosan. Other polymers like poly-ε-caprolactone (PCL), polyglycolic acid (PGA), polylactic acid (PLA) have been synthetically produced. Poly-ε-caprolactone (PCL) is a semi-crystalline aliphatic polyester with good biocompatibility and biodegradation [5] and can be processed easily at the low melting point of around 60 °C. Moreover, the United States FDA approved this polymer as clinically safe [6]. Therefore, it can be an appropriate material for scaffold production. Although PCL presents a gradual degradation of two years among the available biodegradable synthetic polymers, the degradation products can be resorbed through the metabolic pathway and do not cause acidosis to the surrounding tissues [7].
Hydroxyapatite (HA) and beta-tricalcium phosphate (β-TCP) are defined as ceramics that provide chemical similarity to bone mineral components. In addition, these ceramics show the important benefits over autograft and allograft not only in biocompatibility and bioactivity, but also in osteoconduction, unlimited availability and cost effectiveness [8]. The mixtures of HA and β-TCP are referred to as biphasic calcium phosphates (BCP) that have synergistic properties that offer more strength and less resorbability The suitability of BCP granules (β-TCP/HA = 70:30) act as suitable carriers to induce osteoblast cells for bone healing [9]. In addition, the composite scaffolds are made from two distinct scaffolds as ceramics mixed with synthetic polymers to improve the material properties from the benefits of each component, which complement each other [10].
The novel technique of 3-D scaffold fabrication is melt stretching and multilayer deposition (MSMD) which is a melting and blending of the polymer with other compositions. This technique seems to be the appropriate fabrication method because of the cleanliness and the blending is without any solvents or porogens. Therefore, MSMD scaffolds would be suitable as bone inducing materials to enhance osteogenesis which were found to have a microgroove pattern on their surfaces and proved to support cellular conduction and osteoblast attachment as well as protein loading and release [11, 12]. Therefore, this innovation to fabricate scaffolds brings the components of PCL and BCP together to form PCL-BCP composite scaffolds. The significant relevance as potential biomaterials for bone tissue regeneration is due to the improvement of the mechanical and bioactivity of these materials. The proper ratio of 80 PCL and 20% BCP was selected to make the scaffold filaments considering easier fabrication and possible reduction in the amount of BCP [13].
Recent studies reported that stem cells serve as possible treatment options. Human bone marrow stem cells (hBMSCs) are frequently found in clinical reports [14]. Nevertheless, the main issues of hBMSCs are not only the additionally surgical procedures to harvest the cells but also the limited volumes of the cells [15]. Human dental pulp stem cells (hDPSCs) are known as multipotent stem cells that are easily obtained from extracted teeth from routine dental procedures and minimal tissue site morbidity. The patients perceive little pain experience and also obtain an effective procedure. Several hDPSCs studies have shown clonogenic ability, rapid proliferation rate, multipotential plasticity and cryopreservation for a long time as well [16]. In addition, Gronthos and colleagues [17] reported that hDPSCs are similar to bone marrow stem cells (BMSCs) due to the ability to differentiate into various cell types, such as osteoblasts, odontoblasts, adipocytes, or neuron lineage in vitro that depends upon the type of induction media [18]. Moreover, DPSCs that were transplanted in animal experiments could form new bone with extended vascularization [19–21]. These reasons make hDPSCs more pleasant as cell sources in clinical use for bone regeneration.
The rabbit calvarial model was selected from anatomical calvaria similar to the palate and mandible [22]. Eventually, this model proved to be proper for analysis of the graft reconstruction [23, 24]. However, to the best of our knowledge, the behavior of DPSCs on the novel PCL-BCL composite scaffold transplanted into animals has not been proven. Therefore, our aim of this study was to evaluate the modified melt stretching and multilayer deposition of a PCL-BCP scaffold that acts as a suitable scaffold to support the attachment, proliferation and osteogenic potentials of induced hDPSCs in vitro to improve bone tissue regeneration in clinical applications.
2 Materials and methods
2.1 Fabrication of 3D PCL-BCP scaffolds
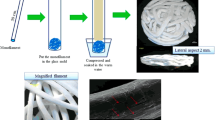
The scaffolds were prepared from monofilaments of PCL-BCP that were composed of PCL and BCP in the ratio of 80/20 by the MSMD technique. Briefly, the raw materials were PCL pellets (\( {{\bar{\rm M}}_{\rm n}} \) 80,000 PC, Sigma Aldrich, USA) and BCP (HA:β-TCP 30/70 particles, particle sizes <75 µm, MTEC, Thailand) [25]. The components were mixed together in the ratio as mentioned above by weight and melted in the melting-extruding machine [11]. The PCL-BCP mixtures were extruded into monofilaments through the spouted tip of the machine and the filaments were then stretched to reduce their size. Thereafter, to fabricate the 3D scaffolds a single filament (50 cm in length) was cut and placed into a 5-cc glass syringe to serve as the mold. The syringe plunger was pushed to reach the reference point of 3 mm from the bottom of the syringe to cause the filaments to be in close contact. The tip of the syringe was sealed with polyvinyl siloxane (3M ESPE, USA) and then the syringe was immerged in warm double distilled water to fuse the contact surfaces of the filaments together and form 3 D structures (diameter 11 mm, height 3 mm) (Fig. 1). All scaffolds were cleaned with ethylene oxide gas before use in the experiments.
Fabrication process of PCL-BCP scaffolds using the mMSMD technique. 1 PCL-BCP monofilament was placed in a glass syringe mold and compressed. 2 The mold was immersed in warm water allowing the contact points of the filament to fuse together and form a 3D scaffold. 3 Superior and lateral aspects of the morphology of the PCL-BCP scaffolds
2.2 Isolation and characterization of hDPSCs
Human dental pulp tissue was harvested from impacted third molars extracted from healthy adults (18–25 years old) due to orthodontic considerations at the Department of Oral Surgery, Dental Hospital, Faculty of Dentistry, Prince of Songkla University. The Research Ethics Committee (REC) of the Faculty of Dentistry, Prince of Songkla University approved this research protocol (project No. EC5709-22-P-HR) on 4 December 2014 and all subjects gave informed consent for the use of their dental pulps. hDPSCs were isolated from five teeth that were cleaned and drilled around the cementum-enamel junction using sterilized dental fissure burs until the pulp chamber was exposed. The pulp tissues were pooled together [26] and soaked in a solution of 3 mg/mL of collagenase type I (Gibco-Invitrogen) and 4 mg/mL of dispase (Gibco-Invitrogen) for 45 min with gentle shaking on a horizontal orbital microplate shaker set at 130 rpm in a CO2 incubator (5% CO2) at 37 °C and 95% humidity. Single-cell suspensions were prepared by filtering through a 70-μm Falcon strainer (Becton & Dickinson, Franklin Lakes, NJ, USA) and centrifuged at 1800 rpm for 5 min [27]. The cell pellets were resuspended in the growth medium containing the alpha modification of Eagle’s medium (α-MEM) (Gibco-Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Gibco-Invitrogen), 100 U/mL penicillin, 100 µg/mL streptomycin (Gibco-Invitrogen) and 1% fungizone (250 µg/mL) (Gibco-Invitrogen). Thereafter, the resuspended cells were cultured in 75 cm2 flasks and incubated in the CO2 incubator (5% CO2) at 37 °C and 95% humidity. The culture medium was replaced every 2–3 days and the cells were subcultured at 70–80% confluence. Non-adherent cells were removed from the culture after 3 days by PBS washing and fresh medium change. Cells at passages 3–5 were used in all experiments. Human gingival fibroblasts (hGFs) and osteoblast sarcoma cell lines (MG-63) were used for negative and positive controls, respectively [28–30].
2.3 Immunophenotypic characterization
The MSCs immunophenotypes of hDPSCs were defined following the International Society for Cellular Therapy (ISCT) protocols [13]. Briefly, examination of the MSC cell surface markers using a cell suspension of approximately 1 × 107 cells of hDPSCs were tested with a preconjugated antibody cocktail in a Human MSC Analysis kit (BD Biosciences) by flow cytometry using FACS Calibur instrument (BD Biosciences). The data processing and analysis used FACS DIVA software (BD Biosciences).
2.4 Self-renewal capacity: colony-forming unit fibroblast (CFU-F) assays
The stemness of the hDPSCs was determined by the colony-forming unit fibroblast (CFU-F) assay. Following the seeding of 100 cells/well in a 6-well plate (Costar), the cells were cultured for 10 days. The cells were then fixed with 4% paraformaldehyde (Sigma) and stained with 0.1% toluidine blue (Sigma). The number of colonies was counted from aggregations of more than 50 cells or greater than 2 mm colonies in diameter. Observation of the cells was performed by a light microscope (Nikon) [31].
2.5 Cell seeding onto the PCL-BCP scaffolds
hDPSCs were seeded onto PCL-BCP scaffolds to determine their ability to differentiate. Gingival fibroblasts were used as the negative control and the MG-63 cell line from ATCC, Rockville, MD, USA was used as the positive control. The cells were seeded onto the scaffolds in each group (n = 5/group/time point). Before starting, the scaffolds were pre-wetted in the culture medium for 24 h. Then 1 × 105 cells were seeded onto the scaffolds (Fig. 2) [13]. The cell-scaffold constructs were cultivated in 5% CO2 at 37 °C for 3 days in order to make sure the cells were attached onto the scaffolds. After that the culture medium was changed to an osteogenic medium supplemented with 5 mM β-glycerophosphate (Sigma, USA), 500 nM dexamethasone (Sigma, USA) and 50 µg/mL ascorbic acid (Sigma, USA). The medium were changed every 2–3 days until the experiment ended.
2.6 Cell proliferation assay (PrestoBlue®: days 1, 3, 5 and 7)
Cell proliferation assays were performed by PrestoBlue®, a resazurin-based solution, according to the manufacturer’s instructions (PrestoBlue® Cell Viability Reagent, Invitrogen, USA) to measure the activity of mitochondrial dehydrogenases through the reducing process in the cytoplasm that reacts with resazurin (purple color) to form resorufin (red color) to estimate the number of viable cells and measured on days 1, 3, 5 and 7. In brief, the constructs were washed twice with PBS, then a 1/10th volume of PrestoBlue reagent was added directly into the culture media and incubated for 1 h at 37 °C. Thereafter, samples (100 µL) of the solution from each well were measured in duplicate by transferring to a 96-well plate at the wavelength absorbance of 570 nM excitation and 600 nM emission using a microplate reader [32].
2.7 Morphology observation: scanning electron microscope (SEM)
The cell morphology and attachment on the scaffolds were monitored by SEM (JEOL 6360 LV) at an accelerating voltage of 15 kV. On days 7, 14 and 21 of the experiment, the constructs were washed with phosphate buffer saline (PBS) and fixed with 2.5% glutaraldehyde in PBS for 2 h. Thereafter, dehydration was processed in an ethanol series of 60–100%, then dried and coated using a gold sputter coater machine (SPI Supplies, Division of STRUCTURE PROBE Inc., Westchester, PA USA).
2.8 Cell viability evaluation: confocal laser scanning microscope (CLSM)
The cell viability of the seeded hDPSCs within the scaffolds and cultured in growth medium was assessed at day 14 using 4 μg/mL of fluorescein diacetate (FDA) (Sigma-Aldrich) combined with propidium iodide (PI) 40 μg/mL (Sigma-Aldrich) staining that showed green and red staining of viable cells and apoptotic cell nuclei, respectively. Thereafter, samples were inspected under a confocal laser scanning microscope (Olympus, FV1000 Fluoview, Japan) [33].
2.9 Alkaline phosphatase activity
Alkaline phosphatase (ALP) activity is an early osteogenic marker of the osteoblasts. ALP activity was quantified at days 3, 7, 14 and 21 as a method to assess osteogenic differentiation. The lysated cell solutions were thawed on ice and 10 µL of the solutions in each tube were mixed with 500 µL of the ALP solutions (60 mmol/L p-nitrophenylphosphate [substrate] in 435 mmol/L 2-amino-2-methyl-1-propanolol [AMP buffer]) (ab83369, Abcam). The cells were incubated for 30 min at room temperature. The optical density of the yellow para-nitrophenol solutions were measured using a microplate reader at 405 nM (Multiscan™ Go, Thermo Fisher Scientific). A triplicate of each sample was analysed. The ALP activity values were normalized to the total protein content (µM p-nitrophenol/mg protein) [34].
2.10 Osteocalcin assay
Osteocalcin (OCN) is considered a late osteogenic marker and a specific protein of the osteoblast. The assay measures the entire OCN synthesized by the osteoblast like cells. Quantification of OCN on days 7, 14 and 21 was performed following the manufacturer’s instructions using the Quantikine Osteocalcin Elisa kit (R&D systems, Inc., MN, USA). The optical densities of the solutions were measured using a microplate reader at 450 nM (Multiscan™ Go, Thermo Fisher Scientific) and the concentrations were calculated from the serial diluted standard solution. Concentrations of OCN were extrapolated from a standard curve of serial dilution of highly purified human OCN and reported as ng/mL [35].
2.11 Mineralization assay
Alizarin red staining assay was used to determine the calcium phosphate mineral nodules (matrix mineralization) on the scaffolds. The constructs were cultured for 3, 7, 14 and 21 days. On the day of the experiment, they were double washed with PBS and fixed with 4% paraformaldehyde for 10 min and 1 mL of alizarin red solution (2 g in 100 mL of distilled water and pH adjusted to 4.1–4.3) was added. The constructs were incubated at room temperature for 20 min with protection from the light. The alizarin red solution was carefully washed from the constructs around four times with distilled water. Mineralization nodules were observed via a microscope [36].
For quantification, the Alizarin red S stained constructs were extracted. In brief, 800 mL of 10% acetic acid was added to the stained constructs for 30 min. After that the supernatant was moved into a 1.5 mL Eppendorf tube and heated for 10 min at 85 °C. Thereafter, centrifugation at 12,500 rpm for 15 min was performed and 100 µL samples were transferred into a 96-well plate and the optical density was measured using the microplate reader at 405 nm (Multiscan™ Go, Thermo Fisher Scientific) [31].
2.12 Statistical analysis
The statistical measurements of samples were tested by one-way ANOVA followed by Tukey’s HSD test (SPSS 16.0 software package). Statistical significance was determined as * = P < 0.01 and # = P < 0.05.
3 Results
3.1 Characterization of hDPSCs by flow cytometric analysis
The levels of surface marker expression were determined by flow cytometry following the ISCT criteria [37]. The cells were shown to be positive for CD44 (99.80%), CD73 (99.79%), CD90 (99.75%), and CD105 (99.98%) with low expression for hematopoietic stem cell markers CD34, CD45, CD11b, CD19 and HLA-DR (1.68%) (Fig. 3a).
3.2 Colony-forming unit fibroblastic (CFU-F) assay
The isolated hDPSCs were able to form colonies from the low seeding density that characterized their self-renewal capacity (Fig. 3b).
3.3 Determination of cell proliferation by PrestoBlue assay
The proliferation of hDPSCs seeded onto the PCL-BCP scaffolds were compared with the negative (hGF) and positive (MG-63) control groups using the PrestoBlue assay at 1, 3, 5 and 7 days (Fig. 4). The results from the first day found that the hGFs had the highest growth that was statistically significant (P = 0.00), while the growth potentials of the hDPSCs and MG63 performed equally well but were statistically insignificant (P = 0.855). On the third day, MG63 control group had a larger proliferation than the others but it was not significant compared to the hDPSCs (P = 0.553). Moreover, the potential of the hDPSCs was greater than the hGFs with statistical significance (P = 0.00). Eventually the fifth and seventh days found similar results in terms of the greatest growth of MG63 cells (P < 0.01 and P < 0.05 when compared to the hDPSCs and hGFs sequentially); nonetheless, the hGFs could perform proliferation nearly as well as the hDPSCs (P = 0.124 and 0.878, respectively).
3.4 Evaluation of cell attachment of hDPSCs seeded on the PCL-BCP scaffolds using scanning electron microscopy (SEM)
The hDPSCs were attached on the PCL-BCP scaffolds from day 7, 14 and 21 that eventually increased and distributed throughout the scaffolds upon the time periods and filopodia connections were found. On day 21, the hDPSCs formed the appearance of a cell sheet (Fig. 5a).
Evidence of hDPSC attachment on the mMSMD PCL-BCP scaffold a SEM on days 7, 14 and 21 b Confocal laser images on day 14: filament surface of the scaffold a and three dimensional structures of cells scaffold construct b, c and d. c Histology of hematoxylin and eosin (H&E) staining of hDPSCs cultured on PCL-BCP scaffolds on day 7. Scale bars = 100 μm (a, b, and c), Scale bars = 50 μm (d)
3.5 Viability evaluation of hDPSCs seeded on the PCL-BCP scaffolds using FDA and PI staining by confocal laser scanning microsopy (CLSM)
The confocal laser images showed that the hDPSCs substantially exhibited viability from the green color of the FDA and had the capability to attach onto the scaffold surface, whereas the red color was found to be insignificant (Fig. 5b).
3.6 Alkaline phosphatase activity of hDPSCs differentiated into osteogenic lineage on scaffolds
Osteogenic medium was used for induction of differentiation of all three cell types at four time points (3, 7, 14 and 21 days). ALP is an enzyme produced by differentiating osteoblasts which is manifested as a differentiation marker of osteoblastic lineage. The hDPSCs and MG-63 groups revealed a relevant increase in ALP activity in the first seven days, while hGF showed an insignificant increase of ALP activity at all time points (Fig. 4).
3.7 Osteocalcin assay
The effect of osteogenic induction to the amount of OCN released from hDPSCs seeded on the PCL-BCP scaffolds constantly rose in 14 and 21 days that was significantly higher than both of the other cells in the same condition on the scaffolds (P < 0.05) (Fig. 4).
3.8 Mineralization assay
Alizarin red S staining determined the presence of calcium mineralization that also revealed the capacity to differentiate toward osteoblasts. The quantification of the levels of Alizarin red S extraction progressively increased in both the hDPSCs and MG-63 cells throughout the culture periods. Moreover, the hDPSCs were remarkably at higher levels at 21 days than the other cells (Fig. 4).
3.9 Histological evaluation
Histological evaluation was used to observe the morphology and to define the cell organization on the cell scaffold constructs. The dental pulp stem cells had clearly spread and were attached thoroughly to the scaffold surface on day 7 (Fig. 5c).
4 Discussion
In the present study, the bioactive mMSMD PCL-BCP scaffolds were used to demonstrate biocompatibility of hDPSCs with a high cellular proliferation assay and osteogenic induction of key osteogenic proteins such as ALP activity and OCN and eventually extracellular matrix (ECM) deposition of Alizarin red staining with quantitative analysis. These three dimensional fabrication techniques of mMSMD are simple and easy with adequate mechanical properties to endure the forces of load bearing implantation sites. The scaffolds can also be fabricated to provide high porosity and a large interconnecting pore system to support cell infiltration and bone tissue ingrowth [38, 39]. Intraoperative fabrication could be done if necessary compared with other methods. In addition, no solvents or substrates are needed during fabrication, so this mMSMD technique allows the two components of the small granules of BCP to interact and fuse closely with the PCL polymers. The most significant advantage of this technique is caused by the simple stretching force that results in reduction of the filament diameter. The alignment and folding in the syringe during the heat curing step generated a complex porosity and space to permit the penetration of nutrients and oxygen in the implantation regions.
The BCP components in the presently fabricated scaffolds play an important role as the filler to increase the strength of the scaffold. Nevertheless, the BCP components are also considered to have bioactive ceramic properties. The composition of the scaffolds also possesses HA and β-TCP which is similar to the mineral phase of natural bone. They provide osteoconductive properties that are also reported to be intrinsically osteoinductive because there were some studies that reported the proliferation and differentiation of osteoprogenitor cells from the degradation products of bioceramics [40]. The ratio of HA/β-TCP in the present scaffold was 30/70 which has been proven to remarkably support cell attachment and growth including osteogenic markers as ALP [32]. However, the disadvantages of BCP were considered as weak for use in defects. So, the limitation is for non-load bearing applications [41]. Therefore, the bioceramics in the present scaffold needed modification of their properties with polymers to increase the toughness of the scaffold.
In this study, the PCL polymer was combined with the BCP. The PCL as a synthetic polymer has been used vastly in drug vehicles and tissue engineering. PCL properties have shown biocompatibility, gradual biodegradation with non-toxicity to the cells from its hydrophobic and semi-crystalline material with a slow hydrolytic degradation rate and also adequate mechanical strength for load bearing areas. Therefore, it has been approved for use by the U.S. Food and Drug Administration (FDA) [42]. In addition, mMSMD PCL-BCP composite scaffolds in a previous study showed similar compressive strength compared to trabecular bone (2–10 MPa) [43].
The cells used in the present study were hDPSCs which are a promising stem cell source for bone tissue engineering. The stem cell characteristics of the hDPSCs were proven prior to the cell seeding process using immunophenotype and colony-forming capacity to confirm the reliability of this study. In addition, hDPSCs were chosen due to easier accessibility compared to other sources. Besides, these cells had minimal tissue site morbidity and were easily isolated and expanded in the culture. Proven evidence showed that stem cells from dental pulp had more clonogenic ability and a rapid proliferative rate equal to human bone marrow stem cells (hBMSCs) [17]. A study by Gronthos and co-workers in 2000 revealed that both hDPSCs and hBMSCs have the multipotential functions that are able to differentiate into diverse cell types such as osteoblasts, odontoblasts, adipocytes, and neuron lineage in vitro that depends on the inductive media [18]. Other studies also reported the ability of DPSCs to differentiate into osteoblasts and endothelial cells and were capable of forming woven bone [20].
The seeded cells could proliferate on the mMSMD PCL-BCP scaffolds as well as the hGFs and MG-63 cells as the negative and positive controls, respectively. Therefore, the PCL-BCP scaffolds that were fabricated by the mMSMD technique would be biocompatible with non-toxicity. Furthermore, the SEM, CLSM and histology demonstrated that the cells could obviously attach and the majority of the cells were distributed with viability on the scaffold. In addition, progressive hDPSCs were found to spread over the scaffold surfaces. After 14 and 21 days, these cells formed a layer of cells on the scaffold surfaces. Therefore, this evidence supported the biocompatibility of the mMSMD PCL-BCP scaffolds that conformed to Zhang and colleagues in 2009 [33]. They studied four other stem cell types such as fetal, umbilical, adipose, and bone marrow. They found that all four types could be viable on the PCL-TCP scaffolds. The cellular viability of the MSCs within the scaffolds was demonstrated by staining with FDA (green) and PI (red) which were retained by live and dead cells at the same time. In addition, the metabolic function of bone markers such as ALP and OCN were investigated during osteogenic differentiation and Alizarin red staining detected mineralization.
The ALP activity of hDPSCs and MG-63 seeded onto the mMSMD scaffolds showed similar characteristics and a peak on day 7 of early phase mineralization. On the other hand, ALP activity of the hGFs was significantly different from other groups which was caused by a lack of differentiation potential. The low proliferation rate of those cells on day 7 was likely due to increased expression of ALP that indicated these cells had started to undergo bone lineage responsibility [44–46]. Goh and colleagues [47] studied human fetal mesenchymal stem cells (hfMSCs) with PCL-TCP that showed ALP expression that peaked at day 7 during osteogenic differentiation as well. The typical MSC differentiation regarding ALP peaking at day 7 was also described by others [48–51]. OCN assay results conformed with late stage mineralization and gradually increased until day 21. Both hDPSCs and MG63 cells readily demonstrated osteogenic differentiation in these scaffolds. Nevertheless, OCN expression from the hGFs showed a low differentiation potential similar to the ALP activity results which was probably caused by a lack of osteogenic differentiation potential. The results of the early and late bone marker activity test in the present study obviously proved that the mMSMD PCL-BCP scaffold radically improves the ability of hDPSCs to organize bone tissue formation. Besides, the functional behavior could be used to discriminate the MSCs from the fibroblasts [52]. The calcified matrix was detected by Alizarin red S staining and carried out through its extraction. The quantitative extracted results also increased gradually to a peak on day 21. The results from the bone marker and mineralization analysis of the cell-scaffold constructs showed similar evidence of new bone formation enhancement. Therefore, these mMSMD PCL-BCP scaffolds could be biocompatible and efficiently used to enhance cell function to promote bone regeneration. The transplantation of cellular scaffold constructs into preclinical critical-sized craniofacial bony defect models will be the next step.
5 Conclusions
This study demonstrated that mMSMD PCL-BCP scaffolds possess the appropriate structure and favorable environment to promote cellular function and osteogenic capacity when seeded with hDPSCs and provide potential benefits for bone tissue regeneration in future clinical applications.
References
Tevlin R, McArdle A, Atashroo D, Walmsley GG, Senarath-Yapa K, Zielins ER, et al. Biomaterials for craniofacial bone engineering. J Dent Res. 2014;93:1187–95.
Zuk PA. Tissue engineering craniofacial defects with adult stem cells? Are we ready yet? Pediatr Res. 2008;63:478–86.
Patel M, Fisher JP. Biomaterial scaffolds in pediatric tissue engineering. Pediatr Res. 2008;63:497–501.
Lang NP, Brägger U, Hämmerle CH, Sutter F. Immediate transmucosal implants using the principle of guided tissue regeneration. I. rationale, clinical procedures and 30-month results. Clin Oral Implants Res. 1994;5:154–63.
Elfick AP. Poly(epsilon-caprolactone) as a potential material for a temporary joint spacer. Biomaterials. 2002;23:4463–7.
Pena J, Corrales T, Izquierdo-Barba I, Doadrio AL, Vallet-Regi M. Long term degradation of poly(ε-caprolactone) films in biologically related fluids. Polym Degrad Stabil. 2006;91:1424–32.
Chuenjitkuntaworn B, Inrung W, Damrongsri D, Mekaapiruk K, Supaphol P, Pavasant P. Polycaprolactone/hydroxyapatite composite scaffolds: preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J Biomed Mater Res A. 2010;94:241–51.
Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31:1465–85.
Lomelino Rde O, Castro-Silva II, Linhares AB, Alves GG, Santos SR, Gameiro VS, et al. The association of human primary bone cells with biphasic calcium phosphate (betaTCP/HA 70:30) granules increases bone repair. J Mater Sci Mater Med. 2012;23:781–8.
O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14:88–95.
Thuaksuban N, Nuntanaranont T, Pattanachot W, Suttapreyasri S, Cheung LK. Biodegradable polycaprolactone-chitosan three-dimensional scaffolds fabricated by melt stretching and multilayer deposition for bone tissue engineering: assessment of the physical properties and cellular response. Biomed Mater. 2011;6:015009
Thuaksuban N, Nuntanaranont T, Suttapreyasri S, Pattanachot W, Sutin K, Cheung LK. Biomechanical properties of novel biodegradable poly epsilon-caprolactone-chitosan scaffolds. J Investig Clin Dent. 2013;4:26–33.
Thuaksuban N, Luntheng T, Monmaturapoj N. Physical characteristics and biocompatibility of the polycaprolactone-biphasic calcium phosphate scaffolds fabricated using the modified melt stretching and multilayer deposition. J Biomater Appl. 2016;30:1460–72.
Sousa BR, Parreira RC, Fonseca EA, Amaya MJ, Tonelli FM, Lacerda SM, et al. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytom A. 2014;85:43–77.
Grassel S, Lorenz J. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: use of mesenchymal stem cells. Curr Rheumatol Rep. 2014;16:452
Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch Oral Biol. 2012;57:1439–58.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–71.
d’Aquino R, De Rosa A, Laino G, Caruso F, Guida L, Rullo R, et al. Human dental pulp stem cells: from biology to clinical applications. J Exp Zool B Mol Dev Evol. 2009;312B:408–15.
Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980;38:176–80.
Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990;1:60–8.
Sweeney TM, Opperman LA, Persing JA, Ogle RC. Repair of critical size rat calvarial defects using extracellular matrix protein gels. J Neurosurg. 1995;83:710–5.
Ebrahimi M, Pripatnanont P, Monmaturapoj N, Suttapreyasri S. Fabrication and characterization of novel nano hydroxyapatite/beta-tricalcium phosphate scaffolds in three different composition ratios. J Biomed Mater Res A. 2012;100:2260–8.
Egbuniwe O, Idowu BD, Funes JM, Grant AD, Renton T, Di Silvio L. P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell Cycle. 2011;10:3912–9.
Mangano C, Paino F, d’Aquino R, De Rosa A, Iezzi G, Piattelli A, et al. Human dental pulp stem cells hook into biocoral scaffold forming an engineered biocomplex. PLoS One. 2011;6:e18721.
Salerno A, Zeppetelli S, Oliviero M, Battista E, Di Maio E, Iannace S, et al. Microstructure, degradation and in vitro MG63 cells interactions of a new poly(ε-caprolactone), zein, and hydroxyapatite composite for bone tissue engineering. J Bioactive Compat Polym. 2012;27:210–226.
Incerti Parenti S, Panseri S, Gracco A, Sandri M, Tampieri A, Alessandri Bonetti G. Effect of low-level laser irradiation on osteoblast-like cells cultured on porous hydroxyapatite scaffolds. Ann Ist Super Sanita. 2013;49:255–60.
Shuai C, Feng P, Zhang L, Gao C, Hu H, Peng S, et al. Correlation between properties and microstructure of laser sintered porous β -tricalcium phosphate bone scaffolds. Sci Technol Adv Mater. 2013;14:055002.
Marrelli M, Paduano F, Tatullo M. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int J Biol Sci. 2013;9:1070–8.
Ebrahimi M, Pripatnanont P, Suttapreyasri S, Monmaturapoj N. In vitro biocompatibility analysis of novel nano-biphasic calcium phosphate scaffolds in different composition ratios. J Biomed Mater Res B Appl Biomater. 2014;102:52–61.
Zhang ZY, Teoh SH, Chong MS, Schantz JT, Fisk NM, Choolani MA, et al. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126–37.
Bakhshandeh B, Soleimani M, Ghaemi N, Shabani I. Effective combination of aligned nanocomposite nanofibers and human unrestricted somatic stem cells for bone tissue engineering. Acta Pharmacol Sin. 2011;32:626–36.
Arpornmaeklong P, Akarawatcharangura B, Pripatnanont P. Factors influencing effects of specific COX-2 inhibitor NSAIDs on growth and differentiation of mouse osteoblasts on titanium surfaces. Int J Oral Maxillofac Implants. 2008;23:1071–81.
Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39–46.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–7.
Jafari M, Paknejad Z, Rad MR, Motamedian SR, Eghbal MJ, Nadjmi N, et al. Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater. 2015.
Roohani-Esfahani SI, Newman P, Zreiqat H. Design and fabrication of 3d printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects. Sci Rep. 2016;6:19468
Muller P, Bulnheim U, Diener A, Lüthen F, Teller M, Klinkenberg ED, et al. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med. 2008;12:281–91.
Wagoner Johnson AJ, Herschler BA. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011;7:16–30.
Chen M, Le DQ, Kjems J, Bünger C, Lysdahl H. Improvement of distribution and osteogenic differentiation of human mesenchymal stem cells by hyaluronic acid and beta-tricalcium phosphate-coated polymeric scaffold in vitro. Biores Open Access. 2015;4:363–73.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43.
Tomlinson MJ, Dennis C, Yang XB, Kirkham J. Tissue non-specific alkaline phosphatase production by human dental pulp stromal cells is enhanced by high density cell culture. Cell Tissue Res. 2015;361:529–40.
Dreesmann L, Mittnacht U, Lietz M, Schlosshauer B. Nerve fibroblast impact on Schwann cell behavior. Eur J Cell Biol. 2009;88:285–300.
Chang MC, Lin LD, Tseng HC, Chang BE, Chan CP, Lee SY, et al. Growth and differentiation factor-5 regulates the growth and differentiation of human dental pulp cells. J Endod. 2013;39:1272–7.
Goh TK, Zhang ZY, Chen AK, Reuveny S, Choolani M, Chan JK, et al. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores Open Access. 2013;2:84–97.
Malaval L, Modrowski D, Gupta AK, Aubin JE. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158:555–72.
Beck GR Jr, Sullivan EC, Moran E, Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. J Cell Biochem. 1998;68:269–80.
Rodriguez JP, González M, Ríos S, Cambiazo V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J Cell Biochem. 2004;93:721–31.
Mathews S, Gupta PK, Bhonde R, Totey S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011;44:537–49.
Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103:197–208.
Acknowledgements
This research was supported by a grant from the Faculty of Graduate Studies, Prince of Songkla University, Hat Yai, Songkhla, Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wongsupa, N., Nuntanaranont, T., Kamolmattayakul, S. et al. Biological characteristic effects of human dental pulp stem cells on poly-ε-caprolactone-biphasic calcium phosphate fabricated scaffolds using modified melt stretching and multilayer deposition. J Mater Sci: Mater Med 28, 25 (2017). https://doi.org/10.1007/s10856-016-5833-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5833-z