Abstract
Traditional chitosan hydrogels were prepared by chemical or physical crosslinker, and both of the two kinds of hydrogels have their merits and demerits. In this study, researchers attempted to prepare one kind of chitosan hydrogel by slightly crosslinker, which could combine the advantages of the two kinds of hydrogels. In this experiment, the crosslinker was formed by a reaction between the isocyanate group of 1,6-diisocyanatohexan and the hydroxyl group of polyethylene glycol-400 (PEG-400), then the crosslinker reacted with the amidine and the hydroxyl group of ethylene glycol chitosan to form the network structure. Physical properties of the hydrogel were tested by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and biodegradation. Biocompatibility was assessed by cell implantation in vitro and the scaffold was used as a cartilage tissue engineering scaffold to repair a defect in rabbit knee joints in vivo. FTIR results show the formation of a covalent bond during thickening of the ethylene glycol chitosan. SEM and degradation experiments showed that the ethylene glycol chitosan hydrogel is a 3-D, porous, and degradable scaffold. The hydrogel contained 2 % ethylene glycol chitosan and 10 μl crosslinker was selected for the biocompatibility experiment in vitro and in vivo. After chondrocytes were cultured in the ethylene glycol chitosan hydrogel scaffold for 1 week cells exhibited clustered growth and had generated extracellular matrix on the scaffold in vitro. The results in vivo showed that hydrogel-chondrocytes promoted the repair of defect in rabbits. Based on these results, it could be concluded that ethylene glycol chitosan hydrogel is a scaffold with excellent physicochemical properties and it is a promising tissue engineering scaffold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years scientists have made great progress to engineer living tissues such as cartilage, bone, skin, and blood. Some clinical researches have been conducted on fabricated tissues and several products will be available for clinical therapy in the near future. The majority of research in tissue engineering is focused on the cells that generate the required tissue and the material scaffold. The purpose of the material scaffold is to provide a 3-D environment for the growth and development of cells and tissues. In order for a material scaffold to function properly it should be biocompatible, easy to sterilize, and its properties should match those of the target tissue [1, 2].
There are two classes of material scaffolds, natural scaffolds and synthetic scaffolds. Chitosan, alginate, bio-ceramics, calcium hydroxyapatite were the main natural scaffolds. And poly (l-lactic acid), poly (ε-caprolactone) were the main synthetic scaffolds [3–12]. Chitosan is a cationic, degradable biopolymer that is derived by partial de-acetylation of chitins. Chitosan has been widely used in biomedical applications, including tissue engineering scaffolds, due to its low cost, large-scale availability, anti-microbial activity, biodegradation, and excellent biocompatibility [13, 14].
Previously, chitosan scaffolds have been made by freeze-drying a solution of chitosan and its derivatives, either alone, or in combination with other polymers or biomaterials [15–17]. Recently, electro-spinning and hydrogel preparation techniques have been used to construct chitosan scaffolds. In general, high voltage electro-spinning may cause high porosity in the scaffold and affect diffusion rates [18]. Beside that, the porosity, the diameter of the fiber and the pore could be controlled [19, 20]. Compared to electro-spinning most hydrogel tissue is a gel-state network that contains proteins and polysaccharides, chitosan hydrogels are a promising material for soft tissue engineering scaffolds.
The solid network within the chitosan hydrogels may be made up by chemical bonds or physical bonds. Chemical covalent bonding takes place between the amino group, hydroxyl group of chitosan and a functional group of the crosslinker. For example, the β-glycerophosphate was the mainly materials for making the thermo-sensitive hydrogel with chitosan [21, 22]. And the thermo-sensitive chitosan-based hydrogels are of great interest as drug delivery systems or tissue engineering scaffolds since they are water-based, non-toxicity, biocompatible and biodegradable [23]. Besides that, polyvinyl alcohol and polyacrylamide are both the main raw materials for prepared hydrogel with chitosan [24, 25]. Covalent cross-linking leads to the formation of hydrogels showing with stable chemical properties and excellent mechanical properties. Zhang et al. [26] prepared one kind of hydrogel with oxidized dextran, teleostean and N-carboxyethyl chitosan. The interpenetrating network was formed by covalent bond-schiff base. And the hydrogel shows excellent mechanical property and stability. However, the main drawbacks of covalently crosslinked these kinds of gels are the toxicity of residual cross-linkers [27, 28]. As mentioned above, the common chitosan hydrogel made with β-glycerophosphate usually need several times β-glycerophosphate than chitosan [21, 22]. And the similar condition was also needed when prepared the chitosan hydrogel with polyvinyl alcohol or polyacrylamide [24, 25].
The physical bonding was resulted by the ionic, hydrogen bonding or hydrophobic associations [29]. The physical hydrogel has low toxicity due to no cross-linkers are not used in the formation of procedure hydrogels with physical bonds and therefore they have low toxicity. Unfortunately, hydrogels lacking covalent bonds have unstable properties. The structure of the gel is easily affected by changes in the environment, such as pH, temperature or solute concentration [30]. As mentioned above, Zhang et al. [26] prepared the hydrogel with oxidized dextran, teleostean and N-carboxyethyl chitosan. At the same time, the teleostean, N-carboxyethyl chitosan hydrogel was also prepared as the control group which was formed by physical bond between the carboxyl group and amino group. The results show that stability and mechanical property were both worse than the oxidized dextran, teleostean and N-carboxyethyl chitosan hydrogel. To improve the properties of chitosan hydrogel for applications in tissue engineering, a new kind of hydrogel which incorporates advantages of both chemical and physical crosslink bonds was prepared.
In this study hydrogel was prepared based on the ethylene glycol chitosan and crosslinker. The ethylene glycol chitosan was one derivative of chitosan, that the ethylene glycol group was grafted to the hydroxy group of chitosan. The crosslinker was prepared by reacting HDI and PEG-400. Only small amounts of crosslinker were necessary to cause the formation of the ethylene glycol chitosan hydrogel. Characteristics of the ethylene glycol chitosan hydrogel were detected and biocompatibility of the hydrogel was tested by implanting chondrocytes in vitro. Finally, the ethylene glycol chitosan hydrogel was used as a tissue scaffold to repair defects in rabbit knee joint cartilage. These results show that this crosslinker forms a desirable chitosan hydrogel tissue that is an excellent material scaffold for tissue engineering.
2 Materials and methods
2.1 Preparation of ethylene glycol chitosan hydrogel
The crosslinker was synthesized through the reaction of the isocyanate group of HDI (Sigma) with the hydroxyl group of PEG-400 (Sigma). A 2:1 mol ratio of HDI and PEG-400 were mixed in a flask. The mixture reacted at room temperature until the transparent mixture turned into an emulsion. 2 g ethylene glycol chitosan powder (Sigma) was dissolved in 100 ml distilled water to form 2 % ethylene glycol chitosan solution. And the 1 % ethylene glycol chitosan solution and 0.5 % ethylene glycol chitosan solution were prepared as the similar method. Different volume crosslinker was added to ethylene glycol chitosan solution, mixed and the ethylene glycol chitosan hydrogel was formed after several minutes at room temperature. The ethylene glycol chitosan hydrogel was lyophilized at −40 °C and 0.1 MPa (Alpha 1-4, Christ, German) and stored at 4 °C.
2.2 Characterization of the ethylene glycol chitosan hydrogel
The lyophilized ethylene glycol chitosan scaffold was pasted on a metal stub and sputter-coated with gold for a period up to 120 s. The specimen was observed with a scanning electron microscope (Quanta 200, FEI, the Netherlands) equipped with a field-emission gun and Robinson detector. The lyophilized scaffold was shaved with a powder of ground hydrogel and potassium bromide crystals. The sample was observed at room temperature using a Fourier transform infrared spectrometer (5700, Nicolet, American).
2.3 In vitro degradation
The degradation of ethylene glycol chitosan hydrogel in buffer solutions was determined by mass loss. Pre-weighted hydrogels were divided into two groups. Hydrogels in group one were placed in 5 ml phosphate buffer (pH 7.4), and hydrogels in group two were placed in 5 ml lysozyme (Sigma) phosphate solution (10,000 U/ml, pH 7.4). The samples were maintained at 37 °C and 100 rpm and the buffer was changed every week. At predetermined intervals the samples were removed, patted dry and weighed.
2.4 In vitro culture of rabbit chondrocytes on ethylene glycol chitosan hydrogel
The costal cartilage of 2 week-old rabbits (male or female) was removed and then digested in 0.25 % trypsogen for 0.5 h at 37 °C and in 0.1 % collagenase for 8 h at 37 °C. The aqueous phase was carefully removed by centrifuging at 1,500 rpm for 5 min. To obtain sufficient chondrocytes the residual tissue was cultured in dulbecco’s modified eagle medium (DMEM) supplemented with 20 % fetal bovine serum (FBS) at 37 °C and 5 % CO2.
The hydrogel contain 2 % ethylene glycol chitosan and 10 μl crosslinker was selected for the biocompatibility research in vitro. The lyophilized ethylene glycol chitosan scaffold was cut into about 1 mm3 sphere and sterilized under ultraviolet light overnight. 100 µl 2 × 106 cells/ml chondrocytes suspension was deposited in the hydrogel and cultured in DMEM, supplemented with 20 % FBS, at 37 °C and 5 % CO2 for 1 week. The cultured ethylene glycol chitosan hydrogel was frozen at −20 °C and 5 μm sections were prepared for normal H&E staining, toluidine blue staining, safranin-O staining and immunochemical staining.
2.5 Immunochemical staining of collagen and aggrecan
Samples were sectioned into 5 μm sections at −20 °C and fixed with acetone for 20 min at 4 °C. A collagen staining kit (Chondrex) was used for immunochemical staining of collagen. Sections were treated with 2 % bovine testicular hyaluronidase for 30 min at 25 °C and then incubated with a dilution of 1:250 and incubated overnight at 4 °C. Streptavid in peroxidase (50 μl diluted in 10 ml of streptavidinperoxidase-dilution buffer) was applied for 1 h at 25 °C. Sections were then color developed with diaminobenzidine (brown color) for 30 min.
Aggrecan was immunochemically stained in a similar manner. Sections were treated with 3 % hydrogen peroxide for 30 min at 25 °C and then incubated with diluted sheep serum (10 μl in 200 μl 5 % BSA solution) for 15 min at 37 °C. The sections were then incubated with mouse monoclonal anti-Aggrecan (Thermo) at a dilution of 1:200 at 4 °C overnight, followed by fluoresce in Texas Red labeled anti-mice IgG (American Qualex) at a dilution of 1:150. Sections were visualized with the microscope (TS100, Nikon, American) equipped with a digital camera.
2.6 Ethylene glycol chitosan hydrogel in vivo repair of cartilage defects
Thirty mature and healthy New Zealand rabbits (male or female 2–2.5 kg mean body weight) were provided and cared for by the Experimental Animals Center of North Sichuan Medical College. The rabbits were randomly divided into three groups. All animals were anesthetized by an ear vein injection of 10 mg/ml pentobarbital (30 mg/kg). A long incision was made over the knee and the patella was dislocated laterally. The knee was flexed 90° to expose the non-weight-bearing area of the medial femoral condyle. A defect 4 mm wide and 3 mm deep was created with a hollow trephine. Rabbit chondrocytes were planted in the hydrogel and cultured 1 week as the methods described above. The hydrogel contained 2 % ethylene glycol chitosan and 10 μl crosslinker was used in this experiment. And the implanted ethylene glycol chitosan hydrogel contains chondrocytes was prepared as described in methods 2.4 and cultured for 1 week in vitro. The wound of the experiment group was filled with hydrogel–chondrocytes, the blank group was filled with ethylene glycol chitosan scaffold without chondrocytes, while the defect of control group was not accepted any treatment. 12 weeks after implantation the rabbits were killed and the joints were collected. The samples were embedded in paraffin, sectioned, and exposed to standard H&E staining, Toluidine blue staining, safranin-O staining and immunochemical staining.
2.7 Statistical analysis
All experiments in vitro were performed in triplicate and statistical differences between groups were calculated using a student’s t test. The data are expressed as mean ± SD and a P < 0.05 was considered to be statistically significant. The experiments in vivo were performed in duplicate and the rank test was adopted for statistical analysis.
3 Results
3.1 Preparation of ethylene glycol chitosan hydrogel
A crosslinked network of ethylene glycol chitosan molecules was formed through a reaction between the amino group, hydroxyl group of ethylene glycol chitosan and the crosslinker (Fig. 1, 2). Firstly, HDI reacted with PEG-400 to form the crosslinker, which ended with isocyanate groups. Then the functional group at the ends of the crosslinker reacted with the amino group or hydroxyl group of ethylene glycol chitosan to achieve the reticulation. The resulting gel was semi-transparent. As shown in Table 1, the gel thickening time increased as the volume of crosslinker decreased, until, eventually, the gel could not be formed. Failure of gel formation in the 0.5 % ethylene glycol chitosan solution may be ascribed to the low concentrations of the amino group, hydroxyl group and isocyanate groups. When the crosslinker volume increased from 1 to 2 %, the gel thickening time decreased from 1.5 ± 0.21 to 1.33 ± 0.14 min.
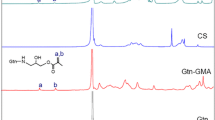
FTIR tests were conducted on various concentrations of ethylene glycol chitosan and various volumes of crosslinkers. Pure chitosan was used as a control. The FTIR spectrum of dried ethylene glycol chitosan gel is shown in Fig. 3. Generally, the characteristic FTIR peaks of ethylene glycol chitosan are a hydrogen bonding peak at about 3,400 cm−1 and an amide absorption peak at about 1,600 cm−1. Both the two peaks shifted noticeably compared with the control, indicating the reaction of the two groups with isocyanate groups (Fig. 3). The amide peak’s transmission weakened with the increase of crosslinker, demonstrating covalent bonds forming between ethylene glycol chitosan and the crosslinker.
FTIR test of ethylene glycol chitosan hydrogel. Samples are labeled as the following: sample 1020 contains 1.0 % chitosan and 20 μl crosslinker; sample 1010 contains 1.0 % chitosan and 10 μl crosslinker; sample 1005 contains 1.0 % chitosan and 5 μl crosslinker; sample 2010 contains 2.0 % chitosan and 10 μl crosslinker; sample 1010 contains 1.0 % chitosan and 10 μl crosslinker; sample 0510 contains 0.5 % chitosan and 10 μl crosslinker
3.2 SEM observation
Hydrogels derived from various concentrations of ethylene glycol chitosan solution and 10 μl of crosslinker were collected and freeze-dried for SEM observation. All the ethylene glycol chitosan hydrogel exhibited a typical, interconnected porous structure (Fig. 4). The pores are irregularly shaped and range in diameter from 100 to 200 μm. The high concentration ethylene glycol chitosan solution has a small pore size and low porosity.
3.3 Degradation of the ethylene glycol chitosan hydrogel
Degradation tests of the ethylene glycol chitosan hydrogel were carried out in PBS and lysozyme medium and there were no significant differences in the outcome of the tests using different mediums (Fig. 5). The degradation curves of sample 2020 showed an identical pattern of increasing weight followed by a weight decrease. After the first week in enzyme medium the samples’ wet weight increased to about 110 % of the initial weight. After 3 weeks, the wet weight decreased to below 100 % of the initial weight and the weight ratio continued to decrease to 73 % after 8 weeks. Samples tested in PBS buffer exhibited the same curve shape. In contrast to sample 2020, the weight of the other two samples decreased, without an initial increase in weight. The degradation of sample 2010 was faster than sample 1020, both in PBS buffer and the lysozyme phosphate medium. After 8 weeks in PBS buffer sample 1020 weighed 52 % of the initial weight, while sample 2010 weighed 71 % of the initial weight.
The degradation of ethylene glycol chitosan hydrogel. a Degradation in phosphate solution (pH 7.4); b Degradation in lysozyme phosphate solution (10,000 U/ml, pH 7.4). Samples are labeled as the following: sample 2010 contains 2.0 % chitosan and 10 μl crosslinker; sample 2020 contains 2.0 % chitosan and 20 μl crosslinker; sample 1020 contains 1.0 % chitosan and 20 μl crosslinker
3.4 In vitro culture of rabbit chondrocytes
According to pathology, the cells could be distinguished by histological staining. And H&E staining was the normal pathology method for distinguished different cells. Histological staining showed that the nucleus of chondrocytes on the scaffold was round and full and existed significant difference from the dead cells, which illustrated that rabbit chondrocytes could survive and proliferate on the hydrogel scaffold (Fig. 6). Except that the results showed that there were no obviously inflammatory cells, so this novel chitosan-based hydrogel exhibits good biocompatibility. Chondrocytes obviously penetrated into the hydrogel scaffold quickly and continued to proliferate over time, as evidenced by increasing cell densities in histological sections. Figure 6b shows the cell could be specific stained by Toluidine blue staining. The H&E stained sections (Fig. 6a) and safranin-O stained sections (Fig. 6c) show that the chondrocytes grew confluently with the scaffold. The safranin-O stained sections (Fig. 6c) also show that the chondrocytes could secrete glycosaminoglycans. The immunohistochemistry and immunofluorescence stains also demonstrate that the chondrocytes can secrete specific extracellular matrix proteins, such as collagen and aggrecan. The scaffold was stained in some sections due to its porous structure, but the chondrocytes could be distinguished clearly.
3.5 In vivo cartilage repair
To further test the biocompatibility of the scaffold, the rabbit chondrocytes grown in hydrogel were implanted into the cartilage of a defective rabbit knee joint. The ethylene glycol chitosan scaffold without chondrocytes was also implanted as the blank groups, and the untreated defects were used as a control. The knee joints were harvested after 12 weeks and stained to observe the biocompatibility of the ethylene glycol chitosan hydrogel scaffold and repairs of the knee joint. The staining of the experimental and blank groups showed no obvious inflammatory cells and indicates that the ethylene glycol chitosan hydrogel has excellent biocompatibility. There was no obvious scaffold in the joint (Fig. 7A), which suggests that the ethylene glycol chitosan hydrogel had degraded.
The defects to the knee joint were being repaired by hydrogel–chondrocytes in the experiment group. Although a boundary between original and renewed cartilage still existed after 12 weeks, the knee joints were filled with new cartilage and the formation of cartilage-like tissue can be observed with toluidine blue and safranin-O staining (Fig. 7B, C). The new cartilage cells were similar to those of normal chondrocytes and the typical structure of hyaline cartilage lacunae was apparent in the regenerated area (Fig. 7A4). Additionally, safranin-O staining indicated a considerable amount of proteoglycan present in the knee (Fig. 7A4). The immunohistochemistry staining showed that brown color was even more obvious than the normal tissue (Fig. 8a). This result indicated that the regenerated tissue not only can secret specific cartilage cells extracellular matrix-collagen, but also secrete vigorously for this tissue rapidly formed.
In the control group, the knee joint defects were still apparent. When the defects section was observed under microscope (Fig. 7B4), there was no obviously structure of hyaline cartilage lacunae. The defect was filled with other cells such as adipocytes, mast cells, and a few chondrocytes had migrated to the knee joint to fill the injured areas. Except that the immunohistochemistry staining results of the filled tissue was negative. There was no evidence showing that cartilage-like tissue had formed in the control group (Fig. 7B4).
Compared with the control group, the defect was repaired partially. The defect was filled and the boundary was not so obviously. The histochemical staining and immunohistochemistry staining results were worse than the experiment group and better than the control group. These data suggested that the ethylene glycol chitosan scaffold without chondrocytes could also improve the repairs for the defect.
Because the histochemical staining and immunohistochemistry staining results demonstrate the same conclusion, so the results was statistical analyzed together. The rank test was adopted for statistical analysis. First, level data using multiple sample comparisons, P = 0.000, according to α = 0.05 level, the difference was statistically significant. Then using multiple comparisons between multiple samples, two-tailed test was selected, PA–B = 0.002, PA–C = 0.000, PB–C = 0.035, according to α = 0.05 level, the difference was statistically significant, can be considered A better than B, B than C (A represent Experiment group; B represent Blank group; C represent Control group).
4 Discussions
Chitosan hydrogels are being used more frequently in biomedical fields as tissue scaffolds, drugs or gene carriers, and smart sensors [31, 32]. As described above, chitosan hydrogels may be obtained by various mechanisms of chemical or physical bonding, such as hydrophobic associations or covalent, ionic, or hydrogen bonding. Both physically and chemically crosslinked hydrogels have their drawbacks, but this research enhances the stable properties of hydrogels and decreases the toxicity of hydrogels by decreasing the amount of crosslinker residual present in the gel.
Recently, poly (ε-caprolactone), hydroxyapatite, gelatin, and collagen were trialed as additions to chitosan hydrogels and successfully stabilized the hydrogen’s properties and lowered the cytotoxicity [33–35]. In this research the similar results can be achieved through a crosslinker formed by the reaction of HDI and PEG-400 (Fig. 1). As shown in Table 1, 1 ml 1 % ethylene glycol chitosan solution just need only 3 μl crosslinker to gel, the volume ratio is just 0.3 %. As described above, the chitosan usually needed several times β-glycerophosphate, polyvinyl alcohol or polyacrylamide to form the hydrogel. Wang et al. [36] prepared chitosan hydrogel with β-glycerophosphate, and the hydrogel contains no other things. In this study, 5 g chitosan needed 28 g β-glycerophosphate to form the hydrogel. Mohamed et al. [24] prepared chitosan hydrogel with polyvinyl alcohol, and the polyvinyl alcohol was 50 times than chitosan. Compared with these chitosan hydrogels, the crosslinker is present at such low quantities that the hydrogel has stable physicochemical properties and excellent biocompatibility. The relationship between the gel thickening time, the crosslinker volume, and the ethylene glycol chitosan solution concentration are attributable to the mechanism of gel formation. If the concentration of related functional groups increases, covalent bonds form quickly and the gel thickening time decreases correspondingly. If the concentration of functional groups is too low, the formed covalent could not thicken the ethylene glycol chitosan solution, and the gel could not be formed. This reason could be explain the failure gelation clearly.
In order to demonstrate the chemical components of ethylene glycol chitosan hydrogels the FTIR spectrum was tested, with chitosan as the control. The FTIR output indicated the action between ethylene glycol chitosan and the crosslinker. The transmission of the characteristic peak changed as the reaction proceeded between –NH2, –OH of ethylene glycol chitosan and isocyanate groups (Fig. 3). For example, the peak at 3,400 cm−1 weakened as the volume of crosslinker increased and the ethylene glycol chitosan concentration remained constant, which indicated the reaction between ethylene glycol chitosan and crosslinker. Although the transmission could be affected by many other factors, the phenomenon of a peak weaken supports the concept of a new covalent bond forming between ethylene glycol chitosan and the crosslinker at some extent. There were no other obvious peak changes or newly formed peaks, which suggests that the ethylene glycol chitosan hydrogel contained no other chemical reagents.
The morphology of ethylene glycol chitosan hydrogel was detected by SEM (Fig. 4). Freeze-dried hydrogel has a typical porous structure and high porosity. Freeze-dried hydrogel with high porosity can swell like a sponge and adsorb cell suspension, increasing the adhesion rate and adding to the biocompatibility of this hydrogel. The concentration of ethylene glycol chitosan affects the pore size and porosity because these pores were formed by the loss of water from the hydrogel when it was lyophilized. A higher concentration of ethylene glycol chitosan results in lower water content and a decrease in pore size and porosity. On the other side, for these pores were formed by the water in the hydrogel, the slightly crosslinker could not decisively effect pore size and porosity, so the other hydrogels containing other amounts of crosslinker were not observed.
Degradation is another important property of a material scaffold for tissue engineering. Lysozyme can break β-1, 4 glycosidic bonds in ethylene glycol chitosan and disrupt the network structure of a hydrogel. Lysozyme was added to the PBS medium to mimic degradation of the scaffold in vivo. While the lattice size of the networks is enlarged in the initial stages of degradation, breakage of the β-1, 4 glycosidic bonds did not damage the hydrogel. As a result, the weight of the hydrogel increased. When the breakage of these bonds reaches a critical value, the cross linking network will become disjointed, resulting in loss of gel weight [37].Compared to the three samples, the degradation rate decreased with an increase in crosslinker volume and ethylene glycol chitosan concentration for more β-1, 4 glycosidic bond and amino bonds formed. That may be explain why a weight increase during the first degradation phase was observed only in sample 2020 (Fig. 5). The other samples may have degraded more quickly and their weight increase phase was not detected in this experiment.
Unusually, there were no significant differences between gel degradation in PBS and in lysozyme solution. This may be due to the low concentration of ethylene glycol chitosan in the gels. Although the ethylene glycol chitosan scaffold contains only slightly crosslinker, but the scaffold in gel status only contains 1–2 % ethylene glycol chitosan and the breakage of β-1, 4 glycosidic bonds could not cause an obvious difference in their strength.
Excellent biocompatibility is a requirement for engineering scaffold tissues. In order to identify the biocompatibility of ethylene glycol chitosan hydrogel, chondrocytes were implanted on the scaffold and relevant cell sections were stained (Fig. 6). Many cells in the hydrogel appear to grow in clusters. Cells in the scaffold maintained morphologic characteristics of chondrocytes and generated viable extracellular matrix. When the hydrogel-chondrocytes were implanted into rabbit knee joints there were no obvious inflammatory cells. All of these results support the premise that ethylene glycol ethylene glycol chitosan hydrogel has excellent biocompatibility.
In this study, ethylene glycol chitosan hydrogel was used as a cartilage tissue engineering scaffold in an attempt to repair knee joint cartilage defects. After a 12-week implantation the cartilage defects showed evidence of repair in the experiment group. The knee joints were filled with newly generated chondrocytes and hyaline cartilage. In the control group the knee joint defects were partially filled and the cartilage defects were still severe. These results suggest that the addition of hydrogel-chondrocytes favors the repair of cartilage defects. It is notable that the ethylene glycol chitosan hydrogel without chondrocytes could also repair the defect though the repairs were worse than the experiment group. This phenomenon may be caused by ethylene glycol chitosan anti-microbial activity.
Although ethylene glycol chitosan hydrogel seemed to stimulate repair in knee joint cartilage, the mechanical function of ethylene glycol chitosan hydrogel remained inferior to native cartilage tissue. However, some scaffolds with poor mechanical properties still display excellent prospects in applications such as autologous chondrocytes implantation and matrix-induced autologous chondrocytes implantation [38–40]. Based on these results, the ethylene glycol chitosan hydrogel-chondrocytes may significantly improve the repair of cartilage defects. Although ethylene glycol chitosan hydrogels may be deficient in mechanical function, this is not the only factor to consider in all tissue engineering applications. The results of the experiments in vitro and in vivo show that ethylene glycol chitosan hydrogel has excellent biocompatibility and may be a promising tissue engineering scaffold.
5 Conclusions
In summary, this research produced a ethylene glycol chitosan hydrogel by forming a novel crosslinker between HDI and PEG-400. The ethylene glycol chitosan solution thickened into a hydrogel after added only a small amount of crosslinker. This hydrogel contains relatively small amounts of chemical agent residues and has excellent biocompatibility properties, compared with common ethylene glycol chitosan hydrogels. An in vivo experiment demonstrated that ethylene glycol chitosan hydrogel can repair the cartilage defect. The preparation method is simple and suitable for controlled experiments. Based on these results, it could be conclude that ethylene glycol chitosan hydrogel is a promising tissue engineering scaffold.
References
Garg T, Singh O, Arora S, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 2012;29:1–63.
Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;36:9622–9.
Peng H, Yin Z, Liu H, Chen X, Feng B, Yuan H, et al. Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs. Nanotechnology. 2012;23:485102.
Amruthwar SS, Janorkar AV. In vitro evaluation of elastin-like polypeptide-collagen composite scaffold for bone tissue engineering. Dent Mater. 2013;29:211–20.
Heiligenstein S, Cucchiarini M, Laschke MW, Bohle RM, Kohn D, Menger MD, et al. In vitro and in vivo characterization of nonbiomedical- and biomedical-grade alginates for articular chondrocyte transplantation. Tissue Eng Part C Methods. 2011;17:829–42.
Jeon O, Powell C, Ahmed SM, Alsberg E. Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng Part A. 2010;16:2915–25.
Vallet RM, Ruiz HE. Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater. 2011;23:5177–218.
Sawada Y, Hokugo A, Yang Y, Kamitani M, Matsuda S, Mao T, Lei D, et al. A novel hydroxyapatite ceramic bone substitute transformed by ostrich cancellous bone: characterization and evaluations of bone regeneration activity. J Biomed Mater Res B Appl Biomater. 2011;98B:217–22.
Tsurushima H, Marushima A, Suzuki K, Oyane A, Sogo Y, Nakamura K, et al. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010;7:2751–9.
Seyednejad H, Gawlitta D, Kuiper RV, de Bruin A, van Nostrum CF, Vermonden T, et al. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly (ε-caprolactone). Biomaterials. 2012;33:4309–18.
Zander NE, Orlicki JA, Rawlett AM, Beebe TP Jr. Quantification of protein incorporated into electrospun polycaprolactone tissue engineering scaffolds. Appl Mater Interfaces. 2012;4:2074–81.
Johari N, Fathi MH, Golozar MA, Erfani E, Samadikuchaksaraei A. Poly (ε-caprolactone)/nano fluoridated hydroxyapatite scaffolds for bone tissue engineering: in vitro degradation and biocompatibility study. J Mater Sci Mater Med. 2012;23:763–70.
Ana RC, Duarte JF, Mano RL. Novel 3D scaffold a of chitosan–PLLA blends for tissue engineering applications: Preparation and characterization. J Supercrit Fluids. 2010;3:282.
Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials. 2005;26:7616–27.
Kim SE, Suh DH, Yun YP, Lee JY, Park K, Chung JY, et al. Local delivery of alendronate eluting chitosan scaffold can effectively increase osteoblast functions and inhibit osteoclast differentiation. J Mater Sci Mater Med. 2012;23:2739–49.
Coimbra P, Ferreira P, de Sousa HC, Batista P, Rodrigues MA, Correia IJ, et al. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int J Biol Macromol. 2011;1:112–8.
Cruz DM, Gomes M, Reis RL, Moratal D, Salmerón-Sánchez M, Ribelles JL, et al. Differentiation of mesenchymal stem cells in chitosan scaffolds with double micro and macroporosity. J Biomed Mater Res A. 2010;95:1182–93.
Chen L, Zhu CH, Fan DD, Liu B, Ma XX, Duan ZG, et al. A human-like collagen/chitosan electrospun nanofibrous scaffold from aqueous solution: electrospun mechanism and biocompatibility. J Biomed Mater Res A. 2011;99:395–409.
Frohbergh ME, Katsman A, Botta GP, Lazarovici P, Schauer CL, Wegst UG, et al. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials. 2012;33:9167–78.
Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Opanasopit P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int Wound J. 2012;11(2):215–22.
Kim S, Nishimoto SK, Bumgardner JD, Haggard WO, Gaber MW, Yang Y. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials. 2010;31:4157–66.
Li CY, Ren SG, Dai Y, Tian FJ, Wang X, et al. Efficacy, pharmacokinetics, and biodistribution of thermosensitive chitosan/β-glycerophosphate hydrogel loaded with docetaxel. PharmSciTech. 2014;15:417–24.
Stephanie S, Nicolas A, Nina S, Marc R, Catherine C, et al. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin Drug Deliv. 2014;11:249–67.
Mohamed AA, Ihab TA. Accelerated wound healing and anti-inflammatory effects of physically cross linked polyvinyl alcohol–chitosan hydrogel containing honey bee venom in diabetic rats. Arch Pharm Res. 2013; 30 (in press).
Nagpal M, Singh SK, Mishra D. Superporous hybrid hydrogels based on polyacrylamide and chitosan: Characterization and in vitro drug release. Int J Pharm Investig. 2013;3:88–94.
Zhang HW, Qadeer A, Mynarcik D, Chen W. Delivery of rosiglitazone from an injectable triple interpenetrating network hydrogel composed of naturally derived materials. Biomaterials. 2011;32:890–8.
Teng DY, Wu ZM, Zhang XG. Synthesis and characterization of in situ cross-linked hydrogel based on self-assembly of thiol-modified chitosan with PEG diacrylate using Michael type addition. Polymer. 2010;3:639–46.
Hu XH, Li D, Gao CY. Chemically cross-linked chitosan hydrogel loaded with gelatin for chondrocyte encapsulation. Biotechnol J. 2011;6:1388–96.
Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–21.
Guo Y, Yuan T, Xiao ZW, Tang PP, Xiao YM, Fan YJ, et al. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J Mater Sci Mater Med. 2012;23:2267–79.
Chiu LL, Reis LA, Radisic M. Controlled delivery of thymosin β-4 for tissue engineering and cardiac regenerative medicine. Ann NY Acad Sci. 2012;1269:16–25.
Wijekoon A, Fountas-Davis N, Leipzig ND. Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater. 2013;9:5653–64.
Zhong X, Ji CD, Chan AK, Kazarian SG, Ruys A, Dehghani F. Fabrication of chitosan/poly (ε-caprolactone) composite hydrogels for tissue engineering applications. J Mater Sci Mater Med. 2011;22:279–88.
Pok S, Myers JD, Madihally SV, Jacot JG. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013;9:5630–42.
Madhumathia K, Shalumona KT, Rania VV, Tamurab WH, Selvamurugana N, Naira SV. Wet chemical synthesis of chitosan hydrogel–hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol. 2009;45:12–5.
Wang Y, Xu N, Luo Q, Li Y, Sun L, et al. In vivo assessment of chitosan/β-glycerophosphate as a new liquid embolic agent. Interv Neuroradiol. 2011;17:87–92.
Hong Y, Song HQ, Gong YH, Mao ZW, Gao CY, Shen JC. Covalently crosslinked chitosan hydrogel: properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 2007;3:23–31.
Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32.
Willers C, Partsalis T, Zheng MH. Articular cartilage repair: procedures versus products. Expert Rev Med Devices. 2007;4:373–92.
Hao T, Wen N, Cao JK, Wang HB, Lu SH, Liu T, et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthritis Cartilage. 2010;18:257–65.
Acknowledgments
This work was supported by the Natural Science Foundation of China (81171472, 81201407, 81071270).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., Zhao, M., Liu, K. et al. Novel chitosan hydrogel formed by ethylene glycol chitosan, 1,6-diisocyanatohexan and polyethylene glycol-400 for tissue engineering scaffold: in vitro and in vivo evaluation. J Mater Sci: Mater Med 25, 1903–1913 (2014). https://doi.org/10.1007/s10856-014-5223-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5223-3