Abstract
Chemical modification of cellulose by phosphorylation enhances its bioactivity and provides new derivatives and materials with specific end uses. In the present study, cellulose derivatized with phosphorous acid was obtained using the reaction of microcrystalline cellulose with phosphorous acid–urea mixture, in molten state, in comparison with others methods that used different solvents and catalysts. Completely water soluble films with a substitution degree close to one were obtained and characterized by analytical and spectral analysis (FT-IR, 31P NMR), contact angle, metallographic microscopy and atomic force microscopy (AFM). 31P NMR spectra of derivatized cellulose showed a signal at 2.58 ppm (assigned to P–O–C6) while the doublets at 4.99–5.29 and at 7.38 ppm were assigned to P–O–C2 and P–O–C3, respectively; thus, the formation of monosubstituted phosphorous acid esters of cellulose is advocated. Contact angle measurements showed that the work of adhesion is more important in water than in ethylene glycol, for the phosphorous acid derivatized cellulose. The cytocompatibility of this hydrosoluble derivatized cellulose was tested by direct contact and also by indirect assays on normal human dermal fibroblasts and on osteoblast-like cells (human osteosarcoma). Cell growth on phosphorylated cellulose pellicle and the results from viability assays had shown a good cytocompatibility and lack of toxicity. Phosphorous acid derivatized cellulose would offer a promising biomaterial, useful as scaffolds for new biopolymer composites, and subject for further development as an ionic crosslinker.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cellulose (C) is a naturally occurring, linear homopolymer of glucose (C6H10O5) n . It is the most abundant, renewable, biodegradable and biocompatible polymer, with the longest and widest history of biomedical applications due to its stability on temperature or pH shift, lack of toxicity, and good mechanical properties. In medicine, cellulose membranes for blood purification were described among the most widely used polymeric devices in therapy [1, 2]. Microcrystalline cellulose Avicel is used in the pharmaceutical and food industry and is Generally Recognized As Safe (GRAS status).
Cellulose viscose sponges have been proposed as implantable matrices for connective tissue regeneration while regenerated cellulose hydrogels showed cytocompatibility as promoting attachment of human bone marrow stromal cells to a good extent [3, 4]. Cellulose regenerated by the viscose process, not only demonstrated bone-matching mechanical properties but also showed osteoconductive characteristics [5–9] even if to obtain a full bioactive character, a material must be osteoinductive. Chemical modification of cellulose materials by phosphorylation continues to provide new derivatives with specific end uses, especially to enhance its bioactivity [10–13].
There are some methods for cellulose phosphorylation, either by reaction of the hydroxyls groups in the parent polymer, or by a second-hand derivatization of an already formed cellulose ether or ester. The synthesis of phosphorus cellulose derivatives has been performed in various solvents [(DMSO)–methylamine, DMAc–LiCl, SO3–triethylamine, formic acid, trifluoroacetic acid, N,N-dimethylformamide, (DMF)–N2O4, paraformaldehyde, DMSO, trimethylchlorosilane–DMF, urea in melt or aqueous-NaOH], with many variants of phosphorous compounds (phosphoric, phosphorus, phosphinic acid, P2O5, amidophosphates, alkyl or aryl derivatives of phosphorous acids) [14–17]. Phosphorylated gels with lower substitution degree were obtained using Isogai method for solving cellulose and subsequent treatment with phosphorous acid or other phosphorous compounds [17, 18]. Higher substitution degree of water soluble phosphorylated cellulose was obtained from the reaction of microcrystalline cellulose with phosphorous acid/urea, either in melt, or subsequent to microwave activation [19, 20]. Most frequently, those derivatives were used as flame retardant additives, and also to increase the stability for aqueous dispersion of sparingly inorganic salts [21–24].
Granja et al. [25] synthesized cellulose phosphate gels with high degrees of substitution and demonstrated their biocompatibility and ability to promote the formation of a granulated hydroxyapatite layer between the surface of the material and osteoblast-like cells. The interaction of cultured cells with cellulose phosphate has firstly been assessed using Chinese hamster ovary cells [14]. Others studies have been carried out on cultured human bone marrow stromal cells [25]. On our knowledge, there are no published data regarding the cytocompatibility of phosphorylated cellulose in direct contact with human fibroblasts. In the present study, the derivatization of cellulose with phosphorous acid was performed using the reaction of microcrystalline cellulose with phosphorous acid–urea mixture, in molten state, in comparison with others methods that used different solvents and catalysts. The resulted product was characterized by analytical and spectral analysis (FT-IR, 31P NMR), contact angle, metallographic microscopy and atomic force microscopy (AFM). The cytocompatibility of this hydrosoluble derivatized cellulose was tested by direct contact and also by indirect assays on normal human dermal fibroblasts and on osteoblast-like cells (human osteosarcoma).
2 Experimental
2.1 Materials
Microcrystalline cellulose Avicel® PH-101 type (analytical grade), and phosphorous acid (purrum p. a.) were purchased from Fluka Chemie (Switzerland, Buchs). Urea was purified by recrystallization from methyl alcohol. Sodium hydroxide (98 %), as in pellets, was obtained from Fluka-Chemie G (CH-9470 Buchs). Dimethylformamide (DMF), dimethylacetamide (DMAc), dicyandiamide (DCDA) and LiCl were maintained at room temperature, and used without further purification.
Normal Human Dermal Fibroblasts (NHDF) and Human Osteosarcoma Cells (HOS) were purchased from Lonza together with reagents for subcultivation (DMEM with 10 % fetal bovine sera-FBS, 1 % antibiotic antimycotic, for NHDF and MEM with 10 % FBS, 1 % antibiotic antimycotic, for HOS). Cell proliferation was performed in 75 cm2 flasks in specific culture media, in a CO2 incubator at 37 °C. Cytoplasmic fluorophore labeling was performed by a filtered tetracycline solution (225 μM in PBS). All cell assays were carried out using six replicates for every condition tested.
2.2 Methods
2.2.1 Synthesis of phosphorylated cellulose
Reaction between cellulose and phosphorous acid was performed according to the method described by Inagaki and al. [19]. Others reactions were developed in different solvents mixed with NaOH, urea and/or catalyst [26].
General procedure: Urea was placed in a three necked flask, equipped with a nitrogen inlet and heated at 150 °C under nitrogen. Microcrystalline cellulose and phosphorous acid were alternatively added in portion to the molten urea preventing the foaming. The reaction was allowed to proceed at 150 °C for various time amounts. The reacted mixture was dissolved in 1 N aqueous sodium hydroxide. The alkaline solution was purified by dialysis in water for several days, using a cellulose tube, in order to remove urea, sodium hydroxide and other impurities using UV absorption spectra any traces may be observed. Afterwards, the aqueous solution was treated with HCl (6 M) and insoluble by-products were removed by filtration. The filtrate was dialyzed again in distilled water for a week, and concentrated in vacuum. Pellets from the viscous solution were dropped on a polyethylene sheet. Following air drying, the pellets were kept in vacuum desiccators over phosphorous pentoxide. Some samples were prepared using dimethylformamide (DMF) as dispersant for cellulose, urea and/or dicyandiamide (DCDA) catalyst. A solution of phosphorous acid in DMF was added in that mixture at 130–140 °C for different periods of time, and in different rates between reactants. Samples were similarly purified.
2.2.2 Methods for characterization of phosphorylated cellulose
FT-IR analysis was performed with a Vertex 7 Spectrometer.
The energy of the hydrogen bonds had been evaluated for cellulose with the following formula [27]:
where: νo is standard frequency corresponding to free OH groups (3,650 cm); ν is the frequency of the bonded OH for cellulose (3554, 3492, 3428, 3359, 3285 and 3222 cm) groups, while k is a constant equal with 1.62 × 10−2 .
For phosphorylated cellulose the frequency of bonded OH was appreciated at 3,380, 3,310 and 3,230 cm−1.
The hydrogen bonding distances were calculated using equation [28].
where Δν = νO − νH, νO = OH stretching frequency (= 3,600), νH = stretching frequency observed in the infrared spectrum of the sample, R = hydrogen bonding distances.
The substitution degree (DS) was calculated using the following deduction [29].
C6H7O2 = 111.1 − Weight of structural unit of cellulose without functional groups, OH = 17 − weight of hydroxyl group, OPH(O)OH = 81 − Weight of substituted phosphonic group, M = C6H7O2[OPH(O)OH]DS, (OH)(3 − DS) = weight of structural unit of phosphorylated cellulose, 3 = number of hydroxyl groups.
Thus,
But phosphorus content
so that
The phosphorus content was determined by the molybdenum-blue method [30].
2.2.3 Static contact angle measurements
Polymer surface properties were evaluated by static contact angle measurements using sessile-drop method and a CAM-101 contact angle measurement system equipped with a liquid dispenser, video camera and drop-shape analysis software (KSV Instruments, Helsinki, Finland). The measurements were performed on a solid film of PC separately prepared from a concentrate solution of PC deposited on a horizontally polyethylene support. The film was dried in air and then kept in desiccators. This film is quite hygroscopic and the determinations should be performed as fast as possible. Prior testing, the film was placed and fixed on the glass support; then, liquid drops with a volume of about 1 μl were placed, with a Hamilton syringe, on the polymer surface and the image was computed. For each drop, 10 photos were recorded at an interval of 0.016 s. All measurements were performed at room temperature. Double distilled water and ethylene glycol were used as solvents. For each liquid, three different surface regions were selected to obtain a statistically significant result.
31 P NMR, and 13 C NMR analysis were performed by using solution in D2O. The NMR spectra have been recorded on a Avance III 400 spectrometer (Bruker Biospin, Rheinstetten, Germany), equipped with a 5 mm inverse detection, z-gradient probe, operating at 161.97 MHz for 31P nuclei. The 31P chemical shifts are electronically referenced to external 85 % H3PO4 (δ = 0 ppm).
The 1D 31P NMR experiments were recorded using standard pulse sequence with proton decoupling, as delivered by Bruker with TopSpin 2.1 PL6 operating software. The NMR parameters were: 128 scans, a 15 μs pulse, 2 s relaxation delays and a spectral window of 400 ppm.
Microscopic surface information was performed on a Leica Microscope DM2500 M (Germany), magnification order 3200 and 3500. AFM microscopy observation was performed by the topographical module of a Park SYSTEMS XE -100 microscope on a phosphorylated cellulose dried film disposed from a volume of 500 μl polymer dissolved in PBS. The film was deposited on a polyethylene terephthalate membrane of 5 × 5 cm and dried in a closed thermostated low velocity shaker.
X-ray diffraction (XRD) patterns of both cellulose and modified cellulose were collected on a Dron-2 apparatus equipped with a Co anticathode, using Co Kα1 radiation at λ = 1.78892 Å.
2.2.4 Cytocompatibility assays
Phosphorylated cellulose cytocompatibility was tested by direct contact with normal NHDF and HOS cells while cell viability was evaluated by MTT assay and PI staining followed by flow cytometry cell count.
2.2.4.1 Phosphorylated cellulose pellet preparation
Round pellets were obtained by pouring 0.5 ml solution of viscous derivatized cellulose on a polyethylene sheet. Pellets were dried under vacuum and kept away from rehydration. While the phosphorylated cellulose formed an acidic gel (pH 5.5) in a 10 mM FBS solution, the resulted pellets were dissolved in NaOH-glycine buffer (1 M) with a pH of 9.8 and incubated for 24 h in a thermostated shaker, at 37° C. The next day, the obtained gel was neutralized by HCl 1 mM solution until the measured pH stabilized at 7.4 (pH determination used a Hanna 120 pH-meter). The gel was poured on thin glass cover slips of 22 × 22 cm and then dried for 24 h in a thermostat at 48° C. Samples were sterilized by an UV transilluminator (UV lamp 100 W) for 15 min and then placed into the six-well plates in sterile conditions (laminar flow hood).
2.2.4.2 Cell culture preparation
NHDF were subcultured for 3 days in 75 cm2 flasks until confluence was obtained. Culture media was removed and cells were detached by tripsin-EDTA (2 ml/flask), washed by HEPES buffer and centrifuged at 300 g for 5 min, then resuspended in 10 ml fibroblast basal medium (FBM) with growth factors and 10 % fetal bovine serum (FBS), l-glutamine and antibiotic/antimycotic. Suspended cells were then poured into three-wells (3 mL medium with cells)—2 with phosphorylated cellulose and one witness (clear) well, as described further for the direct contact assay. Cell behavior was observed by a Nikon Eclipse TE300 phase contrast microscope, with image capture system. The cell growth was examined on an epifluorescence inverted Olympus IX51 microscope.
HOS cells were thawed and washed for DMSO removal, in complete growth medium (MEM with 1 % antibiotic/antimycotic supplemented by 10 % FBS) and then centrifuged at 300 g for 5 min. Resuspension in complete growth medium and seeding for proliferation in 75 cm2 sterile cell culture flask (Corning) was performed.
At 48 h, prior testing onto neutralized derivatized cellulose pellets, both tested cell types were detached by trypsin, resuspended in complete medium, counted and dispensed in six-well culture flasks (Corning) as described further. Plates were covered by their lid and incubated at 37 °C in a 5 % CO2 humid atmosphere. Observations were performed following another 48 h of incubation, by the above described phase contrast/epifluorescence microscopes.
2.2.4.3 Cell culture direct-contact assay
Phosphorylated cellulose pellets were prepared as described in Phosphorylated cellulose pellet preparation and placed into the six-well plates in sterile conditions. To ensure a direct contact with the phosphorylated cellulose pellets, cells were seeded at a density of 1 × 105 cells per well, suspended in a drop of 30 μl complete culture medium. Following 30 min incubation in standard conditions, extra medium was gently added up to a volume of 3 ml/well. Incubation was performed for a maximum of 6 days for NDHF cells and 3 days for HOS cells due to different proliferation rate.
2.2.4.4 Cell viability by MTT assay
is a versatile method used to evaluate the cell survival following incubation with extraction liquid (LEx) from the investigated samples [31]. It is a colorimetric method that uses a tetrazolium salt (MTT) which is transformed by mitochondrial dehydrogenases in purple formazan granules that can be subsequently dissolved by DMSO [32]. Results were counted on spectrophotometric reading plates at 570–590 nm. To demonstrate fibroblast viability in contact with pellets of phosphorylated cellulose, MTT assay was performed on human fibroblasts incubated with extraction fluid (phosphorylated cellulose pellets incubated with fibroblast cell culture media), according to EN 30993-5 protocols. Briefly, UV sterilized phosphorylated cellulose pellets were placed in sterile fibroblast culture media respecting a ratio of 3–6 cm2/ml between the apparent immersed surface and the extraction liquid volume. While direct-contact assays showed cell proliferation (NDHF or HOS) from 24 h of incubation, the extraction liquid was harvested following 72 h of incubation, to allow any toxic leachate to be liberated. Following an 80 % confluence for the cultured fibroblasts in 25 cm2 filter flasks, cells were detached by trypsin and placed in 24 well plates (Corning), at a density of is 2 × 105, while each well received 1 ml of complete culture media. After 24 h when cell confluence was reached, in each of the wells from 1st lane it was added 1 ml extraction liquid, from three concentrations (100, 50 and 25 %). For each concentration, there was a control sample that remained untreated and received an equal volume of medium. Following medium removal, cells were incubated with MTT solution (1 mg/ml in PBS) for 3 h and the resulting formazan was dissolved by DMSO (100 μl). Absorbance was measured at 590 nm using an automated multiplate reader (Pharmacia LKB Ultrospec Plus). Cell viability was expressed as percent compared to control lanes (blank—culture medium without cells; control—culture medium with cells) according to the formula CV = 100 × (ODs − ODb)/(ODc − ODb), where ODs represent the optical density (in units) for the sample, ODb–the optical density for the blank wells and ODc–the optical density for the control wells. Assays for each extract were carried out in six replicates, including untreated cell control and the blank cell-free control.
2.2.4.5 Cell viability assay by flow cytometry
Cell viability was evaluated by observing changes in membrane permeability and/or physiological state, and expressed by the exclusion of vital dyes. The fluorescent, DNA-binding probes propidium iodide (PI) was used and cell count was performed by a FACS CantoII and FACS Diva software (Beckton-Dickinson, USA). Confluence normal human fibroblasts scraped from 24 well plates incubated with extraction liquid from phosphorylated cellulose (100, 50 and 25 %) were distributed in FACS polystyrene tubes, in 1 ml PBS with 2 μg/ml PI. Flow cytometry assays were performed in duplicate and fluorescence emission was read on PerCP-Cy5.5 channel, with an excitation laser line of 488 nm and a maximum emission at 695 nm.
3 Results and discussions
3.1 Reaction of cellulose with phosphorous acid
Cellulose reaction with phosphorous acid was performed at 150 °C for several reaction times and different reaction conditions (Table 1).
The phosphorylation reaction of cellulose in molten urea proceeded at high temperature (150 °C). The maximum DS of cellulose corresponded to a reaction time of 180–300 min. In our experiments, up to this time, only darkly, insoluble products were obtained. In DMF dispersion medium, the phosphorylation proceeded at slightly low temperature, but a part of the resulted product is insoluble. A higher ratio urea–H3PO3 favors a higher content in phosphorus beyond a reaction time of 5 h. While Isogai method was used to dissolve cellulose in aqueous NaOH, the subsequent reaction with phosphorous acid led to a gel-like material with an acceptable DS.
The employment of a catalyst (DCDA) led to lowest DS while Evan Gospodinova reported a DS = 2.8, obtained after 120 min of microwave irradiation at 105 °C, and with no supplementary information regarding the resulted phosphorylated cellulose solubility [20, 33]. The phosphorylated cellulose with a DS = 0.97 was selected for the present study, due to better solubility and film forming properties.
Literature data concerning potentiometric/conductometric titration conclude that cellulose derivatized with phosphorous acid behaves as a monobasic acid and titratable phosphorus is bond by a unique link to the cellulose chain [24, 34]. Cellulose derivatized with phosphorous acid has the same density of surface OH groups compared to unmodified cellulose. OH function on the original cellulose (linked to C6) is substituted by only one OH group provided from phosphorous acid. Thus, the density of OH groups is the same in cellulose and in the modified one. Curtis et al. [35] showed that OH groups are required for cell adhesion, although higher densities lowered the adhesion. With respect to this observation, our choice was the cellulose bearing phosphorous group, with no enhancement for OH groups concentration, compared to cellulose bearing phosphate group.
A comparison between our derivatized cellulose and others cellulose bearing phosphate groups is not adequate. Granja indicate a higher DS (transformation degree) between 1.14 and 2.5 [34]. Highly phosphorylated cellulose gel absorbed water in larger amounts than did amorphous cellulose, confirming that its high water sorption is not solely the result of its low crystallinity. Hence, the high water swelling observed is closely related to the functionality of phosphate groups themselves. Kim et al. [36] reported a surface phosphorylation of cellulosic membrane with a lower DS and related that the surface wettability of samples with different DS is quite similar.
On the other hand, the cellulose modified with phosphorous acid has only one negatively charged function, which OH bonded to phosphorus atom. Hence, negatively charged surface of derivatized cellulose corresponds to the number of phosphorus atoms determined, respectively to DS.
3.2 Characterization of phorphorous acid-derivatized cellulose
3.2.1 FT-IR spectra
A typical infrared spectrum of phosphorylated cellulose (PC), in comparison with starting cellulose (C), for regions of interest, is shown in Fig. 1.
Two regions of the FT-IR spectra, namely 3,800–2,000 cm−1 and 1,800–800 cm−1, were better observed. The infrared bands between 3,800 and 2,000 cm−1 correspond to the OH stretching frequencies, asymmetric and symmetric CH2 valence vibration of cellulose and new stretching frequency of phosphorylated cellulose: a sharp band at 2,384 cm−1 certify the presence of P–H group. The infrared region between 2,000 and 800 cm−1 is more difficult to perform because of superposition of stretching frequencies. FT-IR spectrum of phosphorylated cellulose could be observed bands at 1,216 cm−1 corresponding to P=O bond, at 1,095 cm−1 attributed to the P–O–Alkyl stretching and at 917 cm−1 corresponding to P–OH stretching. In Table 2 there are presented the position and assignments of principal bands from IR spectra of cellulose (C) [37] and phosphorylated cellulose (PC) [19, 37].
The 3,460–3,405 cm−1 range, characterizing intra and intermolecular hydrogen bonds involving C6 position, was moved in PC spectrum to lower wavenumber. A broad peak between 3,600–2,852 cm−1 covered O3–H3–O5 intramolecular H bonds, O6–H6–O3 intermolecular H bonds from cellulose and asymmetric CH2 vibration valence. Comparing energy of hydrogen bonding and hydrogen bonding distances for C and PC (Table 3), some connections with the properties of PC could be achieved.
Data in Table 3 show that energies of hydrogen bonds are lower for PC versus C. Concerning the lower values for energies of hydrogen bonds, they could be related to the disruption of those bonds after the esterification reaction.
3.2.2 The contact angle measurements
The material which contacts a polymer during the film preparation process affects the properties of the polymer surface [38]. To avoid this effect, the film surface opposite to the side contacting the glass plate during film preparation has been used.
Measurement of contact angle in water for pure cellulose films indicates values that vary in connection with crystallinity degree (CI) [39]. For example: a θ = 40.5, for regenerated cellulose films from LiCl-DMAc (CI = 6), while θ = 11.5 for microcrystalline cellulose (CI = 78). A higher θ = 42 was recovered for amorphous cellulose. The highest θ = 54.55 could be also related to the amorphous structure of PC [18].
The contact angle measurements were performed for derivatized cellulose (PC) to demonstrate the relationship between the properties and chemistry of a surface: wettability of phosphorylated cellulose surface varies owing to the polarity of the functional groups P–OH. Measuring the contact angles in two liquids (water and ethylene glycol), interesting data concerning surface free energy (γSV), and work of adhesion (W) or interfacial tension (γSL) could be determined. Table 4 presents the results of contact angle measurements performed for front of phosphorylated cellulose films. These values show that the work of adhesion is more important in water than in ethylene glycol, which means that water adheres better then ethylene glycol to the phosphorylated cellulose. The higher surface free energy indicates a good adherence of studied liquids to the surface of phosphorylated cellulose. A small part of solid–liquid interfacial tension value could be related to a good biocompatibility of the phosphorylated cellulose [40].
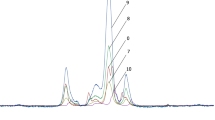
3.2.3 31P and 13C NMR spectra
31P NMR spectra of phosphorylated cellulose was also studied (Fig. 2a). According to published 31P NMR data on phosphorous organic derivatives, the observed resonances in the 2.5–7.5 ppm region correspond to those of monosubstituted phosphorous acid esters [41]. The peaks corresponding to the three positions of substitution were observed in the spectrum of phosphorylated cellulose with DS = 0.97. A signal at 2.58 ppm was assigned to P–O–C6. The doublets at 4.99–5.29 and at 7.38 ppm were assigned to P–O–C2 and P–O–C3, respectively. All the signals split into doublets when proton decoupling is not applied. This result confirms the formation of monosubstituted phosphorous acid esters of cellulose.
Concerning the 13C NMR spectra, data allows to assign the peak at 105 ppm to the C1 and the bands in the 70–80 ppm region to the C2, C3, and C5 carbons of cellulose [42, 43]. A sharper resonance at 89 ppm and the broader resonance at 84 ppm are assignable to C4; the narrower peak at 66 ppm and the broader peak at 63 ppm are due to C6 resonances. The 13C NMR spectrum is presented in Fig. 2b. Chemical shifts of carbon bearing OH groups available for substitution (C6, C2, C3) are moved to lower frequency after the phosphorylation. The peak at 80–84 ppm assigned to C4 in crystalline regions completely disappeared, suggesting the breaking up the crystalline lattice of microcrystalline cellulose after the reaction with phosphorous acid. No comparison with literature data could be performed for 13C NMR cellulose spectra, while solid-state NMR determination was used in this situation.
3.2.4 X-ray diffraction patterns
X-ray diffraction patterns of unmodified cellulose (C) and phosphorylated cellulose in solution (PC-sol) and in melt (PC-m) are presented in Table 5. A crystallinity decrease is observed for the sample phosphorylated in DMF dispersion medium, whereas for sample prepared in molten urea, only an amorphous product was obtained. Using Segal approximate determination of crystalline index, a qualitative comparison could be made between samples with different substitution degree [44].
3.2.5 Microscopic surface information and AFM microscopy
The micrographs of Avicel clearly show its nonfibrous nature and the presence of pinholes at its surface [16]. Figure 3 shows a smooth surface of PC film. Some irregularities resulted from polyethylene substrate used to deposit and dry the film.
3.3 Cytocompatibility of the phosphorylated cellulose using normal NHDF and HOS cell lines
In this study, the cellulose was chemically modified by introducing phosphonic groups, to observe the effect of those groups on cellular grow and survival. The phosphorylated cellulose pellets were prepared and submitted to direct contact and viability assays as described in materials and methods. For direct contact assay on phosphorylated cellulose layer, at 6 days from cultivation it was observed a good fibroblast proliferation with a confluence trend (Fig. 4). However, due to a lack of transparency for the cellulose layer spread on the coverslip, a tetracycline staining solution as fluorophore (225 mM) was used for cytoplasm labeling. Following tetracycline application on the wells, the coverslips are gently washed for five times in PBS, and then examined for cell proliferation using an inverted epifluorescence microscope. At 3 days, few proliferating fibroblasts were present on the phosphorylated cellulose layer (Fig. 5) while at 6 days, the amount of living fibroblasts was significantly increased (Fig. 6).
NHFB grown at 6 days of incubation on a coverslip covered with a thin film of phosphorous acid derivatized cellulose. 1 coverslip limit; 2 cellulose film over the coverslip limits; 3 confluence grown fibroblasts on the clear well bottom in close neighborhood with the phosphorylated cellulose (40 × magnification, phase contrast)
NHFB grown at 3 days following incubation on a coverslip covered by a thin film of phosphorous acid derivatized cellulose. 1 coverslip limit; 2 tetracycline crystal; 3 dead/apoptotic fibroblasts on the phosphorylated cellulose; 4 normal grown fibroblasts; 5 Lamellipodium on a fibroblast (×1000, 225 mM Tetracycline incubation for 1 min as fluorophore)
NHFB grown at 6 days of incubation on a thin film of phosphorous acid derivatized cellulose a 1 derivatized cellulose pellet edge; 2 fibroblast layer (×1,000, 225 mM Tetracycline incubation for 1 min as fluorophore). b Subconfluent fibroblast layer on derivatized cellulose pellet (×1,000, phase contrast)
A thin layer of phosphorylated cellulose show a good cytocompatibility for normal human dermal fibroblasts, following 3 and 6 days of incubation. This represents an encouraging result, mainly because fibroblasts are sensitive to pH variations and our neutralizing method has stabilized the phosphorylated gel at pH 7.4 [45]. Fibroblast proliferation is performed at a slower rate on the biomaterial thin film compared to witness wells or to clear well bottom in close neighborhood of the phosphorylated cellulose.
Direct contact proliferation assay with HOS showed not only a good cytocompatibility of the derivatized cellulose pellicles but also the interesting ability of the osteoblast-like tumor cells to aggregate into spheroid-like structures (Fig. 7). As demonstrated earlier [13], mammalian cells (Chinese hamster ovary-CHO) adhesion to phosphate groups grafted cellulose membranes is impaired and not dependent on cation or protein concentration in the culture medium but on the substrate. Same authors observed that CHO cells in aggregates grown at cellulose phosphate surface show a low proliferation rate. At the same time, lack of adherence is inducing anoikis in normal cells while tumor cells with metastatic potential may escape from this process. HOS cells are proliferating at a higher rate in contact with the proposed derivatized cellulose while they are not forming 2D layers at the pellet surface. Reduced adherence and spheroid formation on the pellet surface is probably due to lack of adherence while escape from anoikis is due to the malignant properties of these cells. This observation suggests further application for this polymer support as an ionic cross-linker into three dimensional culture scaffolds for tumor cells.
Osteoblast-like tumor cells (HOS) aggregated into spheroid-like structures on thin film of phosphorous acid derivatized cellulose (×400). a 1 derivatized cellulose pellet; 2 HOS layer at subconfluence (2 days of incubation) (×400 phase contrast). b HOS forming spheroid-like structures (3 days of incubation) (×400, 225 mM Tetracycline incubation for 1 min as fluorophore)
Fibroblast viability in contact with pellets of phosphorylated cellulose was evaluated by MTT assay. MTT assay results showed very good cell viability in contact with all three concentrations from extraction liquid (LEx 100, 50 and 25 %). Cell viability, expressed as a percentage of the control culture values shows a 94 % survival rate for LEx 100 %, 97 % for LEx 50 % and 98 % for LEx 25 %.
While MTT test is reproducible but not always accurate, an assessment of cell viability by flow cytometry using propidium iodide staining was performed. PI staining reproduced MTT results consisting in very good cell viability in contact with all three concentrations from phosphorylated cellulose extraction liquid (LEx 100, 50 and 25 %) (Fig. 8). Cell viability percentage recorded values were of 86.06 % for LEx 100 %, 89.45 % for LEx 50 % and 90.40 % for LEx 25 %, compared to normal untreated cells (92.92 %). Dead cell presence can be explained by trypsin treatment prior to flow cytometry assay for the untreated cells, and covers a certain ratio from the dead cells in treated samples.
Cell viability following flow cytometry assay using propidium iodide staining. a Unstained cells; b Untreated cells; c Cells treated by extraction liquid 100 % from PC; d Cells treated by extraction liquid 50 % from PC; e Cells treated by extraction liquid 25 % from PC; f cell viability rate, compared to untreated cells (NHDF normal human dermal fibroblasts; PC_100, 50, 25—100, 50, 25 % extraction liquid concentrations from derivatized cellulose)
Viability assays indicate that the new proposed phosphorylated cellulose shows lack of toxicity and a good proliferation support for normal human fibroblasts.
The proposed derivatized cellulose possesses not only a higher phosphorylation degree but is also a hydrosoluble polymer that may be considered as scaffold for in vitro cell culture and in vivo tissue regeneration.
4 Conclusions
The derivatization of cellulose with phosphorous acid was performed using the reaction of microcrystalline cellulose with phosphorous acid-urea mixture, in molten state. Completely water soluble films with a substitution degree close to one were obtained and characterized by FT-IR, 31P NMR and 13C NMR, contact angle measurements, metallographic microscopy and atomic force microscopy and X-ray diffraction. Phosphorous acid derivatized cellulose samples present an amorphous morphology, with a smooth surface of the film. The good cytocompatibility of the tested samples in direct contact with normal human dermal fibroblasts or with human osteosarcoma cells is advocated by cell proliferation at 3 and 6 days of incubation together with viability assays (MTT and flow cytometry using propidium iodide staining). Phosphorous acid derivatized cellulose would offer a promising biomaterial, useful as scaffolds for new biopolymer composites, and subject for further development as an ionic crosslinker.
References
Enderle JD, Blanchard SM, Bronzino JD. Introduction to biomedical engineering. San Diego: Academic Press; 2000.
Kennedy JF, Phillips GO, Williams PA, Cellucon Conferences (Organization), Sen’i Gakkai (Japan). Cellulose : structural and functional aspects. Ellis Horwood series in polymer science and technology. Chichester/New York: Ellis Horwood/Halsted Press; 1989.
Märtson M, Viljanto J, Hurme T, Laippala P, Saukko P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials. 1999;20(21):1989–95. doi:10.1016/s0142-9612(99)00094-0.
Granja PL, De Jeso B, Bareille R, Rouais F, Baquey C, Barbosa MA. Mineralization of regenerated cellulose hydrogels induced by human bone marrow stromal cells. Eur Cell Mater. 2005;10:31–7; discussion 7–9.
Barbosa MA, Granja PL, Barrias CC, Amaral IF. Polysaccharides as scaffolds for bone regeneration. ITBM-RBM. 2005;26(3):212–7. doi:10.1016/j.rbmret.2005.04.006.
Barbié C, Chauveaux D, Barthe X, Baquey C, Poustis J. Biological behaviour of cellulosic materials after bone implantation: preliminary results. Clin Mater. 1990;5(2–4):251–8. doi:10.1016/0267-6605(90)90023-o.
Granja PL, Pouységu L, Pétraud M, De Jéso B, Baquey C, Barbosa MA. Cellulose phosphates as biomaterials. I. Synthesis and characterization of highly phosphorylated cellulose gels. J Appl Polym Sci. 2001;82(13):3341–53. doi:10.1002/app.2193.
Granja PL, Pouységu L, Deffieux D, Daudé G, De Jéso B, Labrugère C, et al. Cellulose phosphates as biomaterials. II. Surface chemical modification of regenerated cellulose hydrogels. J Appl Polym Sci. 2001;82(13):3354–65. doi:10.1002/app.2194.
Fricain JC, Granja PL, Barbosa MA, de Jéso B, Barthe N, Baquey C. Cellulose phosphates as biomaterials. In vivo biocompatibility studies. Biomaterials. 2002;23(4):971–80. doi:10.1016/s0142-9612(01)00152-1.
Granja PL, Barbosa MA, Pouységu L, De Jéso B, Rouais F, Baquey C. Cellulose phosphates as biomaterials. Mineralization of chemically modified regenerated cellulose hydrogels. J Mater Sci. 2001;36(9):2163–72. doi:10.1023/a:1017587815583.
Granja PL, Ribeiro CC, De Jeso B, Baquey C, Barbosa MA. Mineralization of regenerated cellulose hydrogels. J Mater Sci Mater Med. 2001;12(9):785–91.
Mucalo MR, Kato K, Yokogawa Y. Phosphorylated, cellulose-based substrates as potential adsorbents for bone morphogenetic proteins in biomedical applications: a protein adsorption screening study using cytochrome C as a bone morphogenetic protein mimic. Colloids Surf B. 2009;71(1):52–8. doi:10.1016/j.colsurfb.2009.01.004.
Kim SS, Jeong WY, Shin BC, Oh SY, Kim HW, Rhee JM. Behavior of CHO cells on phosphated cellulose membranes. J Biomed Mater Res. 1998;40(3):401–6.
Nifant’ev EE. The Phosphorylation of Cellulose. Russ Chem Rev. 1965;34(12):942–9. doi:10.1070/RC1965v034n12ABEH001577.
McCormick CL, Callais PA, Hutchinson BH. Solution studies of cellulose in lithium chloride and N,N-dimethylacetamide. Macromolecules. 1985;18(12):2394–401. doi:10.1021/ma00154a010.
Ramos LA, Assaf JM, El Seoud OA, Frollini E. Influence of the supramolecular structure and physicochemical properties of cellulose on its dissolution in a lithium chloride/N,N-Dimethylacetamide solvent system. Biomacromolecules. 2005;6(5):2638–47. doi:10.1021/bm0400776.
Isogai A, Atalla RH. Dissolution of cellulose in aqueous NaOH solutions. Cellulose. 1998;5(4):309–19. doi:10.1023/a:1009272632367.
Petreuş O, Bubulac T, Petreuş I, Cazacu G. Reactions of some phosphorus compounds with cellulose dissolved in aqueous alkaline solution. J Appl Polym Sci. 2003;90(2):327–33. doi:10.1002/app.12532.
Inagaki N, Nakamura S, Asai H, Katsuura K. Phosphorylation of cellulose with phosphorous acid and thermal degradation of the product. J Appl Polym Sci. 1976;20(10):2829–36. doi:10.1002/app.1976.070201017.
Gospodinova N, Grelard A, Jeannin M, Chitanu GC, Carpov A, Thiery V, et al. Efficient solvent-free microwave phosphorylation of microcrystalline cellulose. Green Chem. 2002;4(3):220–2.
Inagaki N, Katsuura K. Modification of cellulose phosphonate with N, N-dimethylacrylamide and 4-vinylpyridine, and flame-retardant properties of the products. J Polym Sci. 1978;16(11):2771–9. doi:10.1002/pol.1978.170161105.
Luneva N, Petrovskaya L, Ezovitova T. Synthesis and properties of cellulose phosphates. Russ J Appl Chem. 2007;80(11):1923–7. doi:10.1134/s1070427207110298.
Weil ED, Levchik SV. Flame retardants for plastics and textiles: practical applications. Cincinnati: Hanser; 2009.
Suflet DM, Chitanu GC, Popa VI. Phosphorylation of polysaccharides: new results on synthesis and characterisation of phosphorylated cellulose. React Funct Polym. 2006;66(11):1240–9. doi:10.1016/j.reactfunctpolym.2006.03.006.
Granja PL, Jéso BD, Bareille R, Rouais F, Baquey C, Barbosa MA. Cellulose phosphates as biomaterials. In vitro biocompatibility studies. React Funct Polym. 2006;66(7):728–39. doi:10.1016/j.reactfunctpolym.2005.10.027.
Petreuş O, Cazacu G, Vasile C, Bubulac T. Noi metode de sinteză a celulozei fosforilate şi utilizarea sa ca biomaterial. Celuloză şi Hârtie. 2003;52(20):20–6.
Simionescu C, Butnaru R, Rozmarin G. Investigation the field of the supermolecular structure of cellulose. Cellulose Chem Technol. 1973;7:153–69.
Pimentel GC, Sederholm CH. Correlation of infrared stretching frequencies and hydrogen bond distances in crystals. J Chem Phys. 1956;24(4):639. doi:10.1063/1.1742588.
Rozmarin G, Simionescu C, Bulacovschi V, Butnaru R. Chimia lemnului si a celulozei. Iasi: Litografia Institutului Politehnic; 1973. p. 175–93.
Merz W. Eine mikroanalytische schnellmethode zur bestimmung von phosphor sowie zur gleichzeitigen bestimmung von phosphor und halogen in organischen substanzen. Microchim Acta. 1959;47(3):456–65. doi:10.1007/bf01216866.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4.
Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3(7):207–12.
Suzuki M, Yoshida T, Koyama T, Kobayashi S, Kimura M, Hanabusa K, et al. Ionic conduction in partially phosphorylated poly(vinyl alcohol) as polymer electrolytes. Polymer. 2000;41(12):4531–6. doi:10.1016/s0032-3861(99)00682-5.
Granja P, Pouysegu L, Petraud M, De Jeso B, Baquey C, Barbosa M. Cellulose phosphates as biomaterials. I. Synthesis and characterization of highly phosphorylated cellulose gels. J Appl Polym Sci. 2001;82(13):3341–53.
Curtis A, Forrester J, Clark P. Substrate hydroxylation and cell adhesion. J Cell Sci. 1986;86(1):9–24.
Kim S, Jeong W, Shin B, Oh S, Kim H, Rhee J. Behavior of CHO cells on phosphated cellulose membranes. J Biomed Mater Res. 1998;40(3):401–6.
Popescu C, Tibirna C, Raschip I, Popescu M, Ander P, Vasile C. Bulk and surface characterization of unbleached and bleaced softwood kraft pulp fibers. Cellulose Chem Technol. 2008;42(9–10):525–7.
Bismarck A, Kumru ME, Springer J. Characterization of several polymer surfaces by streaming potential and wetting measurements: some reflections on acid–base interactions. J Colloid Interface Sci. 1999;217(2):377–87. doi:10.1006/jcis 1999.6345.
Yamane C, Aoyagi T, Ago M, Sato K, Okajima K, Takahashi T. Two different surface properties of regenerated cellulose due to structural anisotropy. Polymer. 2006;38(8):819–26.
De Bartolo L, Morelli S, Bader A, Drioli E. Evaluation of cell behaviour related to physico-chemical properties of polymeric membranes to be used in bioartificial organs. Biomaterials. 2002;23(12):2485–97. doi:10.1016/s0142-9612(01)00383-0.
Gunnars S, Wågberg L. Cohen stuart MA. model films of cellulose:I. Method development and initial results. Cellulose. 2002;9(3):239–49. doi:10.1023/a:1021196914398.
Kolovith L, Ingall E, Benner R. Composition and cycling of marine organic phosphorus. Limnol Oceanogr. 2001;46(2):309–20.
Maciel GE, Kolodziejski WL, Bertran MS, Dale BE. Carbon-13 NMR and order in cellulose. Macromolecules. 1982;15(2):686–7. doi:10.1021/ma00230a097.
Segal L, Creely JJ, Martin AE, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J. 1959;29(10):786–94. doi:10.1177/004051755902901003.
Rathna GV. Gelatin hydrogels: enhanced biocompatibility, drug release and cell viability. J Mater Sci Mater Med. 2008;19(6):2351–8. doi:10.1007/s10856-007-3334-9.
Acknowledgments
This paper was supported by the Research Grant IDEI 2560/2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petreus, T., Stoica, B.A., Petreus, O. et al. Preparation and cytocompatibility evaluation for hydrosoluble phosphorous acid-derivatized cellulose as tissue engineering scaffold material. J Mater Sci: Mater Med 25, 1115–1127 (2014). https://doi.org/10.1007/s10856-014-5146-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5146-z