Abstract
In the last few years, the use of biodegradable magnesium (Mg) alloys has evoked great interest in the orthopedic field due to great advantages over long-term implant materials associated with various side effects like allergy and sensitization and consequent implant removal surgeries. However, degradation of these Mg alloys results in ion release, which may cause severe cytotoxicity and undesirable complications after implantation. In this study, we investigated the cytological effects of various Mg alloys on cells that play an important role in bone repair. Eight different magnesium alloys containing varying amounts of Al, Zn, Nd and Y were either incubated directly or indirectly with the osteosarcoma cell line Saos-2 or with uninduced and osteogenically-induced human mesenchymal stem cells (MSCs) isolated from bone marrow specimens obtained from the femoral shaft of patients undergoing total hip replacement. Cell viability, cell attachment and the release of ions were investigated at different time points in vitro. During direct or indirect incubation different cytotoxic effects of the Mg alloys on Saos-2 cells and osteogenically-induced or uninduced MSCs were observed. Furthermore, the concentration of degradation products released from the Mg alloys differed. Overall, Mg alloys MgNd2, MgY4, MgAl9Zn1 and MgY4Nd2 exhibit good cytocompatibility. In conclusion, this study reveals the necessity of cytocompatibility evaluation of new biodegradable magnesium alloys with cells that will get in direct contact to the implant material. Furthermore, the use of standardized experimental in vitro assays is necessary in order to reliably and effectively characterize new Mg alloys before performing in vivo experiments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the last few years, the use of magnesium (Mg) as a material for biodegradable medical implants like bone implants [1] and vascular stents [2] has attracted great attention. Biodegradable bone implants manufactured out of Mg alloys can be employed in orthopedic surgery, because of their adequate stability supporting the bone in the first period after implantation followed by complete biodegradation within the human body. Slowly degrading implants are regarded as promising therapeutic materials to be used instead of titanium (Ti) or steel implants, since reoperation for implant removal can be avoided [3].
Besides their degradation behavior, the cytocompatibility of Mg alloys plays a pivotal role when considered as implant material. Regarding orthopedic surgery, the particular bone implant gets into contact with osteoblasts, fibroblasts as well as myoblasts, but also resident and invading mesenchymal stem cells (MSCs). The latter are of particular interest, because of their ability to differentiate into cells of the osteogenic lineage and consequently provide bone repair [4, 5]. In in vivo animal studies it has also been shown that Mg-based bone implants have a stimulatory effect on bone repair [6]. However, before clinical application numerous physical and biological tests have to be performed to systematically analyze the cytocompatibility of the respective Mg-based alloy with the surrounding tissue.
In vitro studies analyzing the biocompatibility of Mg alloys with different cell types like fibroblasts or osteoblasts showed that fibroblasts exhibited similar viability when compared to the osteosarcoma cell line (MG63) used as control [7]. On the other hand, primary osteoblast showed increased tolerance towards the Mg alloys, than MG63 [8]. Furthermore, human umbilical cord perivascular (HUCPV) cells, a MSC-like cell population [9], exhibited minor sensitivity in contact to Mg alloys than MG63 [10]. As these contradictory results show, more research on the cytocompatibility of Mg-based alloys needs to be done to systematically investigate the effect of biodegradable Mg-based bone implants on cells of the surrounding tissue. Additionally, there is still a great need for standardized test systems allowing in vitro and in vivo characterization of novel Mg alloys to identify Mg alloys showing good clinical performance [11].
In the present study, we systematically analyzed the direct and indirect effects of various magnesium alloys on the osteosarcoma cell line Saos-2 as well as on primary human MSCs of different donors in vitro. Viability and adhesion of cells on the surface of Mg-based plates were studied at different time points.
2 Materials and methods
2.1 Material
Small Mg plates with a diameter of 10 mm and height of 1 mm containing varying amounts of Al, Zn, Nd and Y (Table 1) were prepared as previously described in order to mimic biodegradable Mg alloys [12, 13]. Briefly, the alloys were manufactured without inclusion of additives like for example Mn. Magnesium was melted at 740 °C under an inert gas mixture (Ar 0.3 % SF6), followed by the addition of the pure alloying elements. In order to ensure a homogeneous element distribution, the melt was continuously stirred and subsequently added to a thin-walled mold for die casting at 680 °C for 1 h before cooling to room temperature through a water shower within 5 min. Identical casting parameters and cooling conditions were used to ensure similar microstructures of the alloys [14]. All plates were polished with SiC (silicion carbide) 1200 in ethanol on both sides followed by an ultrasonic cleaning in ethanol for 3 min.
2.2 Tissue culture
The osteosarcoma cell line Saos-2 was obtained from the American Type Culture Collection (ATCC) and subcultured on tissue culture treated plastic flasks with McCoys A5 (PAA, Pasching, Austria) supplemented with 15 % fetal calf serum (FCS; PAA) and 2 mM l-Glutamine (PAA). Confluence was kept between 50 and 80 % and medium was changed every 2–3 days.
Primary human mesenchymal stem cells (MSCs) were isolated from bone marrow samples by density gradient centrifugation according to a previously described method [15]. The bone marrow specimens were obtained from the femoral shaft of patients undergoing total hip replacement. The patients gave their written consent and the study was approved by the Ethical Committee of the Faculty of Medicine of the University of Tuebingen. Cells were tested for the minimal criteria to confirm their stemness [16]. Cells were subcultured on tissue culture treated plastic flasks and fed with DMEM (low glucose; PAA) supplemented with 10 % FCS and 4 mM l-Glutamine. Confluence was kept between 50 and 80 % and medium was changed every 2–3 days. For the tests, cells of the 4th passage were used.
For osteogenic induction of the MSCs, cells were seeded on 12- or 24-well plates (Costar; Corning, USA) and treated with osteogenic induction medium (1 ml for 12-well and 0.5 ml for 24-well plates) consisting of normal tissue culture medium supplemented with 4 μM Dexamethasone (Sigma-Aldrich, Steinheim, Germany), 10 mM ß-Glycerophosphate Disodium Salt Hydrate (Sigma-Aldrich) and 100 μM l-ascorbic acid 2-phosphate (Sigma-Aldrich) [17] for 13 days before the tests. The undifferentiated control cells were seeded in the same manner and fed with normal tissue culture medium without supplements for 13 days before the tests.
2.3 Direct incubation of magnesium plates with cells
Each of the cell types was seeded on 12-well tissue culture dishes (Costar; Corning, USA). Saos-2 cells were seeded overnight with a density of 4 × 104 cells per well for the 24 and 48 h measurements or 2 × 104 cells per well for the 72 h time point in 1 ml of medium to obtain optimal adherence. A total of 2 × 104 MSCs per well were seeded for all time points. Thereafter, Mg plates were placed on the cells for direct incubation. The metabolic activity was measured using an MTT-assay (AppliChem, Darmstadt, Germany) after 24, 48 and 72 h of incubation at 37 °C.
2.4 DAPI staining of the cells
Cells were seeded directly onto the different plates for 24 h and their attachment was visualized microscopically after DAPI (4′,6-diamidino-2-phenylindole; AppliChem) staining. Cells were washed and fixed one time with DAPI-methanol (1 μg/ml; −20 °C), followed by incubation with DAPI-methanol for 15 min. Cells were rinsed 2 times with methanol (−20 °C) and detection was done at a magnification of 63 × under UV excitation. The analysis was done by quantifying the number of attached cells on the plates in comparison to each other.
2.5 Inductively coupled plasma optical emission spectrometry (ICP-OES)
Additionally, the concentrations of ions released from the Mg plates after incubation for 120 h in Saos-2 cell and MSC media was analyzed using ICP-OES. For each sample, 3 ml of preincubated medium was mixed with 0.5 ml HCl (32 %) to clear the precipitates. The samples were then diluted to a final volume of 10 ml. Measuring was done with an ICP-OES (Optima 4300 DV, Perkin Elmer, Rodgau, Germany). Every element was measured at 2 different wavelengths with 3 repetitions. The mean values were used for evaluation.
2.6 Indirect incubation of the alloys with cells
This test was done to reveal the effects of the metal ions that are released from the Mg plates in the media. Three plates of each Mg alloy were preincubated in 7 ml culture media for 120 h at 37 °C. Afterwards, 0.5 ml of each respective media supernatant was transferred to cells seeded in 24-well tissue culture dishes (Costar; Corning, USA). Saos-2 cells were seeded with a density of 1 × 104 cells per well for the 24 and 48 h measurement and 2 × 104 for the 72 h time point overnight, to obtain optimal adherence. Cell density of the MSCs was 0.5 × 104 per well for all time points. The metabolic activity of the cells was measured after 24, 48 and 72 h of incubation at 37 °C.
2.7 MTT-assay (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazoliumbromid-assay)
The MTT-assay is a technique allowing to measure the activity of the metabolic NAD(P)H-dependent cellular oxidoreductases, making it possible to deduce the viability of cells and by that the cytotoxicity of agents from these metabolic activity [18]. The growth media and, if present, the plates were completely removed and replaced by 300 μl RPMI (PAA) without phenol red, containing 0.5 mg/ml MTT. After 4 h of incubation at 37 °C, the MTT-medium was also removed and the blue formazan products were solubilized by adding 150 μl of dimethyl-sulphoxide (DMSO; Serva, Heidelberg, Germany) to each well for 10 min at 37 °C. Afterwards, 100 μl of this DMSO was transferred to Nunc MaxiSorp flat-bottom 96-well plates (NUNC; Roskilde, Denmark). Absorbance was measured at 540 nm by a microplate (enzyme-linked immuno-sorbent assay) reader. Pure DMSO was used as control [19].
During all experiments titanium plates and media without plates were used as controls.
3 Results
3.1 Direct incubation of the Mg alloys with Saos-2 cells and MSCs
Investigation of different Mg alloys (No. 2, 4–8, Table 1) regarding direct effects on cell viability was performed after incubation with Saos-2 cells as well as with uninduced and osteogenically-induced MSCs for 24, 48 and 72 h.
Saos-2 cells displayed no alterations in viability after direct contact with plates No. 2, 6, 7 and 8 for 24, 48 and 72 h when compared to untreated or Ti plate-treated cells (Fig. 1). The alloys 4 and 5 led to a decrease of viability in Saos-2 cells after 24 h of direct incubation. However, this cytotoxic effect declined after 48 and 72 h.
Viability of isolated MSCs from two different patients undergoing total hip replacement was investigated after direct contact with the Mg plates. For both patients, viability of uninduced MSCs decreased after direct incubation with Mg plates number 2, 4–6 and 8 over time, whereas cells incubated with Mg plate number 7 showed high viability at all time points comparable to the Ti control and untreated cells (Fig. 2a, b).
Cell viability assay with primary human MSCs incubated for 24, 48 and 72 h with Mg and Ti plates as well as with medium (“media”). The assay has been repeated with MSCs from two different patients: Patient 1 uninduced (a) and osteogenically-induced (c) and Patient 2 uninduced (b) and osteogenically-induced (d) MSCs. Ti control plates were used as a standard and adjusted to 100 %. Data are given as mean ± SD; n = 3
Interestingly, cytotoxic effects were almost abolished when osteogenically-induced cells were directly incubated with the different Mg alloys. Independent of the magnesium plate used or the duration of incubation, viability of osteogenically-induced MSCs of patient 1 was not altered when compared to the control groups (Fig. 2c). Only two Mg alloys, i.e. 2 and 6, resulted in a slight decrease in viability of osteogenically induced MSCs of patient 2 after 72 h of incubation (Fig. 2d).
To exclude cytotoxic effects caused by pH changes, pH values were measured after 1 and 24 h in all samples. After 1 h a pH of 8.7 was measured in all samples containing Mg plates compared to untreated medium with a pH of 7.9. After 24 h the pH of all samples remained unchanged.
3.2 Cell attachment on the surface of Mg alloys
As a further index for cytocompatibility, colonization of the different Mg plates was investigated with Saos-2 cells and uninduced MSCs (Table 2; Fig. 3). Saos-2 cell growth was best on the Mg plates number 1, 4, 5, 7 and 8, when compared to the colonization of the Ti control. However, on Mg plates number 2, 3 and 6 only a small amount of cells could be detected after 24 h of incubation.
Similar results were found after incubation of uninduced MSCs isolated from two different patients with the Mg alloys. Again, on plates number 1, 4, 5, 7 and 8 the highest MSC colonization was found. Also, on plate number 2, attached MSCs were detected. On the other hand, MSCs of both patients did almost not attach on the alloy No. 3. MSCs of patient 1 did not attach on the alloy No. 6, whereas high colonization was found after incubation with MSCs of patient 2.
3.3 Release of ions from the Mg alloys
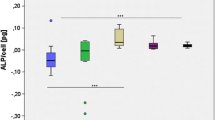
Since corrosion of biodegradable alloys is associated with the release of ions, we measured the concentration of free ions after incubation of eight different biodegradable Mg alloys in Saos-2 cell and MSC media for 120 h. Analysis of the element concentrations in the media revealed an elevated level of Mg ions in all of the samples reaching the highest concentrations in media incubated with plates of the Mg-based alloys 2, 3 and 6 (Fig. 4). Furthermore, Al ions were detected in media incubated with plate 2 and 3. Nd, Y and Zn ions were only detected in traces for some alloys. Nd concentration was highest in DMEM incubated with plate No. 4 with 780 ± 551 μg/l, while the highest Y concentration of 60 μg/l was detected for plate number 8 in McCoy medium.
The highest Zn concentration, i.e. 1.99 ± 0.06 mg/l, was measured in DMEM medium incubated with alloy number 6.
3.4 Indirect incubation of the Mg alloys with Saos-2 cells and MSCs
In order to investigate the effects on cell viability of degradation products released from the different Mg alloys while corrosion occurs, Saos-2 cells as well as uninduced and osteogenically induced MSCs were incubated with medium, which was preincubated with the Mg plates for 120 h.
Incubation of medium preincubated with alloys 2 and 3 with Saos-2 cells led to a decrease in cell viability of almost 100 % after 24 h (Fig. 5), with no recovery after 48 and 72 h. A decline in cell viability after 48 and 72 h was also observed when medium preincubated with the alloy 6 was used. Medium employed after incubation with all other Mg alloys did not affect cell viability when compared to control groups.
In uninduced MSCs, the preincubated medium led to different results of the viability assay in both patients depending on the Mg alloy used. The highest decrease in viability of uninduced MSCs of patient 1 was found when medium of the alloys 2 or 3 was used (Fig. 6a). In uninduced MSCs of patient 2 the highest cytotoxic effects were found when medium preincubated with the alloys 3 or 6 was used (Fig. 6b).
Viability assay with primary human MSCs incubated for 24, 48 and 72 h with fresh media (“media”) or media, which was preincubated with Mg and Ti plates for 120 h. The assay has been repeated with MSCs from two different patients: Patient 1 uninduced (a) and osteogenically-induced (c) and Patient 2 uninduced (b) and osteogenically-induced (d) MSCs. The Ti control was used as a standard and adjusted to 100 %. Data are given as mean ± SD; n = 3
Medium of the alloy No. 1 also strongly decreased viability during 48 h of incubation, but resulted in improved viability after 72 h in patient number 1, whereas viability of uninduced MSCs of patient 2 was not affected. In MSCs of patient 1, medium preincubated with the alloy No. 6 induced a reduction in viability after 48 h, whereas almost no viable cells were detected after already 24 h of incubation with uninduced MSCs of patient 2.
However, viability after treatment with media preincubated with Mg plates 4, 5, 7 and 8 is comparable to the control groups in both patients. Only medium of alloy number 4 resulted in a viability decline after 72 h in uninduced MSCs of patient 2.
Using osteogenically-induced MSCs, viability of cells after incubation with Mg plates varied between the 2 donors. Incubation of osteogenically-induced MSCs of patient 1 with Mg plates number 1, 2, 4 and 5 resulted in a strong and even higher cell proliferation when compared to the control groups (Fig. 6c). After incubation with medium preincubated with plate 3, cytotoxic effects were observed after 72 h. Viability of cells incubated with magnesium plates 6 and 7 already decreased after 24 h, showing recovery after 72 h in the samples incubated with plate No. 7. Regarding osteogenically-induced MSCs of patient 2, plates number 1, 4, 5, 7 and 8 exhibited good cytocompatibility (Fig. 6d). Only with plates number 2, 3 and 6, cell viability declined during incubation.
4 Discussion
In this study, we aimed to identify potential cytotoxic effects on uninduced and osteogenically-induced MSCs caused by direct contact with biodegradable Mg plates or by the emitted ions. In the last years, Mg-based alloys have raised great interest, because of ideal properties as bone implants in orthopedic surgery [1, 3]. Next to their degradation behavior, the cytocompatibility of these alloys is an important factor, which needs to be evaluated before application in animal models and clinical use.
We employed eight different Mg plates containing varying amounts of Al, Zn, Nd and Y, which have been previously investigated regarding their degradation behavior in human whole blood as well as in PBS [13]. In order to evaluate the cytocompatibility of these Mg plates, the osteosarcoma cell line Saos-2 as well as uninduced and osteogenically-induced MSCs were used, since Mg-based bone implants will definitely come into contact with cells of the osseous tissue after implantation. Therefore, in vitro evaluation of potential cytotoxic as well as proliferative effects on MSCs and cells of the osseous tissue are warranted in order to find the optimal Mg-based bone implants exhibiting the greatest benefit and least side effects for the patient.
In the current study, we used the well-described MTT assay, which allows detection of cell viability and by that detection of cytotoxic effects of the test material [18]. In order to avoid false results caused by the interaction of magnesium and its corrosion products with the tetrazolium-based MTT assay and interferences of phenol red with formazan, the media and if present Mg or Ti plates were removed completely from the tissue culture dish before performing the MTT assay [8, 20].
Our data show that Mg alloys releasing high amounts of Mg ions, i.e. plates number 2, 3 and 6 (MgAl3, MgAl9 and MgAl3Zn1), induce cytotoxic effects on MSCs isolated from patients. Furthermore, the release of Al ions, at least in combination with high Mg concentrations, might also increase the cytotoxic effects.
Overall, Mg plates number 4, 5, 7 and 8 (MgNd2, MgY4, MgAl9Zn1 and MgY4Nd2) showed good cytocompatibility after direct and indirect contact with Saos-2 cells and MSCs. These Mg plates also showed a lesser Mg release when compared to the Mg alloys 2, 3 and 6 (MgAl3, MgAl9 and MgAl3Zn1).
The Y and Nd concentrations detected in the release assay are below the lowest concentration used in in vitro test with other primary cells and cell lines [10]. Furthermore, the highest Zn concentration measured is far below cytotoxic Zn concentrations detected in a previous study [21]. Moreover, this concentration is lower than the Zn dosage causing 10 % of cell death in human ECs [22] and thus should not influence cell viability of the MSCs.
Therefore, the observed difference in cytotoxicity caused by the different Mg alloys seems to be mostly dependent on the Mg ions released into the medium during incubation, indicating that a lesser release of degradation products from Mg alloys may contribute to improved cell viability [23]. Furthermore, it has been proposed that next to the degradation rate, the degradation mode also has vital implications determining cytotoxicity and cell attachment [24]. The decrease in cell viability observed after 24 h in some direct incubations followed by a complete recovery after 48 and 72 h could possibly be caused by the massive release of H2 bubbles that occurs in the first 24 h with certain alloys (Fig. 1; No. 4 and 5) [25].
Microscopic analysis of cell attachment on the Mg plates also showed less cell growth on Mg alloys 2, 3 and 6 (MgAl3, MgAl9 and MgAl3Zn1), which can possibly be explained by the fact that these magnesium plates exhibited the highest corrosion degree, which hindered attachment of cells. Furthermore, differences in viability and cell growth caused by pH changes between the Mg plate-treated groups were excluded, because the measured pH values did not vary between the Mg samples.
Differences in viability were also detected when direct or indirect incubation of cells and Mg plates was performed leading to a higher decrease in viability after indirect incubation. One reason might be that the concentration of degradation products in the indirect incubation assay performed with medium preincubated with Mg plates for 120 h was higher compared to direct incubation of cells and Mg plates for up to 72 h only.
Interestingly, direct contact of magnesium alloys with osteogenically-induced MSCs resulted in an overall better viability when compared to uninduced MSCs of the same patient. Apparently, uninduced MSCs are much more sensitive to the degradation products of the Mg alloys than osteogenic progenitor cells. It is therefore absolutely necessary to investigate each type of cell, which potentially gets in contact with the Mg-based implant to get a reliable and complete cytocompatibility evaluation. Furthermore, primary cells need to be used for these tests since cytological performance of Mg alloys is not predictable only by using immortal cell lines.
Recently, we showed that human whole blood has a strong impact on the degradation kinetics of Mg and that these reactions cannot be imitated by simulated body fluids so far [12, 13]. This fact needs to be taken into account when performing cytocompatibility evaluations, since Mg degradation mode and rate gained from in vitro cell culture experiments might differ from the in vivo situation [23, 26].
5 Conclusion
In summary, our data demonstrate the importance of preclinical in vitro tests for cytocompatibility evaluation of Mg alloys. The cytological activity of a biodegradable Mg alloy needs to be evaluated prior implantation with regard to the cell type it gets into contact with. We highly recommend introducing the described testing design to the preclinical test procedures before in vivo application of new Mg-based implants.
References
Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26:3557–63.
Erne P, Schier M, Resink TJ. The road to bioabsorbable stents: reaching clinical reality? Cardiovasc Intervent Radiol. 2006;29:11–6.
Dziuba D, Meyer-Lindenberg A, Seitz JM, Waizy H, Angrisani N, Reifenrath J. Long-term in vivo degradation behaviour and biocompatibility of the magnesium alloy ZEK100 for use as a biodegradable bone implant. Acta Biomater. 2012;9(10):8548–60.
Aldahmash A, Zaher W, Al-Nbaheen M, Kassem M. Human Stromal (Mesenchymal) Stem Cells: basic Biology and Current Clinical Use for Tissue Regeneration. Ann Saudi Med. 2012;32:68–77.
Ciapetti G, Granchi D, Baldini N. The Combined Use of Mesenchymal Stromal Cells and Scaffolds for Bone Repair. Curr Pharm Des. 2012;18:1796–820.
Witte F, Ulrich H, Palm C, Willbold E. Biodegradable magnesium scaffolds: part II: peri-implant bone remodeling. J Biomed Mater Res A. 2007;81:757–65.
Gu XN, Li N, Zheng YF, Ruan L. In vitro degradation performance and biological response of a Mg–Zn–Zr alloy. Mater Sci Eng, B. 2011;176:1778–84.
Fischer J, Pröfrock D, Hort N, Willumeit R, Feyerabend F. Improved cytotoxicity testing of magnesium materials. Mater Sci Eng, B. 2011;176:830–4.
Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–9.
Feyerabend F, Fischer J, Holtz J, Witte F, Willumeit R, et al. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010;6:1834–42.
Liu H. The effects of surface and biomolecules on magnesium degradation and mesenchymal stem cell adhesion. J Biomed Mater Res A. 2011;99:249–60.
Geis-Gerstorfer J, Schille C, Schweizer E, Rupp F, Scheideler L, et al. Blood triggered corrosion of magnesium alloys. Mater Sci Eng, B. 2011;176:1761–6.
Schille C, Braun M, Wendel HP, Scheideler L, Hort N, et al. Corrosion of experimental magnesium alloys in blood and PBS: a gravimetric and microscopic evaluation. Mater Sci Eng, B. 2011;176:1797–801.
Niederlaender J, Rudi P, Schweizer E, Post P, Schille C, et al. Cytocompatibility of magnesium alloys with adult human endothelial cells. Emerg Mater Res. 2013;2:274–82.
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Ardjomandi N, Klein C, Kohler K, Maurer A, Kalbacher H, et al. Indirect coating of RGD peptides using a poly-l-lysine spacer enhances jaw periosteal cell adhesion, proliferation, and differentiation into osteogenic tissue. J Biomed Mater Res A. 2012;100A:2034–44.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Nolte A, Walker T, Schneider M, Kray O, Avci-Adali M, et al. Small-interfering RNA-eluting surfaces as a novel concept for intravascular local gene silencing. Mol Med. 2011;17:1213–22.
Fischer J, Prosenc MH, Wolff M, Hort N, Willumeit R, et al. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater. 2010;6:1813–23.
Goswami M, Yadav K, Dubey A, Sharma BS, Konwar R, et al. In vitro cytotoxicity assessment of two heavy metal salts in a fish cell line (RF). Drug Chem Toxicol. 2014;37:48–54.
Schaffer JE, Nauman EA, Stanciu LA. Cold drawn bioabsorbable ferrous and ferrous composite wires: an evaluation of in vitro vascular cytocompatibility. Acta Biomater. 2013;9:8574–84.
Cipriano AF, Zhao T, Johnson I, Guan RG, Garcia S, Liu H. In vitro degradation of four magnesium-zinc-strontium alloys and their cytocompatibility with human embryonic stem cells. J Mater Sci Mater Med. 2013;24(4):989–1003.
Johnson I, Perchy D, Liu H. In vitro evaluation of the surface effects on magnesium-yttrium alloy degradation and mesenchymal stem cell adhesion. J Biomed Mater Res A. 2012;100A:477–85.
Doepke A, Kuhlmann J, Guo X, Voorhees R, Heineman W. A system for characterizing Mg corrosion in aqueous solutions using electrochemical sensors and impedance spectroscopy. Acta Biomater. 2013;9(11):9211–9.
Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6:1680–92.
Acknowledgments
Special thanks to Dr. N. Hort from Helmholtz Zentrum Geesthacht (Germany) for casting the Mg alloys (AiF-project KF0548101PK7) and to Dr. Richard Schaefer from the Institute of Clinical and Experimental Transfusion Medicine, University of Tuebingen (Tuebingen, Germany) for the kind contribution of the human mesenchymal stem cells.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niederlaender, J., Walter, M., Krajewski, S. et al. Cytocompatibility evaluation of different biodegradable magnesium alloys with human mesenchymal stem cells. J Mater Sci: Mater Med 25, 835–843 (2014). https://doi.org/10.1007/s10856-013-5119-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-5119-7