Abstract
Polyvinyl alcohol (PVA) hydrogel chains were crosslinked by urethane pre-polymer (PPU) in order to fabricate a new substitute for cartilage lesions. The microscopy images showed that the cultured chondrocytes had spherical morphology on PVA–PPU sample after 4 weeks of isolation in vitro. The alcian blue and safranin O staining proved the presence of proteoglycan on the surface of PVA–PPU sample secreted by cultured chondrocytes. This was confirmed by the detection of sulfate ions in the wavelength dispersive X-ray (WDX) analysis. In addition, the expression of collagen type II and aggrecan were observed in chondrocytes cultured on PVA–PPU by RT-PCR. Moreover, the implantation of the PVA–PPU sample with autologous cultured chondrocytes revealed the formation of neocartilage tissue in a rabbit model during 12 weeks follow up. In conclusion, the results verified that isolated chondrocytes cultured on PVA–PPU retain their original phenotype and this composition can be considered as promising substrate for cartilage tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Articular cartilage has a limited capacity of self repair due to its low cellularity and avascular matrix [1]. The major problem with the cartilage healing after the most surgical procedures such as debridement, micro fracture or mosaicplasty, is the formation of fibrocartilage tissue instead of normal hyaline cartilage [2]. It is well known that the rate of metabolic transport in articular cartilage and consequently the rate of natural tissue renewal are relatively low [3]. Therefore, it has been suggested that tissue engineering by the use of hybrid constructs including the autologous chondrocytes and artificial matrixes may be a promising way for the regeneration process of normal cartilage [4]. The mechanical and biological properties of this hybrid structure are the main consideration to promote successful integration between the repaired tissue and the native articular cartilage [5].
A number of biomaterials have been proposed as artificial matrixes including collagen, silk, chitosan, polycaprolactone, polyurethane (PU), polyvinyl alcohol (PVA), poly lactic-glycolic acid and polyethylene glycol [6, 7]. PVA hydrogels are well known for their excellent weight-bearing properties and biocompatibility [8, 9]. However, their resistance to cell attachment is a drawback for tissue engineering application [10]. On the other side, polyurethane compositions are widely used in biomedical application due to their noticeable properties [11]. Studies in animals have revealed that biodegradable polyurethane-based scaffolds can induce healing of the cartilage lesions [12]. Polycaprolactone is used in the synthesis of biodegradable polyurethanes and well believed as a biocompatible polymer [13, 14].
According to the tissue engineering concepts, degradation rate of the scaffold in vivo should be set in proportion with the formation of new extracellular matrix (ECM). Biodegradation decreases the yield strength of the construct and for load bearing tissues such as cartilage; this may lead to the mechanical failure of the structure without healing of the lesion [15]. Therefore, introducing different units with the appropriate degradation profiles in the composition of cartilage scaffolds may improve ECM distribution [16, 17]. In this research, two different units were selected, PVA with lower and polyurethane with higher rate of degradation. It seems that crosslinking of PVA chains with biodegradable urethane chains may be a hopeful idea as a cartilage reconstitution scaffold since the degradation of internal polymeric chains allows the cells to move into the unfilled spaces gradually. Meanwhile, lower degradation rate of PVA may keep the integrity and mechanical properties of the artificial cartilage for longer period of time until the cartilage tissue renewed.
In the previous published work, the physicochemical properties and also cytotoxicity of PVA crosslinked polyurethane were evaluated [18]. In this study, the inductive effect of this composition on chondrocytes activity was analyzed. The autologous chondrocytes from articular cartilage were harvested from humeral head of the rabbits, cultured on this scaffold and implanted in a full-thickness defect created on the femoro-patellar groove of the knee joints for three months.
2 Materials and methods
Polyvinyl alcohol (PVA, Mn = 72000 g/mol, Merck), and Polycaprolactone diol (PCL, Mw = 2000, Aldrich) were dried in a vacuum oven under 50 mbar pressure at 80 °C for 24 h prior to experiment. Hexamethylene diisocyanate (HMDI, Merck) and dimethyl sulfoxide (DMSO, Merck) as a solvent were analytical grades and used as received.
2.1 Sample preparation
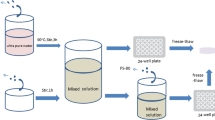
According to the previous published report [18], 12 % solution of PVA in DMSO was prepared using stirrer at 90 °C. NCO terminated urethane chains were synthesized by the reaction of PCL (1 mol) and HMDI (2 mol) in DMSO at 70 °C and titrated according to the ASTM 2572 method. This solution was used as a chemical crosslinker for PVA chains with the mole ratio of 12:1. Three cycles of freeze-thaw process were applied to the PVA samples as a physical crosslinker with 15 h at −25 °C and 9 h at 25 °C (Fig. 1).
2.2 Contact angle measurements
The contact angle formed between a 1 μL drop of MilliQ water and the samples was measured by Kruss K12 measuring system (Germany). On each surface 5 different areas were used to determine the mean value.
2.3 Atomic force microscopy (AFM)
A thin layer of each sample solution was poured on a glass slide and let it to be dried for 7 days. A DualScope™ scanning probe microscope (DS 95-200/50; DME, Denmark) was utilized and surface topography data captured by AFM non-contact mode using AC cantilevers (DME, DS 95-x) with silicon tip (NanoWorld AG, Switzerland).
2.4 Protein adsorption assay
The samples were placed in a protein solution (1 mg/mL) of fetal bovine serum (FBS, Seromed, Germany) and shaken with the speed of 40 rpm for half an hour. They were gently removed from the protein solution, shortly soaked in serial solutions of water, 0.1 and 1 M potassium chloride (KCl) solutions and put in 1 M KCl solution for 24 h. Subsequently, the amount of adsorbed protein was evaluated by Bradford method using coomassie blue G250 (Sigma) dye agent [19]. The SDS–polyacrylamide gel electrophoresis (SDS–PAGE) was used for characterization of the adsorbed proteins by performing the Bio-Rad instruction manual (Mini protein tetracell) and silver nitrate staining procedure.
2.5 Chondrocyte isolation
Articular cartilage shavings were harvested from rabbit proximal humerus and cut into the small pieces. The cartilage slices were predigested with 0.05 % trypsin/EDTA (Sigma) for 1 h following a digestion with 0.08 mg/mL collagenase type II (Sigma) for 12 h in incubator. The isolated chondrocytes were transferred to culture medium and counted with hemocytometer after passing through a 40 μm filter. The culture medium consisted of Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO, Scotland)/Ham’s F12 supplemented with 10 % FBS (Seromed, Germany), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma, USA). Pellet culture was performed by using 4 × 105 cells and the culture medium exchanged every 3 days.
2.6 Microscopy evaluation
The cells (2 weeks after isolation) were initially cultured on the surface of the samples at 2 × 103 cells per a 50 μL of culture medium in 24-well plates (Nunc, Denmark) incubated at 37 °C, 5 % CO2. After 3 h the culture medium (1 mL) was added to each well and kept for 7 and 14 days with the exchange of medium at the 4th day. At the end of the culture, the cells were fixed with the Karnovsky fixation solution for 24 h (2 g paraformaldehyde, 10 mL of 25 % glutaraldehyde solution and 20 mL cacodylate buffer 0.2 M, pH 7.4). In order to observe chondrocytes morphology by SEM, samples were dehydrated in graded alcohols (10, 30, 50, 70, 80, 85, 90, 95, and 100 %) each for 10 min, sputter-coated with gold, and viewed using a scanning electron microscope (SEM, VEGA\\TESCAN). The same protocol were done for local sulfate analysis and observation were done after 14 days of culture with SEM (Philips, XL-30) equipped with the wavelength dispersive X-ray (WDX).
The proteoglycan expression of cultured chondrocytes was determined by staining with alcian blue and safranin O methods with three replications. The stained samples were observed by stereo microscope (Nikon C-SD, Japan).
A solution of 2.5 % glutaraldehyde (diluted from 50 % solution; Merck) in 0.4 M MgCl2 and 25 mM sodium acetate was prepared and alcian blue 8GX (Sigma, Germany) dissolved in this solution to have a concentration of 0.05 %. The samples were washed with acetic acid solution (3 % in distilled water) after staining with alcian blue [20]. The safranin O staining was done according to the protocol published before [21].
2.7 RNA extraction and cDNA synthesis
The isolated chondrocytes were cultured at a density of 5 × 103 cells on the samples (duplicate each one) for two weeks with the medium exchange of every three days. Total RNA was extracted from cultured cells using RNeasy MiniKit (QIAGEN, 74104) according to the manufacturer’s instructions. The RNA concentration was determined by Picodrop at 260 nm wave length with the concentration of 1.9 μg/mL. The cDNA strand was synthesized using 1 μg total RNA in 20 μL of reaction mixture consisting of 4 μL PCR buffer (15×), 2 μL dNTPs (20 mM, Roche, Germany), 1 μL 10 pmol/μL random hexamer (N6; Roche, Germany), 2 μL deionized sterile H2O and 1 μL reverse transcriptase (200 U/μL; Fermentase, Russia). The mixture was incubated at 42 °C for 45 min, followed by 90 °C for 5 min.
2.8 RT-PCR analysis
PCR amplification was performed using specific primers as declared in Table 1 with the marker of GeneRuler™50 bp DNA ladder. The PCR mixture was prepared using 2.5 μL of 10 × PCR buffer, 1 μL of 25 mM MgCl2, 1.5 μL dNTPs (10 mM), 0.5 μL of each primer (10 pmol/μL), 0.1 μL of Taq-DNA polymerase (5 U/μL; CinnaGen, Iran), and 3 μL of cDNA. A number of 37 amplification cycles were applied to the mixture at 92 °C for 30 s, 62 °C for 30 s, 72 °C for 1 min and finally 72 °C for 10 min. The visualization of PCR products were done by running agarose gel (2 %) electrophoresis containing ethidium bromide.
2.9 In vivo experiments
The in vivo study was registered at Faculty of Veterinary Medicine, University of Tehran, Iran and confirmed by its ethics committee. Twelve New Zealand White rabbits weighing 3–3.5 kg (obtained from Pasteur Institute of Iran) were used. Anesthesia was induced with an intramuscular injection of ketamine (50 mg/kg) and xylazine (10 mg/kg). The knee joints of rabbits were exposed through a medial parapatellar approach. The patella was dislocated laterally, and the femoral trochlea was exposed. Full-thickness articular cartilage defects (3 mm in diameter and 2 mm in depth) were created using a drill bit through the articular cartilage.
Autologous chondrocytes were isolated from proximal humerus of each rabbits as described earlier. The isolated cells were cultured on PVA–PPU at a density of 5 × 105 cells per sample and kept for two weeks until implantation. On the experimental side, the samples with cultured chondrocytes were placed into the defect. The other remaining side as a control was filled with a sample without any cell. After implantation, the knee joint synovium, capsule and skin were carefully sutured separately. The rabbits were allowed free movement after operation and sacrificed, 6 after 4 weeks, and 6 after 12 weeks, to assess the cartilage repair regeneration.
2.10 Histological staining
Samples were fixed in neutral buffered formalin (10 %), embedded in paraffin, and observed histologically by hematoxylin- eosin (H&E) staining.
3 Results
Figure 1 shows the schematic representation of PVA crosslinking with biodegradable polyurethane. FTIR spectra confirm the absence of isocyanate peak at 2,270 cm−1 and also presence of carbonyl groups at 1,717 cm−1 in crosslinked sample. The molecular weight between crosslinks was calculated according to the Eq. (1) based on the rubber elasticity theory. Tensile test was performed by preparing a dumbbell-shaped specimen according to ASTM D638 type IV protocols as published previously [18].
where σ is the tensile strength (5.6 × 107 dynes/cm2), α is the deformation ratio (4.14), ρ is the polymer density (1.182 g/cm3), R is the universal gas constant and T is the absolute temperature.
Figure 2a–c shows the dedifferentiation process of isolated rabbit chondrocytes cultured on TCPS after 4 weeks of isolation. These cells have a spherical shape at the time of isolation and their morphology change to the fibroblastic phenotype after 4 weeks. The morphology of these cells on pure PVA is seemed to be spherical. However, due to the hydrophilic structure of the PVA surface, the cells would be attached loosely to the PVA surface and separated with a little turbulence in medium. The SEM images demonstrate the spherical morphology for the cells attached to the PVA and PVA–PPU surfaces (Fig. 3). The chondrocytes attached in a loose manner to the PVA surface (Fig. 3a, b) with the aggregation and colony formation (Fig. 3c). The cell attachment on PVA–PPU surfaces were much more tightly than pure PVA. The chondrocytes used filopodia to form adhesion contacts and distribute on the PVA–PPU substrate (Fig. 3d–i). It seems that addition of polyurethane chains to the PVA improves the cell attachment.
Figure 4 shows the scanned surface of the PVA–PPU sample for determination of sulfate ions (white dots) by WDX. It means that chondroitin sulfate or dermatan sulfate as extra-cellular molecules have been expressed by these cells in the normal condition. The same analysis on pure PVA did not show any sulfate ion on the scanned surface.
According to the contact angle measurements, the water drop made a 55 ± 3 degree angle on PVA–PPU surface, whereas it spread on the PVA surface immediately. The differences between the surface roughnesses of the two samples can be observed by AFM imaging with a higher roughness for PVA–PPU on the range of 0.1–1 μm, while the roughness of PVA sample is under the 0.1 μm (Fig. 5).
The results of protein adhesion test characterized by Bradford method quantified the amount of the attached serum proteins to the surface of the samples (Fig. 6). There was more amount of protein detected for PVA–PPU sample in each washing solution. Evaluation of the washing solutions indicated that these proteins attached more tightly on PVA–PPU sample than pure PVA. These results were confirmed by SDS–PAGE analysis as well and found that the 68 kDa protein attached mainly. The weak band can be observed for 1 M KCl washing solutions of PVA–PPU sample while no band for pure PVA detected (Fig. 7). According to the previous studies, this band is related to the Albumin which its attachment to the surface minimizes the adsorption of other proteins [22].
In order to investigate the phenotypic expression of chondrocytes, two different staining procedures were performed to confirm the existence of proteoglycan on their extracellular matrix (ECM). The alcian blue and safranin O staining showed that the cells attached to the surface of the PVA–PPU sample can secrete proteoglycan molecules after a period of 4 weeks in compare with the pure PVA or TCPS samples (Figs. 8, 9). The consequence of the same procedures for chondrocyte cultured on TCPS also illustrated the presence of proteoglycans at the first two weeks of isolation (Figs. 8a, 9a). However these cells lost their original activity after 4 weeks culture on TCPS or PVA sample (Figs. 8e, f, 9e).
The activity of the chondrocytes, cultured on the PVA–PPU sample, was determined by the analysis of gene expression using RT-PCR method. Collagen type II, collagen type I and aggrecan were selected as the main genetic markers in this assay [23]. Figure 10 indicates that the chondrocytes cultured on the PVA–PPU sample express the collagen type II and aggrecan after 2 and 4 weeks of isolation. In the pellet culture of chondrocytes (as a control), the same bands can be observed. These results obtained without using any growth factor in culture medium. It means that dedifferentiation of the chondrocytes to the fibroblast like cells are controlled by the physicochemical properties of the surface.
Figure 11 shows the procedure of in vivo experiments, isolating chondrocytes from proximal humerus of each rabbits, creating a hole in articular cartilage (Fig. 11a) and implanting the sample (Fig. 11b).
The H&E staining presented in Fig. 12 shows the regeneration of the new cartilage in the cell seeded samples throughout the period of the experiment. This regeneration could not be detected in the control samples without cells after 4 (Fig. 11a, b) and 12 weeks (Fig. 11c, d). On the other hand, the partial and full formation of new tissue can be observed for the samples implanted with autologous cells after 4 weeks (Fig. 11e, f) and 12 weeks (Fig. 11g, h) respectively. Therefore, the presence of the cells is essential for cartilage reconstruction. In rabbit models, this formation started after 4 and completed before 12 weeks with the full renovation of cartilage. In Fig. 13, the old (Fig. 13a, c, e) and the new (Fig. 13b, d, f) cartilage tissues were compared. The new tissue shows much more chondrocytes than the old one which is the outcome of the implantation of autologous chondrocytes in the implantation site. However, the morphology of the chondrocytes is the same with the round shaped appearance.
4 Discussion
In this study, polyvinyl alcohol hydrogel crosslinked by biodegradable pre-polyurethane was successfully investigated as a chondrocyte carrier for articular cartilage reconstruction in a rabbit model. In most cases, the lack of bioactivity of the composition due to inadequate adherence to the cartilage tissue looses the implant. Therefore, appropriate mechanical properties with suturability are essential for complete regeneration in articular cartilage. Moreover, the ability of the cartilage implant to be cut in the size of surgical site at the time of surgery is effective in proper fixation. Several hybrid constructs of biomaterials and cells were proposed for cartilage tissue engineering in recent years [24, 25]. In compare with the autologous cartilage implantation developed by Genzyme, utilizing these materials instead of periosteum may provide a greater degree of fixation in the defect site without an extra surgery [26].
The most important problem in chondrocyte culture is the dedifferentiation of these cells to the fibroblast like cells in a monolayer culture on TCPS [27]. It has been suggested that three dimensional cultures of chondrocytes keep their original morphology and expression. In this research, it was found that chondrocyte may retain their original phenotype even culture in a monolayer and the physicochemical characteristics of the substrate influence on this effect.
According to Nuernberger et al. [28] report, the cell adhesion surface effects on the cell shape and an increase in the contact surface area helps cells to keep their original morphology. The AFM results demonstrated higher contact surface for PVA–PPU samples because of the higher roughness. Consequently, the chondrocytes preserve a spherical morphology on the surface of PVA–PPU as indicated in the SEM observation. The expression of the main chondrocyte genes (collagen type II and aggrecan) also proved the preservation of the original morphology for the chondrocytes cultured on PVA–PPU. In addition, the phenotypic expression was confirmed by alcian blue and safranin-O staining of secreted proteoglycans.
From the other point of view, the surface properties control the protein adhesion and also cell attachment [29]. Several researchers have discussed the correlation of cell and protein attachment to biomaterial properties. It has been reported that the hydrophobic surfaces reveal the higher affinity for the adsorption of proteins such as albumin [30]. Sagvolden et al. [31] declared that although proteins adsorb much better to the hydrophobic surfaces, better cell attachment is seen on hydrophilic substrates. Albumin is the first protein that rapidly moves to the damaged area of a tissue [32]. Ito and Nogawa [33] showed that the amount of immobilized albumin is effective on cellular adhesion. The other main factor, charge density, is critical for the cell attachment. It can be determined by surface functional groups [34]. Scotchford et al. [35] demonstrated that the increase in albumin adsorption due to the presence of OH groups decreases the cell attachment. In this study, more adsorption in serum proteins (particularly albumin) was observed on the sample with urethane and OH groups than the sample with OH alone. In addition to the surface chemistry, other researcher discuss about the surface area [36]. Increasing in roughness produce higher surface area and result in higher protein adsorption which can be seen for PVA–PPU sample.
It has been shown that stronger adhesion force produced between albumin and the surface with higher contact angle [37]. This may confirm our results that in serial washing solutions, higher amount of albumin can be detected for PVA–PPU sample with higher water contact angle.
It has been argued by others that polymeric surfaces with the moderate wettability (water contact angle of 40–70) show the highest amount of cell adherence [38]. Also, the water uptake of 95 % for PVA–PPU and 400 % for pure PVA has been reported [18]. It can be deduced that hydrophobic groups introduced by polyurethane chains make these differences.
Although the chondrocytes attach tightly to the hydrophobic and cyto-compatible structure of a modified polystyrene culture plate, weak attachment of the cells to some types of hydrophobic structure like polytetrafluoro ethylene has been reported [39]. In addition to chemical composition of the surface, the combination of several physical factors determines the cell-surface interaction. The main physical factors are degree of hydrophilicity, roughness, charge density and mechanical strength.
In this research, rabbits have been used as an animal model for in vivo evaluation of hybrid cartilage reconstruction. The autologous transplanted cells had a critical role in the cartilage repair. Therefore, it is essential to use an appropriate biomaterial for cell seeding due to prevention of cell loss from the defect site [39]. Other researchers also used rabbits in their in vivo experiments for evaluation of alginate, chitosan, polylactic-glycolic acid and polycaprolatone in cartilage regeneration [40, 41, 42].
Obviously, this study should be performed on a larger animal with higher number of experiments for statistical evaluation. There are also other aliphatic diisocyanate such as lysine diisocyanate or hydrogenated methylene diisocyanate that can be examined in the composition of polyurethane and the degradation products of these compositions should be considered. The source of the cells could be changed to the mesenchymal stem cells and the role of these substrates on differentiation of the stem cells to chondrocytes evaluated.
5 Conclusion
This study revealed that PVA–PPU composition provides a favorable and supportive structure for chondrocytes in vitro. The comparative studies of PVA–PPU and pure PVA demonstrated the successful role of urethane chains in retaining the chondrogenic phenotype of the cells. Although chondrocytes were cultured in a monolayer on PVA–PPU sample, their spherical morphology retained. Besides it can be effective in neocartilage formation in vivo and suitable for cartilage tissue engineering application. Future studies should be performed on the investigation of mesenchymal stem cells differentiation and larger animal models. It is also suggested that the role of surface roughness (micro or nanometer) on dedifferentiation of cells can be considered as an effective parameter.
References
Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther. 2005;12:1171–9.
Werkmeister JA, Adhikari R, White JF, Tebb TA, Le TP, Taing HC, Mayadunne R, Gunatillake PA, Danon SJ, Ramshaw JA. Biodegradable and injectable cure-on-demand polyurethane scaffolds for regeneration of articular cartilage. Acta Biomater. 2010;6:3471–81.
Ateshian GA. Artificial cartilage: weaving in three dimensions. Nat. Mater. 2007;6:89–90.
Shao XX, Hutmacher DW, Ho ST, Goh JCH, Lee E. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials. 2006;27:1071–80.
Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur. Cell Mater. 2005;9:23–32.
Guo Y, Yuan T, Xiao Z, Tang P, Xiao Y, Fan Y, Zhang X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012;23(9):2267. doi:10.1007/s1085601246845.
Chung C, Burdick JA. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008;60:243–62.
Lee S, Pereira BP, Yusof N, Selvaratnam L, Yu Z, Abbas AA, Kamarul T. Unconfined compression properties of a porous poly(vinyl alcohol)–chitosan-based hydrogel after hydration. Acta Biomater. 2009;5:1919–25.
Chiellini E, Corti A, D’Antone S, Solaro R. Biodegradation of poly(vinyl alcohol) based materials. Prog. Polym. Sci. 2003;28:963–1014.
Nilasaroya A, Poole-Warren LA, Whitelock JM, Martens PJ. Structural and functional characterisation of poly(vinyl alcohol) and heparin hydrogels. Biomaterials. 2008;29:4658–64.
Xu F, Nacker JC, Cronea WC, Masters KS. The haemocompatibility of polyurethane–hyaluronic acid copolymers. Biomaterials. 2008;29:150–60.
Buma P, Ramrattan NN, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25:1523–32.
Zhong X, Ji C, Chan AK, Kazarian SG, Ruys A, Dehghani F. Fabrication of chitosan/poly(e-caprolactone) composite hydrogels for tissue engineering applications. J. Mater. Sci. Mater. Med. 2011;22:279–88.
Gorna K, Gogolewski S. In vitro degradation of novel medical biodegradable aliphatic polyurethanes based on E-caprolactone and Pluronics1 with various hydrophilicities. Polym. Degrad. Stab. 2002;75:113–22.
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6.
Schagemann JC, Chung HW, Mrosek EH, Stone JJ, Fitzsimmons JS, O’Driscoll SW, Reinholz GG. Poly-ε-caprolactone/gel hybrid scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A. 2010;93:454–63.
Oliveira JT, Crawford A, Mundy JM, Moreira AR, Gomes ME, Hatton PV, Reis RL. A cartilage tissue engineering approach combining starch-polycaprolactone fibre mesh scaffolds with bovine articular chondrocytes. J. Mater. Sci. Mater. Med. 2007;18:295–302.
Bonakdar S, Hojjati Emami S, Shokrgozar MA, Farhadi A, Hoshiar Ahmadi SA, Amanzadeh A. Preparation and characterization of polyvinyl alcohol hydrogels crosslinked by biodegradable polyurethane for tissue engineering of cartilage. Mater. Chem. Eng. C. 2010;30:636–43.
Olson BJSC, Markwell J. Assays for determination of protein concentration. In: Coligan JE, Dunn BM, Speicher DW, Wingfield PT, editors. Current Protocols in Protein Science. New York: Wiley; 2007. p. 341–3429.
Picot J. Human Cell Culture Protocols. 2nd ed. Totowa: Humana press; 2004. p. 74.
An YH, Martin KL. Handbook of Histology Methods for Bone and Cartilage. 1st ed. New Jersey: Humana press; 2003. p. 300.
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterial Science. 2nd ed. New York: Elsevier Academic Press; 2004. p. 232.
Lien SM, Ko LY, Huang TJ. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009;5:670–9.
Bahney CS, Lujan TJ, Hsu CW, Bottlang M, West JL, Johnstone B. Visible light photoinitiaion of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cell Mater. 2011;22:43–55.
Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W, Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265–73.
Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res. Ther. 2003;5:235–8.
Vonwil D, Schuler M, Barbero A, Ströbel S, Wendt D, Textor M, Aebi U, Martin I. An RGD restricted substrate interface is sufficient for the adhesion, growth and cartilage forming capacity of human chondrocytes. Eur. Cell Mater. 2010;20:316–28.
Nuernberger S, Cyran N, Albrecht C, Redl H, Vecsei V, Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32:1032–40.
Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28:3074–82.
Wei J, Igarashi T, Okumori N, Igarashi T, Maetani T, Liu B, Masao Y. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009;4:045002.
Sagvolden G, Giaever I, Petterson EO, Feder J. Cell adhesion force microscopy. Proc. Natl. Acad. Sci. USA. 1999;96:471–6.
Krajewski A, Malavolti R, Piancastelli A. Albumin adhesion on some biological and non-biological glasses and connection with their Z-potentials. Biomaterials. 1996;17:53–60.
Ito Y, Nogawa M. Preparation of a protein micro-array using a photo-reactive polymer for a cell-adhesion assay. Biomaterials. 2003;24:3021–6.
Thevenot P, Hu W, Tang L. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008;8:270–80.
Scotchford CA, Gilmore CP, Cooper E, Leggett GJ, Downes S. Protein adsorption and human osteoblast-like cell attachment and growth on alkylthiol on gold self-assembled monolayers. J. Biomed. Mater. Res. 2002;59:84–99.
Nitya Ganesh, Greeshma Nair T, Mony Ullas, Chennazhi Krishna Prasad, Shantikumar Nair V. In vitro evaluation of electrospun PCL/nanoclay composite scaffold for bone tissue engineering. J. Mater. Sci. Mater. Med. 2012;. doi:10.1007/s108560124647.
Xua LC, Siedlecki CA. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials. 2007;28:3273–83.
Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28:3074–82.
Van Wachem PB, Vreriks CM, Beugeling T, Feijen J, Bantjes A, Detmers JP, van Aken WG. The influence of protein adsorption on interactions of cultured human endothelial cells with polymers. J. Biomed. Mater. Res. 1987;21:701–18.
Cho JH, Kim SH, Park KD, Jung MC, Yang WI, Han SW, Noh JY, Lee JW. Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials. 2004;25:5743–51.
Uematsua K, Hattoria K, Ishimotoa Y, Yamauchia J, Habataa T, Takakuraa Y, Ohgushi H, Fukuchi T, Sato M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–9.
Jung Y, Park MS, Lee JW, Kim YH, Kim SH, Kim SH. Cartilage regeneration with highly-elastic three-dimensional scaffolds prepared from biodegradable poly(l-lactide-co-3-caprolactone). Biomaterials. 2008;29:4630–6.
Acknowledgments
The authors would like to express their appreciation to Pasteur Institute for their financial assistance. This study was supported by the research Projects No. 370 and 498.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mohammad Ali Shokrgozar and Shahin Bonakdar contributed equally to this study.
Rights and permissions
About this article
Cite this article
Shokrgozar, M.A., Bonakdar, S., Dehghan, M.M. et al. Biological evaluation of polyvinyl alcohol hydrogel crosslinked by polyurethane chain for cartilage tissue engineering in rabbit model. J Mater Sci: Mater Med 24, 2449–2460 (2013). https://doi.org/10.1007/s10856-013-4995-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-4995-1