Abstract
The primary objective of this study was to evaluate in vitro responses of MLO-A5 osteogenic cells to two modifications of the bioactive glass 13-93. The modified glasses, which were designed for use as cell support scaffolds and contained added boron to form the glasses 13-93 B1 and 13-93 B3, were made to accelerate formation of a bioactive hydroxyapatite surface layer and possibly enhance tissue growth. Quantitative MTT cytotoxicity tests revealed no inhibition of growth of MLO-A5 cells incubated with 13-93 glass extracts up to 10 mg/ml, moderate inhibition of growth with 13-93 B1 glass extracts, and noticeable inhibition of growth with 13-93 B3 glass extracts. A morphology-based biocompatibility test was also performed and yielded qualitative assessments of the relative biocompatibilities of glass extracts that agree with those obtained by the quantitative MTT test. However, as a proof of concept experiment, when MLO-A5 cells were seeded onto 13-93 B3 scaffolds in a dynamic in vitro environment, cell proliferation occurred as evidenced by qualitative and quantitative MTT labeling of scaffolds. Together these results demonstrate the in vitro toxicity of released borate ion in static experiments; however borate ion release can be mitigated in a dynamic environment similar to the human body where microvasculature is present. Here we argue that despite toxicity in static environments, boron-containing 13-93 compositions may warrant further study for use in tissue engineering applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the past decade a vast number of different types of prototype constructs have been designed and tested for use as scaffolds to support the attachment and growth of osteoprogenitor cells for in vitro engineering of functional bone tissue and in vivo repair of bone defects [1–9]. Porous constructs designed as cell support scaffolds for in vitro tissue engineering and in vivo organ repair must satisfy multiple criteria to be deemed satisfactory for clinical applications. Scaffolds must be biocompatible and any degradation products formed must be non-toxic to minimize inflammation and foreign body reactions [7–9]. Scaffolds must possess interconnected pores of at least 100 μm to allow tissue ingrowth and vascularization [10, 11]. A desirable feature of scaffolds used for bone repair and regeneration is bioactivity [12, 13], the ability to bond to host bone without the formation of fibrous encapsulation. Other desirable features include the ability to act as a template for tissue growth and a resorbtion rate that matches bone regeneration [10, 12, 13]. Adequate mechanical strength for use in load bearing sites is another feature that is often desirable for scaffolds designed for bone repair [2].
Bioactive silicate glasses are among the materials that have been used for fabrication of scaffolds for bone tissue engineering [12, 13]. The silicate 45S5 Bioglass® developed by Hench and colleagues and related compositions have particularly received attention due to their osteoconductive and osteoinductive properties [12, 13]. Although 45S5 glass is the most well known, a drawback of 45S5 is its limited viscous flow. At the high temperatures (>1,000 °C) needed to thermally bond 45S5 particles into porous, three-dimensional structures, the glass crystallizes to form combeite (Na2O·2CaO·3SiO2), a form with reduced potential for bioactivity [14]. However, the 13-93 glass composition has been observed to show more simplistic viscous flow characteristics than the original 45S5 glass [15–20]. Due to the wider range between the glass transition temperature and the crystallization temperature of the 13-93 composition compared to that of the 45S5 glass, 13-93 can be more easily pulled into fibers and fabricated into a variety of three dimensional shapes. Work performed previously demonstrated that porous 3D scaffolds fabricated from 13-93 possessed the ability to support the attachment, growth, and differentiation of MLO-A5 osteogenic cells in vitro [21].
The main objective of the present research was to evaluate in vitro cellular response to two modified compositions of bioactive glasses 13-93. The glass compositions tested herein were modified in an attempt to accelerate formation of a hydroxyapatite reaction layer or enhance tissue growth. The modifications of the 13-93 glasses consisted of adding increased amounts of boron to form glasses 13-93 B1 and 13-93 B3. The MTT cytotoxicity assay was utilized for in vitro glass extract experiments performed in the present study.
2 Materials and methods
2.1 Cell line and culture conditions
Mouse late-osteoblast, early-osteocyte MLO-A5 cells [22, 23] were obtained from Dr. Linda Bonewald at the University of Missouri-Kansas City. The MLO-A5 cells were cultured on collagen-coated plates in α-MEM medium supplemented with 5 % fetal calf serum, 5 % calf serum, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin sulfate. All incubations were at 37 °C in a humidified atmosphere of 5 % CO2.
2.2 Glass preparation
Three different glasses were used in the present experiments. The silicate based 13-93 glass was used in original form and two modifications. The 13-93 B1 and 13-93 B3 compositions consisted of the 13-93 composition with one-third and all of the silica replaced with boron respectively (nominal compositions listed in Table 1). Preparation of the glasses by melting and casting were described previously [1, 21].
2.3 Glass extract preparation and addition to culture medium
The various test glasses were crushed in a hardened steel mortar and pestle and size separated with stainless steel sieves to select particles of size ≤45 μm. Batches (0.3 g) of each glass were dry heat sterilized overnight at 300 °C and then placed in 10 ml of sterile distilled water in screw-cap tubes. The sample tubes were incubated for 2 days at 50 °C to initiate and hasten the sequence of ion leaching reactions known to occur when bioactive glasses are placed in contact with body fluids or water [24]. The samples were then centrifuged at 2000×g and the clear supernatant containing the aqueous extract of the pulverized glass was withdrawn. A 3.0 ml portion of each glass extract was adjusted to pH 7.4 with 1.0 N HCl and sterilized by passage through a 0.22 micron filter. Original glass extract and pH adjusted extract were combined with a three-fold concentrated mixture of Minimum Essential Medium, HEPES buffer, and fetal bovine serum to obtain a 1× mix of medium with 25 mM HEPES, 10 % fetal calf serum, and glass extract equivalent to 13.3 mg glass/ml.
2.4 MTT cytotoxicity assay
The quantitative cytotoxic assay with the tetrazolium salt MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was used to assess the cytotoxicity of the glass extracts [25]. MLO-A5 cells were seeded in 96-well plates at a density of 5200 cells/well in 60 μl of complete α-MEM medium. The plates were incubated for 2 h at 37 °C to promote cell attachment. Medium was added (180 μl/well) containing original (pH unadjusted) glass extract up to 10 mg/ml to the cell-seeded plates for a total volume of 240 μl/well (n = 5). All cytotoxicity experiments were repeated using glass extracts adjusted to pH 7.4 with 1.0 N HCl.
After 3 days of incubation, 120 μl of medium was removed from each well and replaced with 120 μl of fresh medium with MTT (0.4 mg/ml) for the last 4 h of incubation. At the conclusion of incubation, the plate was inverted to pour out the medium remaining in the wells and blotted to remove any residual free MTT. The insoluble purple crystals remaining in the wells were solubilized by adding 200 μl 100 % ethyl alcohol per well and measured spectrophotometrically at 550 nm in a BMG FLUORstar Optima plate reader.
2.5 Morphology-based test of biocompatibility
An additional procedure used to assess the biocompatibility of the three different glasses was a qualitative assay involving visual comparison of the morphology and relative density of MLO-A5 cells growing in the presence of neutralized extracts of the three different glasses. MLO-A5 cells suspended in 1 ml of complete α-MEM medium were seeded in 12-well plates (30,000 cells/well). After incubation for 1 h to permit cell attachment, an additional 1 ml of medium with pH adjusted glass extracts (12 mg/ml) was added for a final concentration of 6 mg/ml. The culturing was resumed and at intervals of 1 day and 3 days, digital images of the cells in each well were recorded with a CCD camera (Model DP70; Olympus; Center Valley, PA) mounted on a phase contrast microscope. The morphology and relative density of cells cultured in the presence of glass extracts were visually compared to qualitatively assess the biocompatibility of the various experimental glass types.
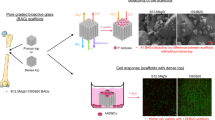
2.6 Cell-seeding of fiber scaffolds and static/dynamic in vitro incubation
Scaffolds fabricated from test glasses were prepared and seeded with MLO-A5 cells as described previously [1]. For static culture, 12-well plates with cell seeded scaffolds were kept stationary for 4 days on a shelf within the CO2 incubator. Dynamic culture involved placing cell seeded scaffolds on a stainless steel mesh disc in 60 mm dishes positioned on a platform rocker in the CO2 incubator. The platform rocker was set at 30 oscillations per min to gently mix the culture medium over and under the scaffold during the 4 day incubation.
2.7 MTT detection of viable cells on scaffolds
Cell-seeded scaffolds were placed in medium containing the tetrazolium salt MTT (50 μg per 250 μl of medium) for the last 4 h of incubation to allow visualization of metabolically active cells on scaffolds. Scaffolds were rinsed with phosphate buffered saline (PBS) at the conclusion of the incubation and blotted dry. A stereo microscope with a digital camera was used to acquire macroimages of the relative amounts of purple formazan-labeled cells, the product of mitochondrial MTT metabolism, on the scaffolds. Lastly, the formazan product was extracted from the scaffolds with 1.0 ml of ethyl alcohol and measured spectrophotometrically at 550 nm in a BMG FLUORstar Optima plate reader. Absorbance intensities of extracts were compared with standard solutions of MTT formazan.
2.8 Statistical analysis
Experiments were performed in triplicate except cytotoxicity experiments which contained five replicates. Data are presented as the mean ± standard deviation. Statistical analyses were performed with Student’s t test. The mean values were considered to be significantly different when P < 0.05.
3 Results
3.1 pH of extracts
The pH of the original unaltered glass extracts was measured to assess alkalinity of the extracts at the conclusion of the 2 day soak at 50 °C. Extracts of 13-93, 13-93 B1, and 13-93 B3 glass had pH values of 7.8, 9.0, and 9.1 respectively.
3.2 MTT cytotoxicity assay
Figure 1 compares the amounts of MTT formazan product formed by MLO-A5 cells cultured in medium with various concentrations of the glass extracts, measurements that reflect different levels of cell growth. The effects on cellular proliferation of addition of unaltered (pH unadjusted) extracts of the 13-93, 13-93 B1, and 13-93 B3 glasses are shown in Fig. 1a. Proliferation of MLO-A5 cells cultured with the 13-93 extracts occurred at 94 to 105 % of control rate over the entire concentration range tested. In contrast, addition of the extract of 13-93 B1 glass at levels of 4, 6, and 10 mg/ml reduced cell proliferation to 82, 70, and 60 % of control, respectively. There was a more pronounced effect of the 13-93 B3 extract on cellular growth. Proliferation of MLO-A5 cells was reduced to 51, 28, 23, and 18 % of the control by addition of 13-93 B3 extract at concentrations equivalent to 2, 4, 6 and 10 mg/ml, respectively.
Evaluation of cytotoxicity of pH unadjusted (a) and pH 7.4 (b) glass extracts of 13-93, 13-93 B1, and 13-93 B3 glasses by MTT hydrolysis. Each bar represents MTT formazan product formed by MLO-A5 cells cultured with extract and is an average of five replicates ±SD. The asterisks indicate statistically significant differences in MTT formazan between culture treatments (P < 0.05)
The aqueous extracts of the 13-93, 13-93 B1, and 13-93 B3 glasses were neutralized and then retested for effects on MLO-A5 growth as measured by MTT hydrolysis. Figure 1b compares the amounts of MTT formazan formed by MLO-A5 cells incubated with different amounts of the neutralized extracts. Addition of the neutralized extract of the 13-93 glass had no discernible effect on cellular proliferation (97 to 107 % of control over the entire concentration range tested). Addition of the neutralized extract of the 13-93 B1 glass at glass equivalent concentrations of 4, 6, and 10 mg/ml decreased cellular proliferation to approximately 80, 73, and 65 % of control, respectively. The neutralized 13-93 B3 extracts had greater effect causing cellular proliferation to drop to approximately 55, 35, 27 and 23 % of control at glass equivalent concentrations of 2, 4, 6 and 10 mg/ml, respectively. Overall, the results presented in Fig. 1b show that addition of the neutralized extract had a similar effect on the rate of proliferation of MLO-A5 cells as that caused by the unaltered extracts.
3.3 Effect of glass extracts on cell morphology and density
The photomicrographs in Fig. 2 show the morphology and relative density of MLO-A5 cells after 1 day and 3 days of culture in the presence of neutralized extracts of pulverized 13-93, 13-93 B1, and 13-93 B3 glasses. The amounts of extract added were equivalent to 6 mg glass/ml and the photos are representative of the cells observed. MLO-A5 cells cultured with extracts of the 13-93 glass were well attached and exhibited the dendritic morphology that is characteristic of the MLO-A5 cell line [22, 23]. In addition, the cells proliferated extensively between day 1 and day 3 of culture and appeared nearly confluent at day 3. An increase in cell density also occurred with the cells cultured in the medium containing 13-93 B1 extract although the increase was less than that which occurred in medium containing 13-93 extract. MLO-A5 cell proliferation at day 3 in 13-93 B1 extract was similar to that found in day 3 control cells. Additionally, the cells cultured with the 13-93 B1 extract lacked the dendritic morphology. MLO-A5 cells cultured with the 13-93 B3 extract showed almost no increase in cell density between day 1 and day 3 of culture and became rounded and detached from the culture plate.
The morphology-based assay, although qualitative in nature, provided in vitro evidence of good biocompatibility of the 13-93 glass. Moderate biocompatibility was evidenced for the 13-93 B1 glass and little or no biocompatibility for the 13-93 B3 glass.
3.4 Static and dynamic culture of MLO-A5 cells on 13-93 B3 scaffolds
Representative images of cell-seeded 13-93 B3 scaffolds incubated with MTT during the last 4 h of a 2 day incubation under static and dynamic culture conditions are shown in Fig. 3. Almost no labeling with insoluble purple formazan was present on the statically cultured 13-93 B3 scaffold. However, purple formazan product was seen on the surface of the dynamically cultured 13-93 B3 scaffolds. This indicated that very few metabolically active MLO-A5 cells were present on the statically cultured scaffolds but metabolically active cells were present on the dynamically cultured 13-93 B3 scaffolds.
3.5 MTT detection of viable cells on 13-93 B3 scaffolds
Absorbance readings corresponding to the amounts of purple formazan removed by ethanol extraction from MTT labeled cells on 13-93 B3 scaffolds are presented in Fig. 4. As shown, increased culture time was accompanied by a nearly linear, statistically significant (P < 0.05) increase in the amount of formazan product extracted from 13-93 B3 and control 13-93 cell-seeded scaffolds. This is an indication of a linear increase in the number of metabolically active cells on and within the scaffolds. Comparisons between static and dynamic culture conditions revealed statistically significant increases on control 13-93 scaffolds and 13-93 B3 scaffolds in the dynamic culture condition. It is notable that absorbance readings on 13-93 B3 dynamically cultured scaffolds were not statistically different from control static cultured 13-93 scaffolds.
Cell proliferation on control 13-93 and 13-93 B3 fiber scaffolds assessed by quantitative measurement of MTT hydrolysis. Each bar represents an average of three replicates ±SD. The asterisks indicate statistically significant differences in MTT formazan between culture intervals (P < 0.05). μmoles MTT formazan/scaffold on 13-93 B3 dynamic and 13-93 static were not statistically different
4 Discussion
Recent studies have demonstrated that it should be possible to modify bioactive glass and scaffold compositions with boron etc. in ways that will improve their ability to support tissue growth and bone regeneration [26–28]. Recent work here involved addition of boron to bioactive glasses 13-93 in attempts to improve their effectiveness for bone repair applications.
It is essential to determine if any changes in the composition of bioglasses or other biocompatible implant materials could have potentially harmful or detrimental effects on cells in vitro and on tissues in vivo. The present work was performed to determine if the additions of this element could have possible cytotoxic effects and at what concentrations. The primary method utilized to assess possible cytotoxic effects was the basic MTT cytotoxicity assay originally developed by Mosmann [25] to quantitatively evaluate the survival and proliferation of cells cultured in the presence of the various glass extracts. Evidence that the MTT cytotoxicity assay is an accurate indicator of potential toxicity has been validated by numerous tests with a variety of known cytotoxic agents [29].
One of the properties of 45S5 Bioglass and other similar bioactive glass compositions when exposed to physiological fluids is the release of ions that cause alkalinization of the surrounding medium [30]. Alkaline pH values recorded for the original unaltered glass extracts are consistent with these reported effects. Inevitable cell death would occur with the addition of high levels of the extracts without some accommodation. Because of the alkalinity of the dissolution products of these glasses, two adjustments were used in the experimental procedures. One was the inclusion of 25 mM HEPES buffer in the culture medium to minimize pH change. The second adjustment was to neutralize some of each extract for re-testing in a neutralized state (pH 7.4).
The outcome of the MTT tests of effects of the glass extracts on the growth of MLO-A5 cells was not the same with all of the glass extracts. However, one thing that is apparent from a comparison of the bar charts presented in Fig. 1a, b is the striking similarity of the patterns of the charts depicting the effects of the original unaltered extracts compared with the effects of the neutralized extracts. As an example, Fig. 1a, which shows the effect of addition of original unaltered extracts of 13-93, 13-93 B1, and 13-93 B3 glasses is very similar to Fig. 1b which shows the effect of addition of neutralized extracts of the same three glasses. This suggests that the partial inhibition of growth of MLO-A5 cells shown in these two figures was not the result of a pH effect but was instead due to some other agent present in these extracts. Addition of extract up to 10 mg/ml of the 13-93 glass, which contained no borate, had no effect on MLO-A5 growth. Apparently, there was either very little ionic material extracted from the 13-93 glass or the extracted components had an innocuous effect on cell proliferation. Addition of the 13-93 B1 and 13-93 B3 extracts did, however, cause a concentration dependent inhibition of cellular growth. The partial inhibition caused by the 13-93 B1 and B3 glass extracts was likely due to effects of borate ion (BO3 3−) extracted from these glasses. Indeed, it has been previously shown that addition of borate ions to MC3T3-E1 cells caused a concentration dependent inhibition of cell growth [31].
In order to clarify whether solely boron dissolution was responsible for cytotoxicity in the MTT assay, the H3BO3 concentration in pH unadjusted glass extracts (Fig. 1a) was calculated for the 13-93 B1 and B3 glasses and presented in Fig. 5. The maximum boric acid concentration reached in the MTT cytotoxicity assay for 13-93 B1 and 13-93 B3 glasses was 0.05 M H3BO3 and 0.15 M H3BO3 (10 mg/ml 13-93 B1 and B3) respectively. These boric acid concentrations may very well be responsible for cytotoxicity as a previous study by Richard found that 0.05 and 0.25 M H3BO3 addition to MC3T3-E1 culture resulted in cell death [32]. For comparison, a study by Heindel determined that 0.004 and 0.007 M H3BO3 was the fetal toxicity limit and maternal toxicity limit in CD-1 mice respectively [33].
Relative MLO-A5 cell density in H3BO3 concentrations calculated from Fig. 1A 13-93 B1 and B3 glass extracts. For comparison, Brown determined that 0.002 M H3BO3 addition decreased MC3T3-E1 cell number 40 % [31]. Richard found that 0.05 M H3BO3 addition to MC3T3-E1 culture resulted in cell death [32]. Heindel determined that 0.004 and 0.007 M H3BO3 was the fetal toxicity limit and maternal toxicity limit in CD-1 mice respectively [33]. Jung subcutaneously implanted 13-93 B3 fiber scaffolds in adult Sprague-Dawley rats to total 0.002M H3BO3. No detectable histological changes were observed in kidney or liver tissue sections
The photomicrographs in Fig. 2 obtained in the morphological test of biocompatibility provide a qualitative indication of the biocompatibility of the various test glasses. The levels of biocompatibility of the various glasses as indicated by the morphologies and relative cell densities shown in this figure is very similar to the levels of biocompatibility indicated by the MTT cytotoxicity tests of the effects of extracts on MLO-A5 cell growth. For example, the qualitative morphological test revealed a high level of in vitro biocompatibility for the 13-93 glass as indicated by an extensive increase in cell density and retention of the characteristic dendritic morphology. The qualitative morphological test also revealed a moderate level of in vitro biocompatibility for the 13-93 B1 glass as indicated by a moderate increase in cell density and only partial retention of the characteristic dendritic morphology. And, finally, the outcome of the qualitative test shows a low level of in vitro biocompatibility for the 13-93 B3 glass as indicated by the lack of an increase in cell density plus the rounded, non-attached appearance of the cells. The levels of in vitro biocompatibility determined by the qualitative test agree with the levels of biocompatibility determined by the MTT cytotoxicity test.
The photomicrographs of MTT labeled 13-93 B3 scaffolds seeded with MLO-A5 cells and incubated statically and dynamically for 2 days in Fig. 3 provide a proof of concept comparison of the abilities of 13-93 B3 scaffolds to support cell growth in an environment more similar to that found in a living organism. The results in Fig. 3 show the pattern of MTT labeling of 13-93 B3 scaffolds subjected to static and dynamic culture and the presence of MTT formazan confirms greater cellular growth with dynamic culture. This is evidence of a higher level of cell proliferation, indicating perhaps a modulation of effects of borate ion under dynamic culture conditions and/or greater nutrient flow for the dynamically cultured scaffold. Perhaps the medium flow due to the rocking platform lowered the amount of borate ion present in the cell microenvironment.
Most in vitro investigations of cell growth on porous scaffolds are conducted using static incubation conditions [1, 2]. However, some investigations of cell growth on porous scaffolds have been conducted using complex perfusion systems [34]. The perfusion of nutrient through the scaffolds mimics the action of the microvasculature of tissue. The placement of cell culture plates on the platform rocker, a procedure far less complex than perfusion, was done in an attempt to enhance cell growth. We speculated that use of the platform rocker would increase nutrient flow through the scaffolds and disperse aggregated borate ion thereby increasing cellular proliferation on scaffolds. The implications of these findings on in vitro engineering of tissue are such that increased nutrient permeation supports increased growth and could possibly improve the survival of pre-seeded cells.
The results of MTT labeling are further evidence of proliferation of osteogenic cells on boron containing 13-93 B3 fiber scaffolds. The increasing amounts of purple formazan on MLO-A5 seeded control 13-93 and 13-93 B3 scaffolds are indicative of the active proliferation of osteogenic cells on both scaffold types. Particularly noteworthy in Fig. 4 is that absorbance readings between dynamically cultured 13-93 B3 scaffolds were not statistically different from statically cultured control 13-93 scaffolds. The increased amount of purple formazan seen on dynamic culture of 13-93 B3 scaffolds is due in part to culturing conditions, however dynamic conditions more closely mimic those of the human body. The interconnectivity and size of the pores of the fiber scaffolds likely accounts for access of nutrient and thereby fulfils one of the main property requirements for scaffolds used for bone tissue engineering applications.
Despite in vitro cytotoxicity, we argue that borate or borosilicate 13-93 fibers and/or cell support scaffolds may find use for in vivo applications. In the human body, greater than 90 % of boric acid is excreted from the body through urine [35, 36]. Additional evidence of this hypothesis is exemplified in work performed by Jung et al. [37] in which up to 16 scaffolds (<~126 mg/kg/day) of 13-93 B3 were subcutaneously implanted into Sprague-Dawley rats. Histological sections of experimental animals were not statistically different from control animals in terms of kidney or liver tissue damage pathological abnormalities.
5 Conclusion
The boron-containing modifications of the bioactive glass 13-93 (13-93 B1 and 13-93 B3) described in this study possess moderate and low biocompatibility in vitro with MLO-A5 late osteoblast cells. However, through the use of culture methods that simulate microvasculature, glasses such as 13-93 B3 may find use in vivo. These findings suggest that despite toxicity in static environments, boron-containing 13-93 compositions may warrant further study for use in tissue engineering applications.
References
Brown RF, Day DE, Day TE, Jung S, Rahaman MN, Fu Q. Growth and differentiation of osteoblastic cells on 13-93 bioactive glass fibers and scaffolds. Acta Biomater. 2008;4:387–96.
Fu Q, Rahaman MN, Bal SB, Brown RF, Day DE. Mechanical and in vitro performance of 13–93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008;4:1854–64.
Burg KJL, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–59.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43.
Kellomaki M, Niiranen H, Puumanen K, Ashamamakhi N, Waris T, Tormala P. Bioabsorbable scaffolds for guided bone regeneration and generation. Biomaterials. 2000;21:2495–505.
Du C, Cui FZ, Zhu XD, de Groot K. Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J Biomed Mater Res. 1999;44:407–15.
Griffith LG. Polymeric biomaterials. Acta Mater. 2000;48:263–77.
Goldstein SA, Patil PV, Moalli MR. Perspectives on tissue engineering of bone. Clin. Orthop. 1999;357S:S419–23.
Kneser U, Schaefer DJ, Munder B, Klemt C, Andree C, Stark GB. Tissue engineering of bone. Min. Invas. Ther. Alli. Tech. 2002;11:107–16.
Jones J, Ehrenfried L, Hench L. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27:964–73.
Freyman TM, Yannas IV, Gibson LJ. Cellular materials as porous scaffolds for tissue engineering. Prog Mater Sci. 2001;45:273–82.
Hench LL, Wilson J. Surface-active biomaterials. Science. 1984;226:630–5.
Hench LL. Bioceramics. J Am Ceram Soc. 1998;81:1705–28.
Clupper DC, Mecholsky JJ, La Torre GP, Greenspan DC. Bioactivity of tape cast and sintered bioactive glass-ceramic in simulated body fluid. Biomaterials. 2002;23:2599–606.
Brink M. The influence of alkali and alkaline earths on the working range for bioactive glasses. J Biomed Mater Res. 1997;36:109–17.
Asikainen AJ, Hagstrom J, Sorsa T, Noponen J, Kellomaki M, Juuti H, Lindqvist C, Heitanen J, Suuronen R. Soft tissue reactions to bioactive glass 13–93 combined with chitosan. J Biomed Mater Res. 2006;83A:530–7.
Ruuttila P, Niiranen H, Kellomaki M, Tormala P, Konttinen YT, Hukkanen M. Characterization of human primary osteoblasts response on bioactive glass (BaG13–93) coated poly-L, DL-lactide (SR-PLA70) surface in vitro. J Biomed Mater Res. 2006;78 B:97–104.
Pirhonen E, Niiranen H, Niemela T, Brink M, Tormala P. Manufacturing, mechanical characterization, and in vitro performance of bioactive glass 13–93 fibers. J Biomed Mater Res. 2006;77:227–33.
Brink M, Turunen T, Happonen R-P, Yli-Urpo A. Compositional dependence of bioactivity of glasses in the system Na2O-K2O-MgOCaO-B2O3-P2O5-SiO2. J Biomed Mater Res. 1997;37:114–21.
Brink M, Yli-Urpo S, Yli-Urpo A.The resorption of a bioactive glass implanted into rat soft tissue. Presented at the 5th World Biomaterials Congress, Toronto, 48, 1996.
Modglin VC. In vitro evaluation of bioactive glass scaffolds and modified bioactive glasses with an osteogenic cell line. In: Biological Sciences, vol. (M.S. Rolla: Missouri University of Science and Technology, Rolla 2009).
Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. Establishment of an Osteoid Preosteocyte-like Cell MLO-A5 That Spontaneously Mineralizes in Culture. Bone and Min Res. 2001;16:1622–33.
Barragan-Adjemian C, Nicolella D, Dusevich V, Dallas MR, Eick JD, Bonewald LF. Mechanism by which MLO-A5 late osteoblasts/early osteocytes mineralize in culture: similarities with mineralization of lamellar bone. Calcif Tissue Int. 2006;79:340–53.
Zeitler T, Cormack A. Interaction of water with bioactive glass surfaces. J. Crystal Growth. 2006;294:96–102.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J. Immun. Meth. 1983;65:55–63.
Gorustovich A, Lopez J, Guglielmotti M, Cabrini R. Biological performance of boron-modified bioactive glass particles implanted in rat tibia bone marrow. Biomed. Mat. 2006;3:100–5.
Dzondo-Gadet M, Mayap-Nzietchueng R, Hess K, Nabet P, Belleville F, Dousset B. Action of boron at the molecular level: effects on transcription and translation in an acellular system. Biol Trace Elem Res. 2002;85:23–33.
Vrouwenvelder WC, Groot CG, de Groot K. Better histology and biochemistry for osteoblasts cultured on titanium-doped bioactive glass: bioglass 45S5 compared with iron-, titanium-, fluorine- and boron-containing bioactive glasses. Biomaterials. 1994;15:97–106.
Burton JD. The MTT assay to evaluate chemosensitivity. Methods Mol Med. 2005;110:69–78.
Silver IA, Deas J, Erecinska M. Interactions of bioactive glasses with osteoblasts in vitro: effects of 45S5 Bioglass®, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials. 2001;22:175–85.
Brown RF, Rahaman MN, Dwilewicz AB, Huang W, Day DE, Li Y, Bal BS. Effect of borate glass composition on its conversion to hydroxyapatite and on the proliferation of MC3T3-E1 cells. J Biomed Mater Res. 2008;88:392–400.
Richard M. Bioactive behavior of a borate glass. Ceramic Engineering, vol. (M.S. Rolla: University of Missouri-Rolla, 2000). p.140.
Heindel JJ, Price CJ, Schwetz BA. The developmental toxicity of boric acid in mice, rats, and rabbits. Environ Health Perspect. 1994;102:107–12.
Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PHM, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal osteoblasts in titanium fiber mesh. J. Biomed. Mat. Res. 2002;64:235–41.
Smallwood CL, Lipscomb J, Swartout J, Teuschler L. Toxicological report of boron and compounds. In: Agency USEP, editor. Washington D.C.: EPA, 2004. p. 134.
Smallwood C. Boron in drinking-water, vol. 2. Geneva: World Health Organization; 2003.
Jung SB, Day DE, Brown RF, Bonewald LF. Potential toxicity of bioactive borate glasses in vitro and in vivo. ICACC, Daytona Beach, 2012 (Proceedings in press).
Acknowledgments
This investigation was supported by funds from the Center for Bone and Tissue Repair and Regeneration at Missouri University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modglin, V.C., Brown, R.F., Jung, S.B. et al. Cytotoxicity assessment of modified bioactive glasses with MLO-A5 osteogenic cells in vitro. J Mater Sci: Mater Med 24, 1191–1199 (2013). https://doi.org/10.1007/s10856-013-4875-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-4875-8