Abstract
Recently, biodegradable magnesium alloys have been introduced in the field of cardiovascular stents to avoid the specific drawbacks of permanent metallic implants. However, the major obstacle of the clinical use of magnesium-based materials is their rapid corrosion rate. In this paper, a composite micro-arc oxidation/poly-l-lactic acid (MAO/PLLA) coating was fabricated on the surface of the magnesium alloy WE42 to improve its corrosion resistance and the cytocompatibility of the modified materials was also investigated for safety aim. In our study, the morphology of materials was analyzed by Scanning electron microscopy. Potentiodynamic polarization was used to evaluate the corrosion behavior of the samples and corrosion weight loss was used to demonstrate their degradation rate. Furthermore, we applied cytotoxicity test in testing the cytocompatibility of the modified samples. The results showed that the PLLA coating effectively sealed the microcracks and micropores on the surface of the MAO coating by physical interlocking to interfere the corrosion ions. The corrosion rate was decreased and the cyototoxicity test showed that the MAO/PLLA composite coating WE42 had good cytocompatibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Magnesium alloys are the most commonly used metal among all the degradable metallic materials. Recently, biodegradable magnesium-based alloys have been introduced in the field of cardiovascular stents to avoid the specific drawbacks of permanent metallic implants, such as thrombogenicity, permanent physical irritation, long term endothelial dysfunction, inability to adapt to growth, etc. [1, 2].
However, the major obstacle of the clinical use of magnesium-based materials is their rapid corrosion rate [3, 4]. This high corrosion rate would release magnesium ions rapidly in short time accompanied with hydrogen bubbles production. This phenomenon would increase the concentration of the magnesium ions of circumferential medium and lead to high local pH value, which would in turn cause chronic inflammatory and thrombogenic reactions [5]. AE21 was the first magnesium alloy used for cardiovascular stents. Although a favorable biocompatibility with little neointimal proliferation and a low thrombogenicity was observed, stents produced from AE21 degraded too rapidly and AE21 was abandoned for use in cardiac implants [6]. Subsequently, WE43 containing zirconium, and various amounts of rare earths (Zirconium (<5%), Yttrium (<5%), and rare earths (<5%)) was used for the production of a degradable cardiovascular stent (Biotronik, Bulach, Switzerland) [7]. Nevertheless, neointimal growth and negative remodeling caused by high corrosion rate limited further clinical application of this kind of stents [8].
The enhancement of the corrosion resistance of magnesium can be achieved by using different modification methods such as ion implantation [9, 10], physical vapor deposition [11], rare-earth conversion films [12, 13], etc. Microarc oxidation (MAO) allowed thick and hard ceramic-like coatings to be obtained with high corrosion resistance, thermal stability and dielectric properties [14] and their alloys in a suitable electrolyte by increasing the anodic voltage to a high stage, usually accompanied by intensive gas evolution and sparking phenomenon at the anode surface [15].
However, without further sealing, the corrosion resistance was not adequate for use in biomedical implants. Because that the MAO coating was composed of one inner compact layer and one outer loose layer. There were many microcracks and microholes on the outer layer, which accelerated the corrosion rate by absorbing electrolyte ions.
Many sealing method such as SiO2–ZrO2 sol–gel layer, multilayer sol–gel coatings and TiO2 coating had been had been researched [16–18].
In this study, we fabricated a MAO/PLLA composite coating to improve the corrosion resistance and its cytocompatibility. The PLLA coating sealed the MAO coating by physical interlocking to interfere the corrosion ions to improve the corrosion resistance. This paper aimed at investigating the feasibility of composite coating in controlling the degradation of magnesium alloy and evaluating cytotoxicity of modified WE42.
2 Experimental
2.1 Materials and processing
The die-cast WE42 magnesium alloys (chemical composition was Y: 1.7–4.1%, RE (Nd: 2.0–2.5%, Heavy RE: balance): 2.4–4.4%, Zr > 0.4%) with the dimensions of 10 × 10 × 2 mm3 were used as the substrate materials in the present study. NaOH and Na2SiO3 solution was prepared. All samples were polished with waterproof abrasive papers, and then ultrasonically cleaned in acetone, absolute ethanol and distilled water, respectively.
The micro-arc oxidation procedures were carried out at room temperature in alkaline silicate solutions and then washed 10 min in distilled water and dried.
Then the MAO samples were submersed vertically in the PLLA dichloromethane solution and withdrawn slowly. This procedure was repeated for three times. This dipping method makes the biocompatible organic PLLA to seal effectively the MAO coating. In this way the MAO/PLLA composite coating was fabricated.
2.2 Corrosion test
2.2.1 Immersion test
All specimens of WE42, WE42-MAO, WE42-MAO/PLLA were submerged in Hanks’ solution at 37°C. 24 h later, the samples were taken out from the Hanks’ solution and the surface morphologies of WE42, WE42-MAO and WE42-MAO/PLLA, both before and after being submerged, were examined with a XL10ESE scanning electronic microscope (SEM).
2.2.2 Corrosion weight loss
The sample surfaces that had been cleaned before the experiment, were submerged in Hanks’ solution at 37°C. At every predetermined time point, the submerging medium was replaced with fresh Hanks’ solution. At every predetermined time point, the corroded specimens were removed from the corrosion products and then cleaned with distilled water and dried and weighed to get the final weight W0. The corrosion weight loss is W = W1−W0.
2.2.3 Electrochemical performance tests
Electrochemical tests were carried out using a PAR Model 2263. The samples were submerged in Hanks’ solution (a simulated body fluid) at 37°C, being the pH value was adjusted to 7.4 with NaOH or HCl. Potentiodynamic polarization was measured at a scan rate of 0.5 mVs−1 to investigate the protective properties of the samples.
2.3 Cytotoxicity test
2.3.1 Cell culture
Endothelial cells were isolated from human umbilical vein by collagenase I (Sigma,USA) digestion and then cultured in endothelial cell medium (ECM, Cat.No.1001, ScienCellTM) at 37°C in an incubator with an atmosphere of 5% CO2 and 95% air. Human umbilical vein endothelial cells (HUVECs) used in the experiments were between two and three passages.
2.3.2 Cytotoxicity test—evaluation of the effect of immersion extracts from different samples on cell viability
The MTT assay is used to determine the cytotoxicity of different materials to human cells. The method was carried out with the immersion extracts in contact with HUVECs. To prepare extracts, each specimen with a size of 10 × 10 × 5 mm3 was sterilized with 60Co radiation and then incubated in a Falcon tube with ECM at 37°C for 24 h. The recommended relation between the material surface and the volume of ECM is 3 cm2/ml, following the ISO10993-1 standard.
The cell suspension was adjusted to a density of 1.5 × 105 HUVECs/ml, and 100 μl cell suspension was added to each well of a 96-well plate and incubated. All wells were divided into four groups including naked WE42 group, WE42-MAO group, WE42-MAO/PLLA group and control group as well. Each group contained 9 wells. Once 90% cells confluenced, the culture medium was then removed and replaced by 50 μl of different immersion extracts and 50 μl ECM. As for control group, 100 μl ECM without any extract was added to each well. After culturing for 24 h, 10 μl MTT solution (1 g thiazolyl blue tetrazolium bromide powder in 1 l phosphate buffered saline) was added to each well and incubated at 37°C for further 4 h. After that, the supernatant in each well was abandoned. The blue formazan reaction product was dissolved by the addition of 150 μl dimethyl sulfoxide (DMSO) and then diluted to 2 ml with phosphate buffered saline. The optical density (OD) value was measured at 570 nm using a spectrophotometer to determine the cell viability in comparison to control group.
3 Results
3.1 Corrosion test
3.1.1 Immersion test
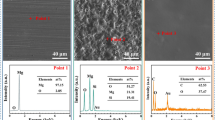
Figure 1 shows the images of the surface morphologies of WE42, WE42-MAO and WE42-MAO/PLLA before and after being submerged in Hanks’ solution for four days. As Fig. 1a and d show the WE42 magnesium alloy was corroded severely by the Hanks’ solution. Figure 1d shows how the surface of the WE42 was destroyed and deeper and wider cracks and holes appeared. Some white flocculent accumulations were deposited with strong corrosion.
Figure 1b shows that micro-pores and micro-cracks were randomly distributed on the surface of MAO coating. Figure 1e shows that the MAO coating was corroded after being submerged, with little white flocculent deposits being distributed on the surface. Figure 1c demonstrates that the biocompatible sealing layer (PLLA) was smooth and uniform, overlaying cracks or pores and on the surface of the MAO coating. Figure 1f shows that there was no obvious change on the surface of the MAO/PLLA. The surface of the sample WE42-MAO/PLLA was still covered by an intact layer with no signs of corrosion.
3.1.2 Corrosion weight loss
Figure 2 shows the corrosion weight loss corrosion weight loss of the two samples WE42 and WE42-MAO/PLLA. From Fig. 2, it can be seen that the rate of corrosion weight loss of the WE42 sample was high at the initial time (<500 h), and then decreasing gradually after 500 h. The rate of corrosion weight loss of WE42-MAO/PLLA sample was also high at the initial time (<500 h) and then decreasing in a long period of time (500 h –2000 h), and became high once again 2000 h later. The average corrosion weight loss of the WE42 was much higher than WE42-MAO/PLLA samples.
3.1.3 Electrochemical behavior
The potentiodynamic polarization curves of WE42, WE42-MAO and WE42-MAO/PLLA are shown in Fig. 3. The corrosion current density Icorr and the corrosion potential Ecorr can be used to evaluate the corrosion resistance of the coating [19].
The corrosion potential of samples with the MAO/PLLA coating was the most positive and the corrosion current density of the MAO/PLLA coating was the lowest. This result demonstrated that the MAO/PLLA coating effectively improved the corrosion resistance of the WE42.
3.2 Cytotoxicity test
MTT results in Fig. 4 show that the WE42 group has lower OD when compared with other groups, that implies decreased cell viability (P < 0.01). However, there is no significant difference between OD values of control group, WE42-MAO group and WE42-MAO/PLLA group.
4 Discussion
Magnesium alloys are expected to be applied in the field of cardiovascular stents as new bioabsorbable metals because Mg is an essential element, even though it can be easily corroded in an aqueous environment. Given the extreme activity of Mg, control of the corrosion rate for safety in the human body is a key factor in the utilization of magnesium alloys for medical devices [20].In this paper, we fabricated a MAO/PLLA composite coating on magnesium alloy WE42 to modify its corrosion. The purpose of this study was to verify the corrosion protection ability of this kind of surface modification to evaluate the biocompatibility of the modified samples.
4.1 Corrosion test
4.1.1 Immersion test
The surface morphology of the samples was demonstrated by SEM. As Fig. 1b shows there are many microcracks and microholes on the surface of the MAO coating. As shown in a previous study [21], the microholes on the MAO coating was formed by the oxygen bubbles and the microcracks were formed because of the thermal stress due to the rapid solidification of the molten oxide in the relatively cooling electrolyte. The MAO/PLLA coating which was formed by sealing the MAO coating using PLLA was smooth and uniform without any cracks. The PLLA coating could penetrate into the microholes and microcracks to inhibit the corrosion process. The SEM images of the samples after being submerged in Hanks’ solution show that the MAO coating improved the corrosion resistance in some extent and the MAO/PLLA improved the corrosion resistance effectively. This can be explained because the MAO coating was composed of one inner layer and one outer layer. The inner layer was compact with no cracks. Consequently, the corrosion resistance of the MAO was increased. But the outer layer was porous and it would decrease the corrosion resistance. By PLLA sealing, the corrosion resistance of the MAO coating is effectively improved.
4.1.2 Corrosion weight loss
The corrosion weight loss demonstrated that the MAO/PLLA coating improved the corrosion resistance of the WE42 substrate. Lower corrosion weight loss of MAO/PLLA coating was caused by the sealing function. At initial time (<500 h), the corrosion weight loss increases sharply because the samples become corroded. Once the surface is destroyed, the surface area which is contacted by corrosion ions will be increased. As a result, the corrosion rate increases. But as corrosion proceeds, white corrosion accumulations become deposited on the surface and form a thick protective layer that interferes with the corrosion process. This deposit process takes place at the same time that a detaching process. So the corrosion weight loss increases more slowly than before.
4.1.3 Electrochemical measurements
The Ecorr of samples with MAO composite coating was much positive than samples with MAO film, and the sample with MAO/PLLA composite coating showed even lower Icorr than that of the sample with MAO film. This indicated that the composite coating on magnesium alloy WE42 served as an effective barrier against the corrosive electrolyte, and that it greatly improved the corrosion resistance of the magnesium alloy WE42. This was because the outer layer of the MAO coating had many microcracks and pores and this would allow more corrosive intermedium adsorbed onto the MAO film decreasing therefore the corrosion resistance of the MAO film on magnesium. The PLLA sealing layer was connected with the outer layer by physical interlocking, and by penetrating into most of the pores and microcracks, it would decrease the microdefects and coarseness and increase the corrosion resistance of the MAO coating on magnesium alloy WE42.
4.2 Cytotoxicity test
The MTT assay used to determine cell viability is based on mitochondrial viability, that is, only alive mitochondria can oxidize MTT, generating a typical blue formazan reaction product. This assay is an indirect method for testing cell viability and proliferation since the absorbance at 570 nm depends on the cell number. After culturing with immersion extracts for 24 h, the OD values in values in the control group. The MAO/WE42 group and the MAO + PLLA/WE42 group were higher than those of WE43 group. This was due to the modification with MAO and MAO/PLLA which improved subsequently the cell compatibility of the alloy. As we know, magnesium alloys without any surface modification will be corroded heavily when contacting with cell culture medium. The corrosion process is followed by the production of hydrogen, Mg2+ and OH−, which in turn elevate the concentration of magnesium ions and the pH value of medium. This change should damage the viability of cells [22]. So a high corrosion rate of WE42 in ECM would increase sharply in a short period of time the pH value and the concentration of Mg2+ that would damage cells. Therefore, current research has proved that the surface modification can improve the biocompatibility of WE42 magnesium alloy.
5 Conclusions
In this paper, the MAO/PLLA composite coating was fabricated to improve the corrosion resistance and cytocompatibility of WE42. As shown in the results, MAO/PLLA composite coating presented more favorable corrosion resistance. The corrosion weight loss of the composite was decreased at long periods of time. The corrosion potential increased from −1.56 to −1.42 V and the corrosion current density decreased. By modifying the corrosion resistance, the concentration of magnesium ions and pH value were more suitable for cell viability. As a result, the MAO/PLLA improved the cytocompatibility of the WE42 in the cytotoxicity test. In a brief, WE42 modified by MAO/PLLA coating revealed good cytocompatibility and corrosion resistance ability.
References
Heublein B, Rohde R, Kaese V, et al. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart. 2003;89:651–6.
Erne P, Schier M, Resink TJ. The road to bioabsorbable stents: Reaching clinical reality? Cardiovasc Intervent Radiol. 2006;29:11–6.
Gray JE, Luan B. Protective coatings on magnesium and its alloys—a critical review. J Alloys Compd. 2002;336(1–2):88–113.
Yamamoto A, Watanabe A, Sugahara K, Tsubakino H, Fukumoto S. Improvement of corrosion resistance of magnesium alloys by vapor deposition. Scr Mater. 2001;44(7):1039–42.
Delva P. Magnesium and coronary heart disease. Mol Asp Med. 2003;24:63–78.
Drynda A, Hassel T, Hoehn R, Perz A, Bach FW, Peuster M. Development and biocompatibility of a novel corrodible fluoride-coated magnesium–calcium alloy with improved degradation kinetics and adequate mechanical properties for cardiovascular applications. J Biomed Mater Res A. 2010;93A:763–75.
Di Mario C, Griffiths H, Goktekin O, et al. Drug-eluting bioabsorbable magnesium stent. J Interv Cardiol. 2004;17:391–5.
Erbel R, Di Mario C, Bartunek J, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369(9576):1869–75.
Wang XM, Zeng XQ, Wu GS, Yao SS. The effect of Y-ion implantation on the oxidation of AZ31 magnesium alloy. Mater Lett. 2007;61:968–70.
Tian XB, Wei CB, Yanga SQ, Fu RKY, Chu PK. Corrosion resistance improvement of magnesium alloy using nitrogen plasma ion implantation. Surf Coat Technol. 2005;198:454–8.
Wu GS, Zeng XQ, Ding WB, Guo XW, Yao SS. Characterization of ceramic PVD thin films on AZ31 magnesium alloys. Appl Surf Sci. 2006;252:7422–9.
Rudda AL, Breslina CB, Mansfeld F. The corrosion protection afforded by rare earth conversion coatings applied to magnesium. Corros Sci. 2000;42:275–88.
Montemor MF, Simo AM, Carmezim MJ. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection. Appl Surf Sci. 2007;253:6922–31.
Guo HF, An MZ. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate–fluoride solutions and evaluation of corrosion resistance. Appl Surf Sci. 2005;246:229–38.
Cai QZ, Wang L, Wei BK, Liu QX. Electrochemical performance of microarc oxidation films formed on AZ91D magnesium alloy in silicate and phosphate electrolytes. Surf Coat Technol. 2006;200:3727–33.
Shang W, Chen BZ, Shi XC, Chen Y, Xiao X. Electrochemical corrosion behavior of composite MAO/sol–gel coatings on magnesium alloy AZ91D using combined micro-arc oxidation and sol–gel technique. J Alloys Compd. 2008;10:1016.
Duan HP, Du KQ, Yan CW, Wang FH. Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim Acta. 2006;51:2898–908.
Tan ALK, Soutar AM, Annergren IF, Liu YN. Multilayer sol–gel coatings for corrosion protection of magnesium. Surf Coat Technol. 2005;198:478–82.
Wang YQ, Zheng MY, Wu K. Microarc oxidation coating formed on SiCw/AZ91 magnesium matrix composite and its corrosion resistance. Mater Lett. 2005;59:1727–31.
Hanawa T. Materials for metallic stents. J Artif Organs. 2009;12:73–9.
Guo HF, An MZ, Huo HB, Xu S, Wu LJ. Microstructure characteristic of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation in alkaline silicate solutions. Appl Surf Sci. 2006;252(22):7911–6.
Ren B, Wang Y, Ndebele K, Zhi Q, Chen FH, Wang YZ, Parangi S. Multiple signaling is involved in endostatin-mediated apoptosis in ECV 304 endothelial cells. Front Biosci. 2005;10:U1084–9.
Acknowledgment
The authors gratefully acknowledge the financial support of Natural Science Foundation of Tianjin in China (08JC2DJC17900).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lu Cao and Ping Lu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, M., Cao, L., Lu, P. et al. Anticorrosion and cytocompatibility behavior of MAO/PLLA modified magnesium alloy WE42. J Mater Sci: Mater Med 22, 1735–1740 (2011). https://doi.org/10.1007/s10856-011-4354-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4354-z