Abstract
A superior drug controlled release system capable of achieving efficient osteogenesis is in imperative demand because of limited bone substitute tissue for the treatment of bone defect. In the present study, we investigated the potential of using poly(ε-caprolactone)–hydroxyapatite (PCL–HA) composite microspheres as an injectable bone repair vehicle by controlled release of alendronate (AL), a medicine that belongs to the bisphosphonates family. The PCL/HA–AL microspheres were prepared with solid/oil/water emulsion technique, which included two processes: (1) AL was loaded on the hydroxyapatite nanoparticles; (2) the HA–AL complex was built in the PCL matrix. The spherical PCL/HA–AL microspheres were characterized with its significantly improved encapsulation efficiency of hydrophilic AL and better sustained release. Human bone mesenchymal stem cells (hMSCs) were cultured on the surface of these microspheres and exhibited high proliferative profile. Specifically, in osteogenic medium, hMSCs on the surface of PCL/HA–AL microspheres displayed superior osteogenic differentiation which was verified by alkaline phosphatase activity assay. In conclusion, by presenting strong osteogenic commitment of hMSCs in vitro, the PCL/HA–AL microspheres have the potential to be used as an injectable vehicle for local therapy of bone defect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The treatment of bone defect originated from trauma, tumor metastasis and skeletal abnormities compromises limited host source of bone repair. In the past three decades, researchers have witnessed the emergence of controlled release technology as an important field of pharmaceutical sciences [1]. Currently, drug-loaded microparticles have been proposed as injectable vehicle for the treatment of some bone or cartilage defects [2, 3]. To fabricate the suitable carriers that can support cell growth, proliferation and differentiation remains challenging. The development and design of biodegradable microspheres carrying drugs or bioactive factors for the therapeutic application also require a fundamental understanding of how the cells and tissues react to the microspheres.
Both polymers and inorganic substances have been tested for their usage as carriers of many drugs. A variety of inorganic materials such as hydroxyapatite (HA) [4], bioactive glasses [5], and bone cement [6] have been employed as the controlled release drug delivery system in humans with limited success. Among them, HA is the principal inorganic constituent of human bones, which has been widely adopted as biomaterials for bone repair and bone tissue regeneration owe to its biocompatibility, non-immunogenicity, non-inflammatory behavior, high osteoconductivity property [7, 8]. However, its poor mechanical strength and slow degradation rate limited the direct application as a drug carrier for local therapy. It is desirable to combine the HA with other biomaterials to overcome these defects [9]. Given that biodegradable polymers such as poly(ε-caprolactone) (PCL), polylactide (PLA), and poly(lactide-co-glycolide) (PLGA) have been used in biomedical field for a long time, they have attracted many researchers to examine the possibility of using them as carriers for controlled release. Specifically, PCL, a commercially available product approved for human application, has been extensively studied as an implantable or injectable biodegradable carrier making use of its excellent processability, good mechanical property and controllable degradability [10–12]. The clinical applications of PCL have demonstrated that it is compatible with physiological environment since it can be hydrolyzed into metabolite byproducts which are innoxious to the human body [13]. The combination of HA with PCL can be expected to combine the advantages of both biomaterials to obtain the optimal vehicles for local therapy.
Prostate cancer is the most commonly diagnosed malignancy in men. A majority of patients suffer from skeletal metastasis after developing androgen-independent, hormone refractory prostate cancer [14, 15]. During the past three decades, bisphosphonates (BPs), such as pamidronate, alendronate (AL), ibandronate, zoledronate, are indispensable for the treatment of both benign and malignant bone diseases such as osteoporosis and other bone defects induced by the metastasis of prostate cancer [16]. Bisphophonates are a family of analogous pyrophosphate compounds that affect cellular processes involved in bone formation and resorption activity [17]. Recently, numerous studies have described the ability of BPs to inhibit tumor cell adhesion and invasion [18]. For instance, AL has been extensively used in clinical cases including malignant cancers, which have showed no severe side effects. It has been proved to be capable of inhibiting the activity of mature osteoclasts and promoting the proliferation of osteoblasts [19, 20]. However, AL is easily lapsed in aqueous conditions during fabrication process because of its high hydrophilicity [21]. In addition, the cytotoxicity of high AL concentration can induce side effect. In order to improve the AL loading amount and avoid its side effect, many researchers resort to HA to prolong the release of AL [22, 23]. Bigi et al. [24] made HA–AL thin films by assisted pulsed laser evaporation, and they found that the deposition of AL modified HA thin films were able to promote osteoblasts differentiation and restrain osteoclast proliferation. Usually, the strong hydrophilic AL exhibits low encapsulation efficiency in polymeric microspheres and processes the rapid release [25]. The AL-laden HA particles encapsulated in polymer not only improve the encapsulation efficiency but also prolong the release period. Shi et al. induced the synovium mesenchymal stem cells (SMSCs) to osteogenesis by controlled release of AL from a mesoporous silica (MS)–HA composite that was embedded in PLGA microspheres. The total encapsulation efficiency of AL was significantly improved, and the hybrid microspheres showed significant controlled release profile of AL [25]. In this study, the AL release system was explored by packaging the AL laden HA–AL construct into the PCL microspheres.
Recently, human bone mesenchymal stem cells (hMSCs) have drawn more attention to the clinical application in the treatment of bone defect because they can self-renew and differentiate into various types of cells such as osteoblasts [26]. Osteogenic differentiation of hMSCs is a mainstream tissue engineering strategy for bone repair [27]. AL possesses higher chemical and physical stability to be utilized in hMSCs osteogenic commitment [28]. It has been reported that AL has a positive effect on the osteogenic differentiation of hMSCs [28]. However, the bioactivity of AL is very low via oral administration due to poor gastrointestinal (GI) absorption and only a small portion of the absorbed drug can be eventually incorporated in bone [29]. In order to pursue efficient osteogenesis of hMSCs by AL treatment, the controlled release system with high AL loading is required.
This study focused on fabricating and characterizing a novel injectable delivery system taking advantage of both PCL and HA, which could be potential for the treatment of bone defect induced by malignant cancer. Double emulsion (D/M) and solid/oil/water (S/O/W) emulsion technique were employed to elaborate the drug encapsulation systems, and the AL encapsulated PCL microspheres were characterized and compared. Furthermore, the cell viability and alkaline phosphatase (ALP) activity of hMSCs on AL loaded PCL microspheres were also examined.
2 Materials and methods
2.1 Materials
PCL with an average molecular weight of 40,000 g/mol was purchased from Daigang Biomaterials. HA was synthesized using the process described in Ref. [30]. AL, re-purified before usage, was obtained from Tianfeng Inc., Henan, China. Methyl cellulose M20 (MC) was purchased from Guoyao Chemical Reagents Limited, Shanghai, China. hMSCs were a gift from Research Center of Stem Cell of Sun Yat-sen University. Bone marrows were donated from sixteen healthy volunteers aged at 25–35 in accordance with guidelines of the Institutional Human Care and Ethics Committee at the University of Sun Yat-sen University. Cell related reagents would be listed exclusively.
2.2 Preparation of HA–AL particles
HA–AL particles was prepared by dispersing 1 g HA particles in 10 ml AL aqueous solution (1 mg/ml) and then stirred continuingly for 24 h under 37°C. The resulted suspension was centrifuged and washed with deionized water for several times before vacuum drying. The obtained powders were stored in the desiccator for further use.
2.3 Fabrication of PCL/HA–AL and PCL/AL microspheres
-
(1)
Solid/oil/water emulsion (S/O/W): 5 g PCL was dissolved in 40 ml methylene chloride before HA–AL particles were added. Subsequently, the amounts of HA–AL particles accounted for 15% or 30% of PCL mass were added to the above PCL methylene chloride solution. The emulsion was poured into 600 ml 0.4% MC aqueous solution and stirred at 600 rpm for 12 h. The resultant PCL/HA–AL microspheres were washed with deionized water and vacuum dried.
-
(2)
Double emulsion (DM): 5 g PCL was dissolved in 40 ml methylene chloride. 1 ml AL (2 mg/ml) aqueous solution was dropped into 8 ml PCL methylene chloride solution. After that, the mixture was homogenized under 10,000 rpm for 30 s. The resultant mixture was poured into 600 ml 0.4% MC aqueous solution and stirred at 300 rpm for 4 h. The ultimate PCL/AL microspheres were isolated, washed with deionized water and vacuum dried.
-
(3)
Oil/water (O/W): The control group of pure PCL microspheres was prepared by O/W method. 5 g PCL was dissolved in 40 ml methylene chloride. After that, the solution was poured into 600 ml 0.4% MC aqueous solution and stirred at 600 rpm for 12 h. The resultant microspheres were treated as the same way as PCL/HA–AL and PCL/AL microspheres did.
2.4 General characterizations of PCL based microspheres
Size distribution of the microspheres were determined by laser light scattering (Malvern, MS2000, USA). The lyophilized particles (1 g) were suspended in large amount of distilled water under stirring and analyzed. The morphology of three kinds of PCL based microspheres was conducted by scanning electron microscopy (SEM, FEI Quanta 200, The Netherlands). The microspheres were immobilized on a cupreous stub and coated with gold for two times before observation. The morphology of HA–AL particles was performed by Transmission Electron Microscopy (TEM, Philips CM300). Three individual experiments were performed for each batch.
2.5 Fourier transform infrared spectroscopy
Fourier-transformed infrared spectroscopy (Icolet Nexus FT-IR, Thermo Electron Corporation) was used in the wave number range of 4,000–400 cm−1. Experimental spectra of solid samples were taken as about 1 mg samples suspended in about 100 mg of KBr. Three parallel samples were analyzed for each batch.
2.6 Encapsulation efficiency of D/M and S/O/W made microspheres
For the S/O/W made microspheres, the content of HA in the PCL/HA–AL microspheres was determined by the following method: 50 mg of vacuum dried PCL/HA–AL microspheres were dropped in 20 ml acetic acid with a pH of 4.5 for 6 h. Weighed the microspheres together with the vessel, the microspheres’ weight loss after the immersion could be calculated and considered as the HA content. Five parallel samples were repeated for each batch.
-
1.
AL in HA–AL particles: The AL encapsulation efficiency of HA–AL chelate was measured by pouring 50 mg HA–AL into 2 ml PBS buffer (pH 7.2) and keeping at 37°C during which the supernatant was periodically analyzed until stable following the method recorded in Ref. [31].
-
2.
AL in PCL/HA–AL microspheres: PCL/HA–AL microspheres (200 mg) were dissolved in methylene chloride (30 ml) to deposit the insoluble HA–AL particles. The deposited HA–AL particles and the supernant were collected respectively. AL was measured by two parts: first, the part (Cpart 1) encapsulated in HA–AL; second, the part (Cpart 2) encapsulated in the collected supernatant which was composed of PCL/methylene chloride and AL. AL in the HA–AL particles was measured using the method described above. The second part was measured according to Ref. [21]. Briefly, the collected supernatant was rinsed in iron(III) chloride/perchloric acid solution to extract the AL into the aqueous phase. The AL in the iron(III) chloride/perchloric acid solution extraction was quantified using UV spectrophotometer. The above two parts together represented the whole AL encapsulated in PCL/HA–AL microspheres. The encapsulation efficiency was calculated as follow: Cpart 1+part 2/Ctheory. Five parallel samples were repeated for each batch.
2.7 In vitro drug release evaluation
AL release experiments were performed in a shaking incubator at 87 rpm under 37°C. 50 mg AL-loaded microspheres were soaked in 20 ml PBS (pH 7.2). 2 ml supernatant of the sample media was collected at regular time intervals and the same volume of fresh PBS was added. The AL concentration in the supernatant was evaluated using the quantification method described in Ref. [20]. Briefly, the obtained supernatant was rinsed in an iron (III) chloride/perchloric acid solution so that the AL can be entirely extracted into the aqueous phase. The AL concentration was examined using UV spectrophotometer according to the method described in Ref. [20]. Five parallel samples were examined for each batch.
2.8 Cell culture
hMSCs were propagated in Dulbecco’s modified eagle’s medium (DMEM) with supplements of 1.5 mg ml−1 sodium bicarbonate, 10% (v/v) fetal bovine serum (FBS), 3 mg ml−1 4-(2-hydroxyerhyl)piperazine-1-erhanesulfonic acid (HEPES). The cells were incubated in a humidified incubator at 37°C with 5% CO2. The medium was changed every 3 days. All the reagents in this section were purchased from Gibco (Invitrogen, USA).
2.9 Cells seeding on the microspheres
The PCL based microspheres were first sterilized. Each kind of microspheres (containing 100 μg AL) was dispersed in 48-well TCPS (tissue culture polystyrene) plates with the bottom pre-coated with 2% sterile agarose gels. 20 μl passage 5 hMSCs in suspension (5 × 104 cells/well) were seeded on each microspheres. The cells/microspheres construct was incubated at 37°C with 5% CO2 in a humidified incubator. After 3 days, the medium was changed to remove non-adherent cells, and the medium was subsequently changed every 3 days. To observe the hMSCs adhering on the surface of the microspheres, the hMSCs and the microsphere samples were harvested, washed with PBS, fixed with 2.5% glutaraldehyde for 20 min, dehydrated in a series of graded concentrations of ethanol, vacuum-dried, and then imaged by SEM. Meanwhile, hMSCs cultured on the surface of the various types of microspheres were analyzed by a Live/Dead assay kit (Invitrogen) according to the manufacture’s instruction using inverted fluorescence microscope as described previously [32]. Before cell staining, the microspheres were washed three times by PBS. Three parallel samples were analyzed for each batch.
2.10 Cell viability on the drug carriers
Cell viability on the microspheres was measured after 3, 7 and 14 days of culture using a broadly used method named MTT (Sigma) assay. The cells/microspheres constructs were washed three times with PBS and then incubated at 37°C for 4 h in 200 μl 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) solution. The following assay was conducted following the recommendatory protocol. Cells seeded on the pure PCL microspheres were used as control. The experiment was performed in triplicate and three independent experiments were conducted.
2.11 ALP activity
Osteogenic differentiation was induced by incubating cell laden microspheres in osteogenic medium (DMEM supplemented with 10% FBS, 100 nM dexamethasone, 0.2 mM ascorbic acid, and 10 mM β-glycerophosphate; OGM) for 7 days and 14 days. The medium was changed every 3 days. The experiment was performed in triplicate. On day 7 and 14, the ALP activities were measured. At the time points, the microspheres with cells were pre-washed with PBS and the adherent cells were removed from the microspheres. 300 μl of a lysis buffer was added, incubated on ice for 10 min and the lysates were centrifugated at 4°C for 5 min at 13,000 rpm using an ultracentrifuge (Thermo, CA, USA). Each sample (20 μl supernatant) was incubated with 200 μl 5 mM p-nitrophenyl phosphate liquid substrate (pNPP, Sigma) at 37°C for 15 min. The reaction was terminated by adding 200 μl 0.1 M NaOH aqueous solution. The absorbance of the samples was detected at 405 nm using a Thermo plate reader (Thermo, CA, USA). Each ALP activity measurement was normalized by the protein content, which was measured by the BCA protein assay reagent (Invitrogen, USA). Pure PCL microspheres was used as control. Three completely individual experiments were conducted.
2.12 Statistical analysis
Data were expressed as mean ± standard deviations. The statistical significance was determined using one way analysis of variance (ANOVA). A comparison between two means was analyzed by Tukey’s test. P value <0.05 were considered statistically significant.
3 Results and discussion
3.1 Particle size distribution of microspheres
The particle size distribution (PSD) of four kinds of PCL based microspheres were shown in Fig. 1. The diameters of most PCL/15%HA–AL, PCL/30%HA–AL and pure PCL microspheres were about 100 μm, and the most DM-PCL/AL microspheres were smaller than 100 μm. The diameters of PCL/15%HA–AL, PCL/30%HA–AL and pure PCL microspheres were distributed in relative narrow span while the diameters of DM-PCL/AL microspheres displayed a wide distribution. A wave was observed in the PSD of both PCL/30%HA–AL and pure PCL microspheres shown in Fig. 1, indicating that some microspheres with smaller diameters appeared. The sizes of microspheres made by S/O/W, O/W and DM method were determined by several factors such as stirring speed, solution concentration, concentration of surfactant etc. We speculated that the DM-PCL/AL microspheres with relatively smaller diameter may be attributed to the high homogenization speed, which might be beneficial to longer release of the drug [33].
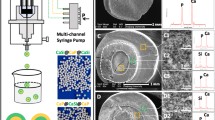
3.2 Morphology of four kinds of microspheres
The SEM images of four kinds of microsphere and TEM image of HA–AL particles were shown in Fig. 2. All of the microspheres maintained a spherical shape, but apparently, the pure PCL and DM-PCL/AL microspheres possessed a smoother surface than the microspheres loaded with HA–AL particles. Some solid particles could be obviously observed on the surface of PCL/HA–AL microspheres. On the basis of the images, a conclusion could be drawn that the microspheres’ surface became rougher with the enrichment of HA–AL particles. PCL/15%HA–AL showed comparable particle size with PCL/30%HA–AL, suggesting that the enrichment of HA–AL particles had little influence on the particle size of PCL/HA–AL microspheres. No significant difference was observed between the morphology of pure PCL and DM-PCL/AL microspheres. TEM image of HA–AL particles demonstrated that the HA–AL particles presented a polygon shape with a diameter of about 50 nm.
3.3 Fourier transform infrared spectra of the microspheres
The Fourier transform infrared (FT-IR) spectrum of microspheres was shown in Fig. 3. In the spectrum of pure PCL microspheres (Fig. 3a), bands at 1,727 cm−1 was due to ν(C=O) in the polymer carbon main chain. Broad peaks ranging from 1,000 to 1330 cm−1 were attributed to ν(C–O–C), and the methane stretching vibration ν(C–H) were observed at 2,946 and 2867 cm−1. Figure 3b illustrated the FT-IR spectrum of the DM-PCL/AL microspheres. All the peaks, which were assigned to PCL, could be observed. However, the signals of AL were hardly to be obtained by FT-IR spectra probably because the ratio of AL in DM-PCL/AL microspheres was very low. In the FT-IR spectra of both PCL/15%HA–AL microspheres (Fig. 3c) and 30%HA–AL microspheres (Fig. 3d), the PO4 3− bands belong to HA were located at 602, 961, 1,040 and 1,097 cm−1, and the low intensity O–H derived vibration was observed at 3,569 cm−1. Meanwhile, the typical peaks caused by PCL could be found in the spectra. The strong intensities of the P–O stretching vibration peaks, especially at around 1,040 cm−1, suggested that HA was successfully blended with PCL matrix. An additional feature should be mentioned that compared with the spectrum of PCL/15%HA–AL microspheres, the peaks’ areas corresponding to the absorption of HA increased in PCL/30%HA–AL microspheres, indicating that the content of HA in PCL/30%HA–AL microspheres was obviously increased.
3.4 Encapsulation efficiency of DM-PCL/AL, PCL/HA–AL microspheres
In our study, AL was firstly loaded onto the HA particles and furtherly encapsulated in the PCL matrix, which facilitated the higher encapsulation efficiency of PCL/HA–AL microspheres. The AL encapsulation efficiency of DM-PCL/AL and PCL/HA–AL microspheres was shown in Table 1. The PCL/15%HA–AL microspheres enwrapped 11.05 ± 1.02% (HA mass/PCL mass) HA particles with an drug encapsulation efficiency of 77.31 ± 3.35%, which showed higher encapsulation efficiency than that of PCL/30%HA–AL microspheres. The drug encapsulation efficiency of PCL/HA–AL microspheres was significantly higher than that of DM-PCL/AL, which was only 4.75 ± 0.91%. The high drug encapsulation efficiency of PCL/HA–AL microspheres should be ascribed to HA–AL complexes while the very low encapsulation efficiency of DM-PCL/AL microspheres may be because of the strong hydrophilicity of AL.
3.5 In vitro release of AL
The in vitro cumulative release profiles of AL within 21 days from three kinds of PCL related microspheres were illustrated in Fig. 4. Just as shown in Fig. 4, the profiles of AL release from the PCL/15%HA–AL and PCL/30%HA–AL microspheres exhibited the comparable curve, which were very different from that of the DM-PCL/AL microspheres. All kinds of microspheres exhibited a burst AL release with remarkably high release rate during the first 4 days. For the PCL/HA–AL microspheres, about 30% of the total loading AL was released during these days. However, nearly 70% of the AL was released in the DM-PCL/AL microspheres within the first 4 days, indicating that this release system had little effect on sustained release of AL. In the next 3 days, almost all of AL release had been accomplished from the DM-PCL/AL microspheres while after the curve inflexion at around day 4–6, the PCL/HA–AL microspheres significantly reduced AL release rate so that it took another 17 days to fulfill another 40% of total AL release. Up to day 21, the cumulative AL release reached around 70–80% from the PCL/HA–AL microspheres. The initial burst release was probably caused by the rapid diffusion of drug adsorbed on the microsphere surface while the subsequent durative release might be attributed to the HA–AL complexes and the degradation of microspheres. The release profile of PCL/HA–AL system may involved the following two processes [34]: first, the AL release from HA–AL was partly controlled by the solubility of HA; second, the HA–AL particles were enwrapped in the bulk of PCL microspheres, which could further retard AL release.
3.6 Cell viability on the DM-PCL/AL and PCL/HA–AL microspheres
Attachment and adhesion occured in the first phase of material/cells construct interactions, which will influence the cell’s capacity to proliferate and differentiate on the materials [35]. As shown in Fig. 5, the green fluorescence (Fig. 5a–d) and long, spindle-shaped cells (Fig. 5e–h) were respectively evident on the surface of microspheres, indicating hMSCs attached to the microsphere surface. A little amount of hMSCs were observed on the surface of pure PCL (Fig. 5a) and DM-PCL/AL (Fig. 5b) microspheres after 5 days of culture. In comparison, the PCL/HA–AL microspheres exhibited the more viable cells (green fluorescence) on the surface (Fig. 5c, d). The results were consistent with that of SEM (Fig. 5e–h), suggesting that the PCL/HA–AL microspheres could promote the attachment of hMSCs compared with the pure PCL and DM-PCL/AL microspheres. It may stem from the possibility that introducing HA to the PCL microsphere system, which resulted in a rough surface, facilitated the attachment of hMSC. The cell viability on the PCL based microspheres was analyzed by measuring the activity of the mitochondrial dehydrogenases, which is referred to the MTT assay. hMSCs were incubated with DM-PCL/AL and PCL/HA–AL microspheres to evaluate the cytotoxicity of the three carriers. Figure 6 showed the results of MTT tests. All of the four microspheres showed an increasing trend of absorbance of formazan as the culture day went longer, suggesting a trend of increasing cell proliferation for all the microspheres. According to cell proliferation on the microspheres, all of the microspheres presented good biocompatibility. Cells on the DM-PCL/AL microspheres exhibited the highest viability at day 3 while at 7th and 14th day cells on PCL/15%HA–AL microspheres proliferated rapidly and showed the highest viability, indicating that PCL/15%HA–AL microspheres exhibited best biocompatibility. Compared with the pure PCL and DM-PCL/AL microspheres, the cells on PCL/15%HA–AL microspheres showed better viability at 7th and 14th day, which suggested that appropriate amount of HA had no cytotoxicity and maybe promoted the proliferation of hMSCs.
The fluorescence images and SEM micrographs of hMSCs adhering to the surface of the pure PCL (a, e), DM-PCL/AL (b, f), PCL/15%HA–AL (c, g) and PCL/30%HA–AL (d, h) microspheres after 5 days of in vitro culture. The fluorescence images were determined by Live/Dead staining. Representative images were from three individual experiments
Viability of hMSCs after 3,7 and 14 days of culture on the samples of pure PCL, DM-PCL/AL, PCL/15%HA–AL and PCL/30%HA–AL microspheres. Pure PCL microspheres without AL was used as control. Columns mean of three individual experiments (n = 3); Bars ISE. Asterisk and Pound sign indicate statistical significant when compared with the pure PCL microspheres and DM-PCL/AL microspheres, respectively (p < 0.05)
3.7 ALP activity
hMSCs underwent osteogenic differentiation on the microspheres, which was assessed by pNPP assay. ALP is an important enzyme of osteogenic differentiation that specifically degrades the organic phosphoesters and promotes the calcium deposition in bone [36, 37]. The increase of ALP activity indicates the occurrence of osteogenesis [36]. Figure 7 showed the ALP activities of hMSCs cultured on pure PCL, DM-PCL/AL, PCL/15%HA–AL and PCL/30%HA–AL microspheres in OGM. The ALP activity levels of hMSCs cultured on all the microspheres upregulated during 14 days of culture, respectively. However, hMSCs cultured on the PCL/15%HA–AL microspheres presented higher ALP activities than the pure PCL microspheres (control) and displayed the highest ALP activity at both 7th and 14th day after inducement, indicating the strongest osteogenic capacity of hMSCs cultured on the PCL/15%HA–AL microspheres. It was worthwhile to mention that hMSCs on both the PCL/15%HA–AL and PCL/30%HA–AL microspheres exhibited much higher ALP activities in contrast to hMSCs on the DM-PCL/AL microspheres at both 7th and 14th day, which was probably attributed to the rapid release of AL encapsulated in DM-PCL/AL microspheres, suggesting that the sustained release of AL significantly facilitated the osteogenic differentiation of hMSCs in OGM [38].The results indicated that the PCL/15%HA–AL microspheres might be promising as an injectable bone repair vehicle for the treatment of bone defect.
ALP activity of hMSCs after 7 and 14 days of culture on the pure PCL, DM-PCL/AL, PCL/15%HA–AL and PCL/30%HA–AL microspheres in OGM. Pure PCL microspheres was used as control. Columns mean of three individual experiments (n = 3); Bars ISE. Asterisk and Pound sign indicate statistical significant when compared with the pure PCL microspheres and DM-PCL/AL microspheres, respectively (p < 0.05)
4 Conclusion
In this study, AL loaded PCL/HA–AL microspheres were fabricated by a solid/oil/water (S/O/W) emulsion method for the treatment of bone defect. The PCL/HA–AL microspheres presented a narrow size distribution which could guarantee a high production. The FT-IR spectra of the PCL/HA–AL microspheres verified that the HA–AL particles were successfully hybridized into PCL microspheres. PCL/HA–AL microspheres exhibited sustained release profile after a slight burst release for more than 21 days. The spherical PCL/HA–AL microspheres, especially PCL/15%HA–AL microspheres, showed little cytotoxicity. Comparison between DM-PCL/AL and PCL/HA–AL microspheres confirmed the advantage of latter microspheres. The results of ALP activity of hMSCs cultured on these microspheres in OGM indicated that the PCL/HA–AL microspheres could facilitate the differentiation of hMSCs. To sum up, the PCL/HA–AL microspheres have the potential to be used as an injectable vehicle for the treatment of bone defect.
References
Habraken WJ, Wolke JG, Jansen JA. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:234–48.
Dhanaraju MD, Gopinath D, Ahmed MR, Jayakumar R, Vamsadhara C. Characterization of polymeric poly(ε-caprolactone) injectable implant delivery system for the controlled delivery of contraceptive steroids. J Biomed Mater Res A. 2006;76:63–72.
Shen H, Hu X, Yang F, Bei J, Wang S. An injectable scaffold: rhBMP-2-loaded poly(lactide-co-glycolide)/hydroxyapatite composite microspheres. Acta Biomater. 2010;6:455–65.
Boonsongrit Y, Abe H, Sato K, Naito M, Yoshimura M, Ichikawa H, et al. Controlled release of bovine serum albumin from hydroxyapatite microspheres for protein delivery system. Mater Sci Eng B. 2008;48:162–5.
Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–55.
Link DP, van den Dolder J, van den Beucken JJ, Wolke JG, Mikos AG, Jansen JA. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-β1 loaded gelatin microparticles. Biomaterials. 2008;29:675–82.
Huang J, Lin YW, Fu XW, Best SM, Brooks RA, Rushton N, et al. Development of nano-sized hydroxyapatite reinforced composites for tissue engineering scaffolds. J Mater Sci Mater Med. 2007;18:2151–7.
Chen F, Lam WM, Lin CJ, Qiu GX, Wu ZH, Luk KD, et al. Biocompatibility of electrophoretical deposition of nanostructured hydroxyapatite coating on roughen titanium surface. In vitro evaluation using mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2007;82:183–9.
Yunoki S, Ikoma T, Monkawa A, Ohta K, Kikuchi M, Sotome S, et al. Control of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growth. Mater Lett. 2006;60:999–1002.
Pitt CG. Poly(ε-caprolactone) and its copolymers. In: Chasin M, Langer R, editors. Biodegradable polymers as drug delivery systems. New York: Marcel Dekker; 1990. p. 71–120.
Chang HI, Lau YC, Yan C, Coombes AG. Controlled release of an antibiotic, gentamicin sulphate, from gravity spun polycaprolactone fibers. J Biomed Mater Res A. 2008;84:230–7.
Oh SH, Lee JY, Ghil SH, Lee SS, Yuk SH, Lee JH. PCL microparticle-dispersed PLGA solution as a potential injectable urethral bulking agent. Biomaterials. 2006;27:1936–44.
Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735–40.
Gulley J, Dahut WL. Novel approaches to treating the asymptomatic hormone-refractory prostate cancer patient. Urology. 2003;62(Suppl 1):147–54.
Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81.
Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97(Suppl 3):840–7.
García-Moreno C, Serrano S, Nacher M, Farré M, Díez A, Mariñoso ML, et al. Effect of alendronate on cultured normal human osteoblasts. Bone. 1998;22:233–9.
Benyettou F, Lalatonne Y, Sainte-Catherine O, Monteil M, Motte L. Superparamagnetic nanovector with anti-cancer properties: gamma Fe2O3@Zoledronate. Int J Pharm. 2009;379:324–7.
van Beek ER, Löwik CW, Papapoulos SE. Effect of alendronate treatment on the osteoclasto-genic potential of bone marrow cells in mice. Bone. 1997;20:335–40.
Halasy-Nagy JM, Rodan GA, Reszka AA. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone. 2001;29:553–9.
Shi X, Wang Y, Ren L, Gong Y, Wang DA. Enhancing alendronate release from a novel PLGA/hydroxyapatite microspheric system for bone repairing applications. Pharm Res. 2009;26:422–30.
Boanini E, Torricelli P, Gazzano M, Giardino R, Bigi A. Alendronate-hydroxyapatite nanocomposites and their interaction with osteoclasts and osteoblast-like cells. Biomaterials. 2008;29:790–6.
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–27.
Bigi A, Boanini E, Capuccini C, Fini M, Mihailescu IN, Ristoscu C, et al. Biofunctional alendronate–hydroxyapatite thin films deposited by MatrixAssisted Pulsed Laser Evaporation. Biomaterials. 2009;30:6168–77.
Shi X, Wang Y, Varshney RR, Ren L, Zhang F, Wang DA. In vitro osteogenesis of synovium stem cells induced by controlled release of bisphosphate additives from microspherical mesoporous silica composite. Biomaterials. 2009;30:3996–4005.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36.
Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7:23–33.
von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, et al. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–9.
Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev. 2000;42:175–95.
Guo X, Gough JE, Xiao P, Liu J, Shen Z. Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J Biomed Mater Res A. 2007;82:1022–32.
Kuljanin J, Janković I, Nedeljković J, Prstojević D, Marinković V. Spectrophotometric determination of alendronate in pharmaceutical formulations via complex formation with Fe(III) ions. J Pharm Biomed Anal. 2002;28:1215–20.
Choong CS, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12(9):2521–31.
Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282:1–18.
Huang W, Wang Y, Ren L, Du C, Shi X. A novel PHBV/HA microsphere releasing system loaded with alendronate. Mater Sci Eng C. 2009;29:2221–5.
Serrano MC, Pagani R, Vallet-Regí M, Peña J, Rámila A, Izquierdo I, et al. In vitro biocompatibility assessment of poly(e-caprolactone) films using L929 mouse fibroblasts. Biomaterials. 2004;25:5603–11.
Wang DA, Williams CG, Yang F, Cher N, Lee H, Elisseeff JH. Bioresponsive phosphoester hydrogels for bone tissue engineering. Tissue Eng. 2005;11:201–13.
Xue W, Krishna BV, Bandyopadhyay A, Bose S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007;3:1007–18.
Cenni E, Granchi D, Avnet S, Fotia C, Salerno M, Micieli D, et al. Biocompatibility of poly(d,l-lactide-co-glycolide) nanoparticles conjugated with alendronate. Biomaterials. 2008;29:1400–11.
Acknowledgments
This work was supported by Chinese National Natural Science Foundation 30772178 (to X.G.) 30973011 (to X.G.), 30901496 (to J.P.), Key Project of Guangdong Provincial Science and Technology Research 7117362 (to X.G.) and Chinese National Hi-Tech Research and Development Program 2007AA021906 (to X.G.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xin Gao is the main corresponding author.
Jianhong Chen and Yun Luo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, J., Luo, Y., Hong, L. et al. Synthesis, characterization and osteoconductivity properties of bone fillers based on alendronate-loaded poly(ε-caprolactone)/hydroxyapatite microspheres. J Mater Sci: Mater Med 22, 547–555 (2011). https://doi.org/10.1007/s10856-011-4232-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4232-8