Abstract
Nanometer size biphasic calcium phosphate (BCP) powders with various Ca/P molar ratios satisfied with appropriate phase ratios of HA/β-TCP were prepared by high temperature flame spray pyrolysis process. The BCP powders had spherical shapes and narrow size distributions irrespective of the ratios of Ca/P. The mean size of the BCP powders measured from the TEM image was 38 nm. The composition ratio of Ca/P was controlled from 1.500 to 1.723 in the spray solution, and required phase ratios of HA/TCP are controlled systematically. The calcium dissolution of the pellets obtained from the BCP powders directly prepared by flame spray pyrolysis in buffer solution increased with the decrease of Ca/P ratios except with the Ca/P ratio of 1.713. The pellet surface with Ca/P ratio of 1.500, which consisted of β-TCP, was eroded dramatically for 7 days. On the other hand, the pellet surface with Ca/P ratio of 1.667 was stable and did not disintegrate after immersion in Tris–HCl buffer solution based on the SEM observation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Calcium phosphate based bioceramics have been widely used for orthopedic applications due to their chemical similarity to natural bone [1–5]. Among them, Biphasic Calcium Phosphate (BCP) powders have recently attracted for their ideal bone graft substitute. BCP is commonly composed of hydroxyapatite (HA) and a more resorbable material like tricalcium phosphate (α or β-TCP) or calcium carbonate in different proportions depending on the characteristics required for the specific medical applications [6–9]. In order to apply appropriate BCP to meet specific biological needs, it is crucial to control the BCP with various phase ratios of HA/TCP. Furthermore, recently nano bioceramics have attracted for their effect of bioactive properties [10–20]. Many studies have shown that bone-forming cells for specific applications are interacting with nanoscale surfaces of biomaterials which is critical to keep the body from rejecting artificial parts [21, 22] and promote the adhesion, proliferation and differentiation of osteoblasts [23, 24].
Currently, several synthetic routes have been utilized for the preparation of BCP powders. Nilen et al. [25] synthesized HA mechanically mixed with β-TCP, then calcinated to form a suite of BCP materials with a wide range of HA/β-TCP phase content ratios. Kannan et al. [26] prepared HA and biphasic ceramics of two different HA and β-TCP proportions with substituted potassium through the aqueous precipitation method. Lee et al. [27] synthesized HA and BCP nano powders by the microwave assisted process. Raynaud et al. [28] synthesized biphasic mixtures composed of a HA and CaHPO4 (Ca/P < 1.5) or Ca(OH)2 (Ca/P > 1.667) using wet method. Yasuda et al. [29] synthesized HA/α-TCP composites by colloidal process.
Nanometer size HA and TCP powders were also prepared by gas phase reaction methods, such as spray pyrolysis and flame spray pyrolysis [30–32]. However, BCP powders were not well studied in the gas phase reaction methods.
In this study, BCP ceramics with various Ca/P molar ratios satisfied with appropriate phase ratios of HA/TCP were firstly prepared by flame spray pyrolysis applying ultrasonic spray system. The prepared BCP ceramic powders had spherical shape, nanometer size and non-aggregation characteristics. In the previous report, calcium phosphate powders were also prepared by flame spray pyrolysis applying nozzle spray system. The calcium phosphate powders prepared by flame spray pyrolysis applying nozzle spray system had hardly aggregated structure. The degradability of the synthesized BCP was studied by dissolution of calcium ion in Tris–HCl buffer solution.
2 Materials and method
Figure 1 shows the schematic diagram of the flame spray pyrolysis apparatus. The system of flame spray pyrolysis has an ultrasonic droplet generator, flame nozzle, quartz reactor, powder collector and blower. Propane and oxygen were used each as the fuel and oxidizer gases to create the diffusion flame. The flame nozzle has five concentric pipes. Droplets generated from the precursor solution are supplied to the diffusion flame through the center pipe by oxygen used as a carrier gas. The flow rates of fuel, oxidizer and carrier gas were each 5, 40 and 10 l/min. The starting materials in the synthesis of biphasic calcium phosphae (BCP) powders were Ca(NO3)2·4H2O and (NH4)2HPO4. The total concentration of Ca and P components was fixed at 0.4 M. The mixing molar ratios of Ca(NO3)2·4H2O to (NH4)2HPO4 was adjusted accordingly to the desired Ca/P ratios from 1.500 to 1.723 in the mixed solvent. The mixture of ethyl alcohol and distilled water was used as the solvent. The volume ratio of ethyl alcohol to water was 60% to prepare the spray solution. The BCP powders with various Ca/P molar ratios were pelletized at 320 MPa for 15 min into a 10 mm diameter and subsequently calcinated at a temperature of 800°C for 2 h under air atmosphere and cooled naturally to room temperature while furnace power was off. The pellets were immersed in Tris–HCl buffer solution. The 0.1 M Tris–HCl buffer solution was prepared by dissolving analytical reagent grade Tris(hydroxymethyl) aminomethane in distilled water and then was buffered at pH 7.4 at 37°C with hydrochloric acid.
The crystal structures of the as-prepared and post-treated BCP powders with various Ca/P molar ratios were investigated by X-ray diffraction (XRD, RIGAKU, D/MAX-RB) with Cu Kα radiation (λ = 1.5418 × 10−10 m). Fourier transform infrared (FT-IR) transmittance spectra were recorded between 400 to 2000 cm−1. The morphological characteristics of the BCP powders with various Ca/P molar ratios were investigated using scanning electron microscopy (SEM, JEOL, JSM-6060) and high resolution transmission electron microscope (TEM, FEI, TECHNAI 300 K). The compositions of the BCP powders with various Ca/P molar ratios were analyzed by using energy dispersive X-ray (EDX). Calcium content in buffer solution was analyzed by using inductively coupled plasma atomic emission spectroscopy (ICP-AES, VARIAN, VISTA AX).
3 Results and discussion
Biphasic calcium phosphate (BCP) powders of various Ca/P molar ratios were synthesized by flame spray pyrolysis process. In the conventional spray process, one powder was formed from one droplet by drying, decomposition and crystallization process. However, nanometer size powders could not be prepared by conventional spray pyrolysis process because droplets generated by ultrasonic spray generators had several microns size. Therefore, in this study, high temperature diffusion flame was applied to the preparation of nanometer size BCP powders. Evaporation of the components composing the BCP powders entered by droplets into the high temperature diffusion flame occurred. Nano-sized BCP powders were formed from the evaporated vapors by nucleation and growth processes. The formation process of the nanometer size powders from the evaporated vapors was well known as chemical vapor deposition (CVD) process. Figure 2 shows the SEM images of the calcium phosphate nanopowders with various Ca/P molar ratios prepared by flame spray pyrolysis. In the SEM images, all the prepared calcium phosphate powders had nanometer size and regular morphology irrespective of the Ca/P molar ratio. Figure 3 shows the TEM images of the powders prepared from the spray solutions with Ca/P molar ratio of 1.500 and 1.667. The prepared powders had nanometer size, spherical shape and narrow size distribution. The mean size of the powders as shown in Fig. 3a, b were 32 and 38 nm. The mean sizes and morphologies of the powders observed from the TEM images were not strongly affected by the Ca/P molar ratios.
The compositions of the nanometer size powders prepared by flame spray pyrolysis from the spray solutions with various Ca/P molar ratios were examined by EDX spectra. The compositions measured by EDX are regarded as approximate values because of the inherent measurement error range in the EDX analysis. The theoretical and determined Ca/P molar ratios were described in Fig. 4. The good accordance between the Ca/P ratios in the spray solution and obtained Ca/P molar ratios was observed in Fig. 4. Consequently these data validate that flame spray pyrolysis is an appropriate synthesis process of forming BCP powders with controlled Ca/P ratios by simple method by controlling the compositions of the spray solution.
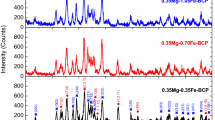
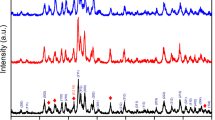
The XRD patterns of the powders directly prepared by flame spray pyrolysis were shown in Fig. 5. The powders prepared from the spray solutions with Ca/P ratios larger than 1.585 had pure phase HA crystal structure. The peaks of β-TCP were shown in the powders prepared from the spray solutions with Ca/P ratios of 1.543 and 1.500. On the other hand, phase pure β-TCP powders were not directly prepared by flame spray pyrolysis. The powders prepared by flame spray pyrolysis were post-treated at a temperature of 800°C for 2 h under air atmosphere. The crystal structures of the powders changed after post-treatment as shown in Figs. 5 and 6. The post-treated powders prepared from the spray solution with Ca/P ratio of 1.667 had phase pure HA crystal structure without impurity peaks. The sample obtained from the spray solution with Ca/P ratio of 1.713 had main crystal structure of HA with small impurity peak of CaO. The peaks of β-TCP observed from the sample obtained from the spray solution with Ca/P ratio of 1.627, and the peak intensities of the β-TCP crystal increased with decreasing the Ca/P ratio. The phase pure β-TCP powders were obtained from the spray solution with Ca/P ratio of 1.500. Phase pure β-TCP powders were not directly prepared by flame spray pyrolysis because of short residence time of the powders inside the high temperature diffusion flame. Therefore, the powders directly prepared by flame spray pyrolysis from the spray solution with Ca/P ratio of 1.500 had mixed crystal structures of HA and β-TCP phases. HA and un-reacted P component reacted to form the phase pure β-TCP powders during the post-treatment process. The phase ratios were estimated from the peak ratios of main peaks of XRD patterns. Table 1 shows the phase ratios of HA and β-TCP depending on the Ca/P ratios of the spray solution. At 1.5 Ca/P ratio, monolithic β-TCP powder was obtained without any other phases. However, the phase of β-TCP decreased with increasing the Ca/P ratio. The pure HA powder was obtained at 1.667 Ca/P ratio. However, the real phase ratios of HA and β-TCP should be analyzed with Rietveld analysis as described in the previous paper [33].
Figure 7 shows the FT-IR spectra of the BCP powders should be analyzed with various Ca/P ratios post-treated at a temperature of 800°C. The powders displays vibrational bands associated with two calcium phosphate phases identified with XRD. In the FT-IR analysis, mainly the peaks for [OH−] and \( \left[ {{\text{PO}}_{ 4}^{ 3- } } \right] \) units in HA and β-TCP can be identified [34, 35]. The IR bands observed at 1040 and 962 cm−1 are characteristic of the phosphate stretching vibration, and the bands observed at 601, 571 cm−1 are due to the phosphate bending vibration. And OH stretching is observed at 630 cm−1 The bands observed at 551 and 606 m−1 are derived from the phosphate bending vibration, and the bands around 945–1025 cm−1 are characteristics of the phosphate stretching vibration of β-TCP. This data demonstrates a gradual variation in the β-TCP/HA ratios starting from β-TCP as a single phase and ending with a HA one.
The degradability of the fabricated BCP bioceramics was determined by dissolution characteristics of calcium ion in Tris–HCl buffer solution. BCP pellets obtained from the powers directly prepared by flame spray pyrolysis were calcinated at 800°C for 2 h in air atmosphere and subsequently placed in polystyrene bottles containing Tris–HCl buffer solution. The bottles with the pellets and Tris–HCl were maintained at 37.0°C in a continuously shaken bath to help maintain uniform ion concentrations. The pellets were immersed for 1, 3, 7, 14, 21 h and 1, 3, 4, 5, 6, 7 days, respectively. In Fig. 8, calcium dissolution in buffer solution increased with the decrease of Ca/P ratios except with the Ca/P ratio of 1.713. It is owing to the increase of the proportion of β-TCP with high solubility in the biphasic materials. Therefore, the pellet with the Ca/P ratio of 1.667 containing single phase of HA shows minimum Ca2+ ion concentration in buffer solution due to its high stability in buffer solution. However, in the case of Ca/P ratio of 1.731, the samples show the maximum Ca2+ ion concentration in the buffer solution at all immersion time. It is because of CaO content of the powders as shown in Fig. 6. CaO had the property of high solubility in the buffer solution.
The morphologies of pellet surface after being immersed for 7 days in the buffer solution were shown in Fig. 9. After immersion, the pellets were recovered and subsequently dried at 30°C. Most of pellets, erosion of the surfaces increased step by step with the decrease of Ca/P ratios. The pellet surface with Ca/P ratio of 1.500, which consisted of pure β-TCP, was eroded dramatically for 7 days as shown in Fig. 9a. On the other hand, the pellet surface with Ca/P ratio of 1.667 was stable and hardly eroded after immersion based on the SEM observation. These results of SEM images were well corresponded with the fact that erosion of pellets increased step by step with the increase of proportion of β-TCP with high resorption in the biphasic materials.
4 Conclusions
BCP powders were directly prepared by flame spray pyrolysis process from the spray solutions with various Ca/P ratios. Nanometer size BCP powders with spherical shape and non-aggregation characteristics were prepared from the evaporated vapors by nucleation and growth process. The flame spray pyrolysis was an appropriate synthesis process of forming BCP powders with controlled Ca/P ratios by simple method by controlling the compositions of the spray solution. The degradability of the synthesized BCP powders was studied by dissolution of calcium ion in buffer solution at similar human atmosphere. It was determined that the calcium dissolution progressively increased with the decrease of Ca/P ratios owing to the increasing portion of β-TCP in BCP powders because of high resorption property of β-TCP.
References
Jarcho M. Biomaterial aspects of calcium phosphates. Properties and applications. Dent Clin North Am. 1986;30:25–47.
Fernandez E, Gil FJ, Ginebra MP, Driessens FCM, Planell JA, Best SM. Calcium phosphate bone cements for clinical applications. Part I: solution chemistry. J Mater Sci Mater Med. 1999;10:169–76.
Oonishi H. Orthopaedic applications of hydroxyapatite. Biomater. 1991;12:171–8.
Lange DG, Putter DC. Structure of the bone interface to dental implants in vivo. J Oral Implantol. 1993;19:136–7.
Groot KD. Macroport tissue ingrowth: a quantitative and qualitative study on hydroxyapatite ceramic. Biomater. 1986;7:137–44.
Moore DC, Chapman MW, Manske D. The evaluation of a biphasic calcium phosphate ceramic for use in grafting long-bone diaphyseal defects. J Orthop Res. 1987;5:356–65.
Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomater. 1998;19:1473–8.
Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003;14:195–200.
Piattelli A, Scarano A, Mangano C. Clinical and histologic aspects of biphasic calcium phosphate ceramic (BCP) used in connection with implant placement. Biomater. 1996;17:1767–70.
Fathi MH, Hanifi A. Evaluation and characterization of nanostructure hydroxyapatite powder prepared by simple sol–gel method. Mater Lett. 2007;61:3978–83.
Zhou ZH, Zhou PL, Yang SP, Yu XB, Yang LZ. Controllable synthesis of hydroxyapatite nanocrystals via a dendrimer-assisted hydrothermal process. Mater Res Bull. 2007;42:1611–8.
Zhang X, Vecchio KS. Hydro-thermal synthesis of hydroxyapatite rods. J Cryst Growth. 2007;306:133–40.
Kannan S, Rocha JHG, Agathopoulos S, Ferreira JMF. Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. Acta Biomater. 2007;3:243–9.
Wang A, Liu D, Yin H, Wu H, Wada Y, Ren M, et al. Size-controlled synthesis of hydroxyapatite nanorods by chemical. Precipitation in the presence of organic modifiers. Mater Sci Eng C. 2007;27:865–9.
Wang A, Yin H, Liu D, Wu H, Ren M, Jiang T, et al. Size-controlled synthesis of hydroxyapatite nanorods in the presence of organic modifiers. Mater Lett. 2007;61:2084–8.
Tüyel U, Öner ET, Özyegin S, Oktar FN. Production and characterization of bioceramic nano-powders of natural-biological origin. J Biotechnol. 2007;131:S65.
Yoshimura M, Suda H, Okamoto K, Ioku K. Hydrothemal synthesis of biocompatible whiskers. J Mater Sci. 1994;29:3399–402.
Eshtiagh-Hosseini H, Housaindokht MR, Chahkandi M. Effects of parameters of sol-gel process on the phase evolution of sol-gel-derived hydroxyapatite. Mater Chem Phys. 2007;106:310–6.
Lin K, Chang J, Cheng R, Ruan M. Hydrothermal microemulsion synthesis of stoichiometric single crystal hydroxyapatite nanorods with mono-dispersion and narrow-size distribution. Mater Lett. 2007;61:1683–7.
Cao L, Zhang C, Huang J. Synthesis of hydroxyapatite nanoparticles in ultrasonic precipitation. Ceram Inter 2005;31:1041.
Price RL, Gutwein LG, Kaledin L, Tepper F, Webster TJ. Research article osteoblast function on nanophase alumina materials: influence of chemistry, phase, and topography. J Biomed Mater Res A 2003;67:1284.
Price RL, Ellison K, Haberstroh KM, Webster TJ. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J Biomed Mater Res A. 2004;70:129–38.
Washburn NR, Yamada KM, Simon CG, Kennedy SB, Amis EJ. High-throughput investigation of osteoblast response to polymer crystallinity: influence of nanometer-scale roughness on proliferation. Biomater. 2004;25:1215–24.
Wan YQ, Wang Y, Liu ZM, Qu X, Han BX, Bei JZ, et al. Adhesion and proliferation of OCT-1 osteoblast-like cells on micro- and nano-scale topography structured poly(L-lactide). Biomater. 2005;26:4453–9.
Nilen RWN, Richter PW. The thermal stability of hydroxyapatite in biphasic calcium phosphate ceramics. J Mater Sci Mater Med. 2008;19:1693–702.
Kannan S, Rocha JHG, Ventura JMG, Lemos AF, Ferreira JMF. Effect of Ca/P ratio of precursors on the formation of different calcium apatitic ceramics—an X-ray diffraction study. Scr Mater. 2005;53:1259–62.
Lee BT, Youn MH, Paul RK, Lee KH, Song HY. In situ synthesis of spherical BCP nanopowders by microwave assisted process. Mater Chem Phys. 2007;104:249–53.
Raynaud S, Champion E, Bernache-Assollant D, Thomas P. Calcium phosphate apatites with variable Ca/P atomic ratio. I. Synthesis, characterisation and thermal stability of powders. Biomater. 2002;23:1065–72.
Yasuda HY, Mahara S, Nishiyama T, Umakoshi Y. Preparation of hydroxyapatite/α-tricalcium phosphate composites by colloidal process. Sci Tech Adv Mater. 2002;3:29–33.
Cho JS, Kang YC. Nano-sized hydroxyapatite powders prepared by flame spray pyrolysis. J Alloy Compd. 2008;464:282–7.
Ana GH, Wanga HJ, Kim BH, Jeong YG, Choa YH. Fabrication and characterization of a hydroxyapatite nanopowder by ultrasonic spray pyrolysis with salt-assisted decomposition. Mater Sci Eng A. 2007;449:821.
Haman JD, Lucas LC, Crawmer D. Characterization of high velocity oxy-fuel combustion sprayed hydroxyapatite. Biomater. 1995;16:229–37.
Vallet-Regi M, Rodriguez-Lorenzo LM, Salinas AJ. Synthesis and characterisation of calcium deficient apatite. Solid State Ionics. 1997;101–103:1278.
Victor SP, Sampath Kumar TS. BCP ceramic microspheres as drug delivery carriers: synthesis, characterisation and doxycycline release. J Mater Sci Mater Med. 2008;19:283–90.
Pena J, Vallet-Regi M. Hydroxyapatite, tricalcium phosphate and biphasic materials prepared by a liquid mix technique. J Eur Ceram Soc. 2003;23:1687–96.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, J.S., Ko, Y.N., Koo, H.Y. et al. Synthesis of nano-sized biphasic calcium phosphate ceramics with spherical shape by flame spray pyrolysis. J Mater Sci: Mater Med 21, 1143–1149 (2010). https://doi.org/10.1007/s10856-009-3980-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-009-3980-1