Abstract
A novel tri-component composite membranes of chitosan/carboxymethyl cellulose (CS/CMC) polyelectrolyte complex membranes filled with different weight ratios of nano-hydroxyapatite (n-HA)(0, 20, 40 and 60 wt%), namely, n-HA/CS/CMC composite membrane, were prepared by self-assembly of static electricity. The structure and the properties of the composite membranes were investigated by Fourier transformed infrared spectroscopy(IR), X-ray diffraction(XRD), Scanning electron microscopy(SEM), mechanical performance measurement, swelling behavior test, and soaking behavior study in phosphate buffered saline (PBS) and simulate body fluid (SBF). The results showed that the n-HA/CS/CMC composite membrane was formed though superficial static electricity interaction among n-HA, CS and CMC. For the n-HA/CS/CMC composite membrane, the microstructure compatibility, mechanical property, swelling behavior, the degradation and bioactivity in vitro of the composite membrane were improved by the addition of n-HA, compared with CS/CMC polyelectrolyte complex membrane. Moreover, the n-HA/CS/CMC composite membrane with 40 wt% n-HA had the most highest mechanical property, which suggested that the novel n-HA/CS/CMC composite membrane with 40 wt% n-HA was more suitable to be used as guided bone tissue regeneration membrane than CS/CMC polyelectrolyte complex membrane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
To avoid the non-union caused by appearance of connective tissue during the formation of new bone in bone healing, a barrier membrane named as guided bone tissue regeneration membrane is designed to be placed at the bone defect area to exclude the competing non-osteogenic soft tissue cells from the bone defect site so as to promote the osseous healing of bone defects [1, 2]. In this sense, it is very important to develop an ideal barrier membrane used for guided bone tissue regeneration. As we know, an ideal barrier membrane is strongly desired to have good mechanical property, swelling behavior, biocompatibility, bioactivity, and so on. At present, some non-bioabsorbable membranes, for example, expanded polytetrafluoroethylene (e-PTFE), have been clinically employed as barrier membranes [3, 4]. However, it must be removed out from the body by a second surgical procedure after the healing of bone defect, which will result in the risk of tissue morbidity. Therefore, bioabsorbable membranes, such as polylactic acid [5], polyglatin [6], alginate [7], collagen [8], have more interest than non-bioabsorbable membranes. Among the bioabsorbable membrane materials, chitosan (CS), a natural biodegradable cationic polymer with good biocompatibility, has also been widely used as guided bone tissue regeneration membrane [9–11]. However, the mechanical property is not satisfactory.
Carboxymethyl cellulose (CMC), another natural biodegradable anionic polymer with good biocompatibility, has also been used as biomedical membrane [12, 13]. Moreover, CMC can interact strongly with CS due to the structural similarity with CS. Consequently, CS/CMC composite membrane with good mechanical property prepared by using glutaraldehyde as linkage reagent was widely investigated in the fields of pervaporation separation in water–ethanol mixture, separator in electro-generation, anti-adhesion after operation [14–16]. However, whether CS/CMC polyelectrolyte complex membrane can be used as guided bone tissue regeneration membrane is still not reported.
Based on the above consideration, the CS/CMC polyelectrolyte complex membrane is expected to develop to be used as guided bone tissue regeneration membrane in this work. Additionally, it has been reported that nano-hydroxyapatite (n-HA) has osteoinductive during the healing bone defect, due to the resemblance of its chemical and crystallographic with the inorganic component existing in nature bone [17, 18]. So n-HA was designed to be filled into the CS/CMC polyelectrolyte complex membrane to improve the bioactivity of guided bone tissue regeneration membrane.
To obtain successfully the inorganic/organic hybrid composite membrane, among the preparation methods, the biomimetic mineralization is a simple method, that is to say, CS/CMC composite membrane is obtained by co-solution method, then CS/CMC composite membrane is mineralized by soaking in SBF, and HA/CS/CMC composite membrane may be obtained. However, it is difficult for this biomimetic mineralization method to ensure the content and dispersibility of HA deposited on the CS/CMC composite membrane. Alternatively, co-solution method is a simple and universal method in preparing composite membrane, namely, n-HA slurry is added to CS/CMC solution, and a uniform membrane formation solution containing n-HA, CS and CMC composition may be obtained. However, this method cannot be used to prepare n-HA/CS/CMC composite membrane in this system, because the acidity of CS/CMC membrane formation solution is very strong, in which n-HA would be dissolved or changed as other Ca–P phase. Therefore, in this paper, the self-assembly technology of static electricity was adopted to prepare n-HA/CS/CMC composite membrane, which is a simple and promising technique for fabricating biomedical membrane because it avoids adding other harmful cross-linking agents [19, 20]. As a result, a serials of CS/CMC polyelectrolyte complex membranes filled with different weight ratios of n-HA (0, 20, 40 and 60 wt%) were obtained. In addition, their structure and properties were characterized by Fourier transformed infrared spectroscopy (IR), X-ray diffraction (XRD), Scanning electron microscopy (SEM), mechanical performance measurement, swelling behavior test, and soaking behavior study in phosphate buffered solution (PBS) and simulate body fluid (SBF). The main purpose in this work is to develop a novel biodegradable inorganic/organic hybrid composite membrane by adding n-HA into CS/CMC polyelectrolyte complex membrane, which would be used as guided bone tissue regeneration membrane.
2 Experimental
2.1 Materials
An 80-mesh chitosan powder with a molecular weight of about 3.5 × 106 and a N-acetylation degree of 95% was purchased from Haidebei Bioengineering Co. Ltd, Jinan, China. Sodium carboxymethyl cellulose was purchased from Kelong Chemical Agent Factory, Chengdu, China, with a molecular weight of about 4.2 × 108 and a substitution degree of 0.7. n-HA was prepared in our laboratory [21]. All other reagents used here were of analytical grade.
2.2 Preparation of n-HA/CS/CMC composite membrane
The n-HA/CS/CMC composite membranes were prepared by the following procedure, and the original amount of three composition used here were listed in Table 1. Firstly, some amount of n-HA was added into 2 wt% CMC aqueous solution and the stirring was kept for 4 h, and the homogeneous mixture was poured into glass plate to form a liquid membrane. Secondly, 2 wt% CS solution(obtained by dissolving CS in 2 wt% acetic acid solution) was cast slowly on the n-HA/CMC composite membrane mentioned above, so as to form a tri-component composite membrane, and then was dried at room temperature. Finally, the dried composite membrane was cross-linked with 1 wt% CaCl2 solution for 30 min and rinsed to neutrality with deionized water, and dried again in vacuum oven at 40°C.
2.3 Characterization of membrane
2.3.1 Fourier transform-infrared (FT-IR) spectroscopic studies
The Infrared (IR) spectra of n-HA, CS, CMC and n-HA/CS/CMC composite membranes with different weight ratios of n-HA were recorded with a FT/IR spectrophotometer (American Perkin Elmer Co.) by attenuated total reflection (ATR) techniques. The spectra were collected over the range of 4,000–600 cm−1.
2.3.2 X-ray diffraction (XRD) patterns
X-ray diffraction patterns of n-HA, CS, CMC and n-HA/CS/CMC composite membranes with different weight ratios of n-HA were conducted with Cu–Kα radiation using X’pert Pro MPD (Philip, The Netherlands)at a voltage and current of 20 KV and 30 mA. The relative intensities were recorded within the range of 10°–70° (2θ) at a scanning rate of 4° min−1.
2.3.3 Scanning electron microscope (SEM) studies
The surface of n-HA/CS/CMC composite membranes were gold-coated and observed with SEM (JSM-5900LV, Japan) at an accelerated voltage of 20 kV.
2.3.4 Mechanical property test
The tensile strength of n-HA/CS/CMC composite membranes in dry and wet condition were tested by using a universal materials testing instrument (AG-10AT, DaoJin Japan) at room temperature and a relative humidity of 40%. The crosshead speed was set as 20 mm/min. Five parallel samples of each membrane were test and the mean values were given.
2.3.5 Swelling behavior
The swelling behavior of n-HA/CS/CMC composite membranes were determined by using a gravimetric method. The samples dried in vacuum oven at 40°C to a constant weight (noted as W 0) with 2 × 2 cm2 in size were immersed in deionized water for 48 h until the membranes adsorbed water to equilibration, and the samples were removed out from the fluid, cleaned with filter paper to get rid of water on the surface and weighed again (noted as W 1). The swelling ratio of membranes (Ws) were calculated based on the formula of Ws(%) = (W 1 − W 0 )/W 0 × 100%. Three specimens were measured for each membrane to obtain an averaged value.
2.3.6 In vitro degradation in PBS
The degradation was investigated by phosphate buffer solution (PBS) soaking. The n-HA/CS/CMC composite membranes samples with 2 × 2 cm2 in size were dried and weighed. The samples were immersed in tube containing 10 ml of PBS, kept oscillating at 37.0 ± 0.5°C. After soaking for 7, 10 and 14 days, the samples were withdrawn, rinsed with deionized water, dried and weighed again. The weight loss (W L) was calculated according to the formula of W L = (W 0–W 1)/W 0 × 100%, where W 0 and W 1 denote the weights of sample before and after soaking, respectively. Five parallel samples of the n-HA/CS/CMC composite membranes were also carried out.
2.3.7 In vitro bioactivity in 1.5SBF
The n-HA/CS/CMC composite membrane with a weight ratio of 40 wt% n-HA was selected as a representative sample for the further analysis and comparison with CS/CMC membrane in the study of bioactivity in vitro.
The 1.5SBF solution containing nearly 1.5 times the inorganic ion concentration of human blood plasma was prepared by dissolving reagent chemicals of NaCl(11.994 g), NaHCO3(0.525 g), KCl(0.336 g), K2HPO4 · 3H2O(0.342 g), MgCl2 · 6H2O(0.4575 g), CaCl2(0.417 g) and Na2SO4(0.1065 g) into deionized water. Solution pH value was adjusted with hydrochloric acid. The final pH value of the solution was 7.4, and the solution was metered volume to 1,000 ml scale [22].
The specimens with 2 × 2 cm2 in size were immersed in 10 ml of 1.5SBF. All the test tubes were placed in a rocking water bath with a constant temperature (37 ± 0.5°C). The super-saturation conditions in the 1.5SBF solution were maintained by periodic replacement with a fresh solution. The surface microstructure of specimens was observed by SEM after soaking 5 and 10 days. The composition of the apatite layer on the two kinds of membranes was analyzed by XRD.
3 Results and discussion
3.1 Membrane structure characterization
3.1.1 IR analysis
IR spectra of pure n-HA, CS, CMC and n-HA/CS/CMC composite membranes were given in Fig. 1. Though comparison between these IR spectra, it can be seen that the specific peaks of pure CMC and CS all appeared in the spectrum of CS/CMC composite membrane except for slight band-shifts (Fig. 1c). However, an amino absorption at 1,599 cm−1 in CS was shifted to 1,579 cm−1 in the CS/CMC composite membrane, which may be the result of the formation of NH3 +. Moreover, the symmetry stretching absorption bands at 1,607 cm−1 of COO− in CMC shifted to 1,644 cm−1, the asymmetry stretching of group at 1,420 cm−1 in CMC was shifted to 1,413 cm−1, and a new absorption was observed at 1,540 cm−1, which shows that there was strong static electric interaction between NH3 +of CS and COO− of CMC. In addition, when n-HA was added in CS/CMC composite membrane, the PO4 3− characteristic peak in n-HA spectrum had little shift, and there were weak peaks at about 1,589 cm−1 and 1,420 cm−1 in n-HA/CS/CMC composite membranes (see in Fig. 1d–f), which shows that the main characteristic peaks of n-HA still existed in n-HA/CS/CMC composite membranes, suggesting that there was no change of chemical component after the composite membranes were prepared. Moreover, there might be other static electric interaction between the PO4 3− of n-HA and positive ion of CS-CMC polyelectrolyte complex. That is to say, the static electric interaction existed among n-HA, CS and CMC.
3.1.2 XRD analysis
Figure 2 shows the X-ray diffraction patterns of pure n-HA, CS, CMC and n-HA/CS/CMC composite membranes. It can be seen that the main specific peaks for CMC at 2θ = 32° and 45° disappeared evidently in CS/CMC composite membrane (Fig. 2c), resulting from the interaction of CS and CMC. In addition, when n-HA was added in CS/CMC composite membrane, the specific peaks for CS at 2θ = 10° and 20° were weakened. However, the specific peaks for CMC at 2θ = 32° appeared, and some main specific peaks of n-HA all appeared except for a slight weak in 002 crystal plane, comparing with the pure n-HA(see Fig. 2d–f), which indicates that n-HA was not be changed as other Ca–P phase during the self-assembly of static electricity. On the contrary, it shows that there was strong electrostatic interaction among n-HA, CS and CMC composition in the n-HA/CS/CMC composite membranes. For the slight weak in 002 crystal plane of n-HA, it may be caused by the material used to perform the diffraction of pure n-HA was different than the one incorporated in the CS/CMC.
3.1.3 SEM observation
The SEM photographs of the surface for n-HA/CS/CMC composite membranes with different n-HA content are shown in Fig. 3. From the surface of composite membrane, it could be seen that CS/CMC composite membrane had many granulations when CS and CMC was compounded (as shown in Fig. 3a). However, with the addition of n-HA, the compatibility was greatly improved, and there was no obvious agglomeration on n-HA on the composite membrane surface, which may result from the fact that the superficial Ca2+ and PO4 3− of n-HA had also an electrostatic interaction with the positive and negative charge of CS-CMC polyelectrolyte complex, so that it weakened the electrostatic interaction between CS and CMC. Obviously, n-HA had an important effect on the microstructure of n-HA/CS/CMC composite membrane.
According to the structural analysis and discussion presented previously, the possible mechanism of self-assembly among n-HA,CS and CMC of n-HA/CS/CMC composite membrane was given in Fig. 4.
3.2 Membrane properties
3.2.1 Mechanical property
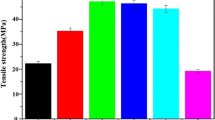
The mechanical property of the membrane is an important requirement for the guided bone tissue regeneration membrane, and the tensile strength is an important parameter to evaluate the mechanical property of membrane. The tensile strength of the n-HA/CS/CMC composite membranes in dry and wet state were both given in Fig. 5. It can be seen that the tensile strength of the composite membranes increased with the addition of n-HA, and the tensile strength of the n-HA/CS/CMC composite membrane with 40 wt% n-HA was the highest, which could reach 40 MPa in dry and 12 MPa in wet state. Theoretically, it may keep good structural stability and display the barrier function in vivo. Additionally, when n-HA content is too high, the tensile strength of the n-HA/CS/CMC composite membrane is slight reduced, the reason is that the higher content of n-HA may dilute the chemical interaction and the mechanical interlocking, which causes a poor stress transfer in the composite material. In a word, n-HA has a positive effect on improving the mechanical property of n-HA/CS/CMC composite membrane.
3.2.2 Swelling behavior
The swelling behavior is also one of important aspects to be investigated for barrier membrane. The moderate hydrophilicity of the membrane will keep the wound arescent so as to accelerate healing. Figure 6 shows the results obtained in swelling behavior test. According to the results, it could be seen that the CS/CMC composite membrane had very high swelling ratio due to the fact that CS and CMC are both hydrophilic polymers, however, the swelling ratio of n-HA/CS/CMC composite membrane decreased with increasing n-HA content, because n-HA has little hydrophilicity. Moreover, the interaction of n-HA, CS and CMC has a little effect on the swelling behavior, which can decrease the conjugate between the hydrone and polar bond of CS and CMC polymers. So that n-HA/CS/CMC composite membrane have a slight lower swelling ratio than that of CS/CMC composite membrane, which is more beneficial to keep the mechanical property of the composite membrane in vivo.
3.2.3 In vitro degradation in PBS
To investigate the degradation and structural stability of the composite membrane, monitoring the weight-loss of the composite membrane is a suitable and reliable method, and it has been widely employed for the investigation of degradation behaviors in vitro [23]. In this study, we chose PBS as the soaking solution, which is a balanced salt solution, providing the cell with energy and inorganic ion. The weight loss of n-HA/CS/CMC composite membranes as a function of soaking time in PBS was given in Fig. 7. According to the weight loss tendency, it could be found that the weight loss of the membrane increased gradually, which showed that the composite membranes were degradable with the soaking time, and the n-HA/CS/CMC composite membrane had more evident weight loss than CS/CMC composite membrane due to a worse cohesion of composite membrane. However, after PBS soaking for 14 days, it could be found that the n-HA/CS/CMC composite membrane still remained the original shape, which indicated that the composite membrane still has good structural stability so that it can display the barrier function.
3.2.4 In vitro bioactivity in 1.5SBF
Figure 8 shows the SEM microstructures of the n-HA/CS/CMC composite membranes with 40 and 0 wt% n-HA content after soaking in 1.5SBF for 5 and 10 days, respectively. For the 40 wt% n-HA content of n-HA/CS/CMC composite membrane, it can be seen that a few apatite particles deposited on the surface after 5 days soaking, shown in Fig. 8a. With the time going, more and more apatite particles appeared and aggregated to form apatite layer on the surface, which can be seen in Fig. 8b. However, for the 0 wt% n-HA content of n-HA/CS/CMC composite membrane, there was a few apatite particles deposited on the surface even it was soaked for 10 days (as shown in Fig. 8c). The major reason for enhancement of apatite formation might be that the tri-component composite membrane presented apparent n-HA particles, which acted as nucleation sites in 1.5SBF, a metastable calcium phosphate solution supersaturated with respect to apatite. Therefore, apatite could be formed more efficiently on the n-HA/CS/CMC composite membrane than on the CS/CMC composite membrane in the same interval of time.
The composition of the apatite layer on the two composite membranes were analyzed by XRD. The XRD patterns of the two composite membranes after soaking in 1.5SBF were shown in Fig. 9. From Fig. 9, it can be seen that the characteristic peaks of apatite layer appeared in the n-HA/CS/CMC composite membrane, and the characteristic peak of 002 crystal plane got more obvious with the soaking time going, while there was few characteristic peaks of an apatite in the CS/CMC composite membrane after soaking in 1.5SBF for 10 days, which is in agreement with the results of SEM observation after soaking.
4 Conclusion
In the paper, the n-HA/CS/CMC composite membrane with different n-HA contents (0, 20, 40 and 60 wt%) were prepared by self-assembly of static electricity. From the analyses and discussion above, it can be concluded that the formation of composite membrane mainly resulted from the static electricity interaction among n-HA, CS and CMC. The addition of n-HA into the CS/CMC membrane not only had a positive effect on the structure but also greatly improved the properties, such as mechanical property, swelling behavior, the degradation and bioactivity in vitro of the tri-component composite membrane, and the n-HA/CS/CMC composite membrane with 40 wt% n-HA had the most highest mechanical property. In addition, the self-assembly technology of static electricity was a suitable and simple method in preparing n-HA/CS/CMC composite membrane. In a word, the CS/CMC polyelectrolyte complex membrane filled with n-HA was successfully developed by self-assembly technology of static electricity in this paper, and the novel n-HA/CS/CMC composite membrane with a weight ratio of 40 wt% had better properties for guided bone tissue regeneration comparing with the CS/CMC polyelectrolyte complex membrane.
References
Fujihara K, Kotaki M, Ramakrishn S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials. 2005;26:4139–47.
Hirotaka M, Toshihiro K, Larry LH. Preparation of poly(l-lactic acid)-polysiloxane-calcium carbonate hybrid membranes for guided bone regeneration. Biomaterials. 2006;27:1216–22.
Piattelli A, Scarano A, Paolantonio M. Bone formation inside the material interstices of e-PTFE membranes: a light microscopical and histochemical study in man. Biomaterials. 1996;17:1725–31.
Marouf HA, El-Guindi HM. Efficacy of high-density versus semipermeable PTFE membranes in an elderly experimental model. Oral Surg, Oral Med, Oral Pathol, Oral Rad & End. 2000;89:164–70.
Imbronito AV, Todescan JH, Carvalho CV, Arna-Chavez VE. Healing of alveolar bone in resorbable and non-resorbable membrane-protected defects. A histologic pilot study in dogs. Biomaterials. 2002;23:4079–86.
Soncini M, Rodriguez R, Baena R, Pietrabissa R, Quaglini V, Rizzo S, et al. Experimental procedure for the evaluation of the mechanical properties of the bone surrounding dental implants. Biomaterials. 2002;23:9–17.
Yoshiya U, Kunio I, Takamitsu M, Takahiro K, Hitoshi N, Kazuomi S, et al. Usefulness as guided bone regeneration membrane of the alginate membrane. Biomaterials. 2002;23:2027–33.
Ozmeric N, Bal B, Oygur T, Balos K. The effect of a collagen membrane in regenerative therapy of two-wall intrabony defects in dogs. Periodontal Clin Investig. 2000;22:22–30.
Mi FL, Shyu SS, Wu YB, Lee ST, Shyong JY, Huang RN. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials. 2001;22:165–73.
Kuo SM, Chang SJ, Lin LC, Chen CJ. Evaluating chitosan/β-tricalcium phosphate/poly (methyl methacrylate) cement composites as bone-repairing materials. J Appl Polym Sci. 2003;89:3897–904.
Shyh MK, Shwu JC, Ta WC, Tang CK. Guided tissue regeneration for using a chitosan membrane: an experimental study in rats. J Biomed Mater Res A. 2006;76:408–15.
Bowler PG, Jones SA, Davies BJ, Coyle E. Infection control properties of some wound dressings. J Wound Care. 1999;8:499–502.
Ryan CK, Sax HC. Evaluation of a carboxymathyl cellulose sponge for prevention of postoperative adhesion. Am J Surg. 1995;169:154–9.
Yan YJ, Wang JW, Wang W, Ding MT. Pervaporation of ethanol-water through polyelectrolyte complex membranes of chitosan-carboxymethyl cellulose. J Func Mater. 1998;29:642–4.
Xu CX, Chen RY, Zheng X, Huang ZX, Huang XH, Chen Z. Preparation of CS-CMC polyelectrolyte membrane and its application to electro-generation of FeO4 2−. Acta Chimica sinica. 2006;64:784–8.
Xiao HJ, Hou CL, Gu QS, Liu YP. Preparation and evaluation of chitosan-carboxymethylcellulose membrane for prevention of postoperative intestinal adhesion: an experimental study. Chin J Rep & Recon Surg. 2006;20:762.
Xua JL, Khora KA, Dongb ZL, Guc YW, Kumarc R, Cheang P. Preparation and characterization of nano-sized hydroxyapatite powders produced in a radio frequency (rf) thermal plasma. Mater Sci Eng, A. 2004;374:101–8.
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. In vitro preparation and ellipsometric characterization of thin blood plasma clot films on silicon. Biomaterials. 2001;22:1803–8.
Zhu ZQ, Feng XS, Penlidis A. Layer-by-layer self-assembled polyelectrolyte membranes for solvent dehydration by pervaporation. Mater Sci Eng. 2007;C 27:612–9.
Lia J, Lia X, Ni XP, Wang X, Li HZ, Leong KW. Self-assembled supramolecular hydrogels formed by biodegradable PEO–PHB–PEO triblock copolymers and a-cyclodextrin for controlled drug delivery. Biomaterials. 2006;27:4132–40.
Wang XJ, Li YB, Wei J, de Groot K. Development of biomimetic nano-hydroxyapatite/poly(hexamethylene adipamide) composites. Biomaterials. 2002;23:4787–91.
Chen Y, Mak AF, Li J, Wang M, Shum AW. Formation of apatite on poly(alpha-hydroxy acid) in an accelerated biomimetic process. J Biomed Mater Res B Appl Biomater. 2005;73:68–76.
Lei LJ, Ding T, Shi R, Liu QY, Zhang LQ, Chen DF, et al. Synthesis, characterization and in vitro degradation of a novel degradable poly((1,2-propanediol-sebacate)-citrate) bioelastomer. Polym Degrad Stabil. 2007;92:389–96.
Acknowledgment
This research is supported by China 973 founds (2007CB936102).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liuyun, J., Yubao, L. & Chengdong, X. A novel composite membrane of chitosan-carboxymethyl cellulose polyelectrolyte complex membrane filled with nano-hydroxyapatite I. Preparation and properties. J Mater Sci: Mater Med 20, 1645–1652 (2009). https://doi.org/10.1007/s10856-009-3720-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-009-3720-6