Abstract
Fabrication of net shape load bearing implants with complex anatomical shapes to meet desired mechanical and biological performance is still a challenge. In this article, an overview of our research activities is discussed focusing on application of Laser Engineered Net Shaping (LENS™) toward load bearing implants to increase in vivo life time. We have demonstrated that LENS™ can fabricate net shape, complex metallic implants with designed porosities up to 70 vol.% to reduce stress-shielding. The effective modulus of Ti, NiTi, and other alloys was tailored to suit the modulus of human cortical bone by introducing 12–42 vol.% porosity. In addition, laser processed porous NiTi alloy samples show a 2–4% recoverable strain, a potentially significant result for load bearing implants. To minimize the wear induced osteolysis, unitized structures with functionally graded Co–Cr–Mo coating on porous Ti6Al4V were also made using LENS™, which showed high hardness with excellent bone cell–materials interactions. Finally, LENS™ is also being used to fabricate porous, net shape implants with a functional gradation in porosity characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Musculoskeletal disorders are recognized as among the most significant human health problems that exist today. Over 200,000 total hip replacements (THRs) are performed in the United States each year. Shorter implant life is a significant problem especially for the growing number of younger patients because of their active lifestyle. The world wide population of people younger than 40 years of age who receive hip implants is expected to grow significantly in the coming years [1], which is likely to create a need for implants with longer in vivo lifetime. Even though significant research and development have gone toward understanding musculoskeletal disorders, there is still a lack of bone replacement material that is appropriate for restoring lost structure and function, particularly for load-bearing applications. For example, average life time of hip implant is 7–12 years. Major factors limiting the life of current load bearing implants include (i) mismatch of the Young’s modulus between bone (10–30 GPa) and metallic implant materials (110 GPa for Ti and over 200 GPa for Co–Cr–Mo alloy) leading to stress-shielding; (ii) poor interfacial bond between the host tissue and the implant due to bioinert surface; (iii) wear induced osteolysis and aseptic loosening in metal-on-polymer implants, and (iv) absence of high recoverable strain (∼2%) as well as hysteresis similar to natural bone. Therefore, fabrication of functional load bearing implants with complex anatomical shapes and desired biomechanical performance is still a challenge.
Stress-shielding and weak interfacial bond between the tissue and the implant can be eliminated by the use of porous metals. Use of porous materials can effectively reduce the modulus mismatch [2] and provide stable long-term anchorage for biological fixation of the implant due to bone tissue ingrowth through the pores [3, 4]. Among various biomedical alloys such as Ti and its alloys, only NiTi alloy (Nitinol) exhibits hysteresis in loading–unloading cycles as well as a 8% recoverable strain similar to natural bone [5]. This similarity in the deformation behavior between Nitinol and bone can improve in vivo lifetime of load bearing implants due to excellent biomechanical compatibility [5]. Conventional powder metallurgical (PM) processing has been used in the past to fabricate surface treated or fully porous metals, including Ti, Ti alloys [6–8], and NiTi alloys [9–11] for biomedical applications. These conventionally sintered metals are often very brittle and pore size, shape, volume fraction, and distribution are difficult to control, which have major influence on mechanical and biological properties. Other fabrication techniques that use foaming agents or molten metal suffer from typical limitations such as contamination, impurity phases, limited, and predetermined part geometries, and limited control over the size, shape, and distribution of porosity. Considerable modification of composition and structure of PM processed NiTi alloys from the feedstock powder also significantly deteriorate the mechanical as well as shape memory properties. For example, embrittling oxide (Ti4Ni2Ox: 0 < x ≤ 1) content of sintered alloys is generally high compared to melt-cast alloys [9]. Moreover, PM NiTi alloys usually contain a large amount of undesirable secondary phases such as Ti2Ni, Ni4Ti3, and Ni3Ti [11]. Therefore, there is significant interest for fabrication methods which can ensure uniform size, shape and distribution of porosity, high levels of purity in porous metals and good shape memory property in NiTi alloys for load bearing applications.
High wear rate of ultrahigh molecular weight polyethylene (UHMWPE) liner used in current hip replacements is another cause of serious concern due to osteolysis and aseptic loosening [12, 13]. Due to these concerns, there is considerable interest in the alternative wear resistant systems such as metal-on-metal (MM) configurations. MM configurations showed 40 times lower linear wear rate and 200 times lower volumetric wear rate than conventional UHMWPE bearings [14]. Wear debris essentially originate from the interface of femoral head-acetabular liner and acetabular liner-shell. A wear resistant alloy coating on acetabular shell not only reduces its wear rate but also eliminate the use of UHMWPE liner. The benefit of this is obvious: elimination of wear induced osteolysis and possible use of large diameter femoral heads. While a wear resistant alloy coating on acetabular shell seems plausible there is only one metallic alloy combination, i.e., Co–Cr–Mo and Ti6Al4V, suitable for surgical implant, which shows metallurgical incompatibility in terms of intermetallic compound formation. In addition to elastic moduli, mismatch in coefficient of thermal expansion and hardness between the coating and the substrate material could lead to excessive residual stresses in the coatings and consequent delamination/cracking and premature failure.
Functionally gradient materials (FGM) are characterized by gradual changes in composition, crystallinity, and/or grain structure from one interface to another. This uniform structural change across the interface provides a unique functionality and performance for biomedical applications [15, 16]. For example, implants with gradients in porosity and pore sizes that can allow on one side of the implant high vascularization and direct osteogenesis, while promoting osteochondral ossification on the other, is appealing in terms of reproducing multiple tissues and tissue interfaces on the same implant. Functional gradation of porosity across the implant section not only reduces the stiffness of the implant but also improves the adhesion to surrounding tissue. In addition, unitized structure with porosity on one side, which will be in contact with bone, can improve cell–material interactions, and the hard coating on the other side can increase the wear resistance of the structure in contact with femoral head. We have used LENS™ to successfully fabricate such functional FGM implants with compositional as well as porosity variations to allow bone tissue ingrowth.
2 Laser engineered net shaping (LENS™)
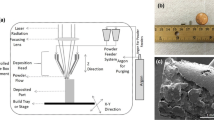
Direct fabrication of functional components using rapid prototyping (RP) from a CAD file is a viable and promising near-net shape manufacturing approach. One such process is the LENS™, which uses metal/ceramic powders to create functional parts. A schematic representation of the LENS™ process is shown in Fig. 1. Initially, a three-dimensional model of a component to be built is generated using CAD, subsequently a computer program slices the model into a number of horizontal cross-sections or layers. These cross-sections are sequentially created on a substrate producing a three-dimensional object. The deposition process begins with directing a focused Nd–YAG laser beam onto a metal substrate placed on a numerically controlled X–Y table. The laser first generates a small molten pool of material on the substrate. A predetermined amount of metal/ceramic powder is injected directly into the molten pool to increase its volume as the X–Y table moves based on the CAD file. Molten material line solidifies rapidly as the laser beam moves away, forming a thin track of solidified material strongly bonded to the substrate or the previous layers along the line of laser scanning. A layer is generated by a number of consecutive overlapping tracks. After each layer is formed, the laser head, along with the powder delivery nozzle, moves up by one layer thickness and the subsequent layer is generated. This procedure is repeated many times until the entire object represented in the three-dimensional CAD model is produced on the substrate, which can be tailored to be a solid or a porous object.
LENS™ process is characterized by high solidification rates in the range of ∼103–105 K/s leading to several microstructural benefits such as (i) suppression of diffusion controlled solid-state phase transformations, (ii) formation of supersaturated solutions and nonequilibrium phases, (iii) formation of extremely fine, refined microstructures with little elemental segregation, and (iv) formation of very fine second phase particles such as carbides. Since the fabrication is carried out in a protective atmosphere with oxygen content less than 10 ppm, LENS™ processed materials can retain high purity and desired phases of the feedstock powder. Also, LENS™ allows us to tailor microstructure, porosity, shape, and size of the part in one operation by controlling different process parameters. This process also allows the user to fabricate functionally graded materials such as materials with gradient in composition and/or porosity across the section. Gradient in porosity can be achieved by changing the tool path and is useful to replicate bone structure/porosity into load bearing metal implants thus eliminating the stress-shielding effect. Finally, the process is reliable and has the potential for direct low-volume manufacturing.
3 Porous metals for stress-shielding
Our design philosophy to fabricate complex shaped implants with designed and functionally graded porosity, to suit natural bone, using LENS™ is described in detail in ref. [2]. There are two ways to introduce porosity in the parts processed via LENS™. One is inter-particle porosity and other is tool path based porosity. Extent of powder melting in each track/scan decides the achievable porosity in the final part depending on laser energy input. At appropriate LENS™ process parameters, one can achieve optimal energy input which ensures lower working temperatures and a low amount of liquid phase around the powder particles due to partial melting of the powder. These surface melted powders join together in the presence of a liquid metal at the particle–particle interfaces, leaving some inter-particle porosity. Formation of porosity in each track is schematically shown in Fig. 2. Particle bonding in this case is a direct result of localized melting and subsequent solidification. Structures with different porosity parameters/internal architecture with designed gradient across the part can be fabricated by optimizing the distance between two successive metal roads and the thickness of each metal layer. Moreover, by changing the deposition angles of laser scans for each layer, the tool path based pores can be oriented layer by layer. Porous samples with different total porosities in various biomedical materials such as pure Ti, Ti6Al4V, Co–Cr–Mo, and NiTi alloys have been fabricated using LENS™ with laser powers between 150 and 300 W, scan speeds between 5 and 25 mm/s, powder feed rates in the range of 15 and 38 g/min, and scan spacing between 0.76 and 1.27 mm. Typical porous Ti samples fabricated using different design procedures are shown in Fig. 3. Young’s modulus of laser processed porous samples is shown in Fig. 4. This results show that the modulus of laser processed Ti samples can be tailored in the range of 2 and 45 GPa by changing the LENS™ process parameters [2]. The modulus of Ti samples having porosities between 35% and 42% is almost the same as that of human cortical bone. These samples also display open pore volume fraction of 53–72 volume% of total porosity. In vitro cell–materials interactions of laser processed porous Ti samples [3] showed enhanced bone cell proliferation by forming more extracellular matrix and high level of alkaline phosphatase expression than observed on fully dense Ti.
LENS™ has also been used to fabricate porous NiTi alloy samples with 12–36% porosity. Laser processing did not induce any intermetallic compound formation as shown in Fig. 5. Laser processed samples showed both B2 and B19′ peaks at room temperature with relatively high amount of B2 than observed in as-received powder. These results also confirm the absence of undesirable intermediate phases such as Ti2Ni, Ni4Ti3, and Ni3Ti in laser processed samples. The transformation sequence in laser-processed samples was one-stage B19′ → B2 on heating and B2 → B19′ on cooling. The high B2 phase in laser processed samples was due to high cooling rates associated with laser processing, which suppressed the complete B2 → B19′ transformation upon cooling. The two-stage transformation of as-received powder is attributed to the presence of Ti3Ni4 grain boundary precipitates, as evidenced in our earlier work on this powder [17]. Since the chemical composition of feed stock powder is unaltered, the inherent biocompatibility of Nitinol alloy will also be retained in these laser-processed samples. Porosity found to have strong influence on maximum recoverable strain, which decreased from 6% to 4% with an increase in the pore volume. Porous NiTi alloy samples exhibited low Young’s modulus between 2 and 18 GPa as well as high compressive strength and up to 4% recoverable strain. These porous alloy samples are thus promising biomaterials for hard tissue replacements as they can eliminate the problem of potential plastic deformation at pore necks due to stress concentration in conventional porous metallic biomaterials. Another aspect of these laser processed porous metals is that by changing the pore shape from spherical to more irregular, one can reduce the modulus of these porous samples [2]. For example, due to irregular pore shape in our samples, we have achieved a lower modulus of ∼11 GPa for NiTi samples with only a total porosity of 16% as against ∼15 GPa for samples with identical porosity and equiaxed pores in earlier work [18]. Therefore, LENS™ provides more flexibility for designers to tailor the modulus of porous samples without changing their bulk density or total pore volume.
4 Functionally graded structures for wear resistance
Independently controllable multiple powder feeders in LENS™ enable variation of composition and porosity simultaneously in one operation to manufacture such novel implant structures. Functionally graded structures with hard and wear resistant Co–Cr–Mo alloy coating on porous Ti6Al4V alloy with metallurgically sound interface have been produced using LENS™. A 100% Co–Cr–Mo transition from Ti6Al4V resulted in severe cracking due to metallurgical incompatibility. However, using optimized LENS™ processing parameters crack free coatings containing up to 86% Co–Cr–Mo have been deposited on Ti6Al4V alloy with excellent reproducibility [19]. These coatings were found to be nontoxic and biocompatible. Composition gradation in the transition region was achieved by gradually increasing the feed rate for Co–Cr–Mo alloy and accordingly decreasing the feed rate of Ti6Al4V alloy powder over 5–7 layers of deposition. The graded structures exhibited good bonding between individual layers without any gross porosity, cracks or lack of fusion defects as shown in Fig. 6. Elimination of intermetallic compounds in these coatings, due to rapid cooling rates, is beneficial in terms of better wear resistance and biocompatibility [20]. Gradient coatings with 86% Co–Cr–Mo in top surface showed ∼184% increase in the surface hardness [19]. Moreover, the porosity on the Ti6Al4V alloy side, which will be in contact with bone, can improve bone tissue ingrowth [3] and hard coating on the other side increases the wear resistance of the structure in contact with Co–Cr–Mo femoral heads. From our work it is apparent that LENS™ can be used to manufacture a variety of load bearing implants with tailored microstructures and compositions while maintaining the size and the shape for specific applications or patients.
5 Summary
We have demonstrated that application of LENS™ to fabricate novel porous and unitized structures with functional gradation in composition and/or porosity can potentially eliminate the long standing issues such as stress-shielding, poor interfacial bond between the host tissue and the implant, and wear induced bone loss, in load bearing implants to increase in vivo life time. Porosities, pore characteristics and mechanical properties of laser processed structures can be tailored to suite various biomedical applications by changing LENS™ process parameters. Apart from other processed materials, porous NiTi alloy samples having density in the range of 64–88% with 2–18 GPa moduli and 2–4% recoverable strain has significant potential in next generation load bearing implants.
References
A. Sargeant, T. Goswami, Mater. Des. 27, 287 (2006)
B.V. Krishna, S. Bose, A. Bandyopadhyay, Acta Biomater. 3, 997 (2007)
W. Xue, B.V. Krishna, S. Bose, A. Bandyopadhyay, Acta Biomater. 3, 1007 (2007)
M. Assad, F. Likibi, P. Jarzem, M.A. Leroux, C. Coillard, CH.-H. Rivard, Mat.-wiss. u. Werkstofftech. 35, 219 (2004)
S.A. Shabalovskaya, Biomed. Mater. Eng. 12, 69 (2002)
I.H. Oh, N. Nomura, N. Masahashi, S. Hanada, Scr. Mater. 49, 1197 (2003)
R.M. Pillar, Int. J. Powder Metall. 34, 33 (1988)
C.E. Wen, M. Mabuchi, Y. Yamada, K. Shimojima, Y. Chino, T. Asahina, Scr. Mater. 45, 1147 (2001)
K. Otsuka, C.M. Wayman, in Shape Memory Materials (Cambridge University Press, Cambridge 1998)
L. Korne, J. Mentz, M. Bram, H. Buchkremer, D. Stover, M. Wagner, G. Eggeler, D. Christ, S. Reese, D. Bogdanski, M. Koller, S.A. Esenwein, G. Muhr, O. Prymak, M. Epple, Adv. Eng. Mater. 7, 613 (2005)
S. Wu, C.Y. Chung, X. Liu, P.K. Chu, J.P.Y. Ho, C.L. Chu, Y.L. Chan, K.W.K. Yeung, W.W. Lu, K.M.C. Cheung, K.D.K. Luk, Acta Mater. 55, 3437 (2007)
H.G. Willert, H. Bertram, G.H. Buchhorn, Clin. Orthop. Relat. Res. 258, 95 (1990)
A.A. Edidin, C.M. Rimnac, V.M. Goldberg, S.M. Kurtz, Wear 250, 152 (2001)
M. Jacobs, R. Gorab, D. Mattingly, L. Trick, C. Southworth, J. Arthroplasty 19(7), Suppl. 2, 48 (2004)
W. Pompe, H. Worch, M. Epple, W. Friess, M. Gelinsky, P. Greil, U. Hempel, D. Scharnweber, K. Schulte, Mater. Sci. Eng. A 362, 40 (2003)
R.J. Narayan, L.W. Hobbs, C. Jin, A. Rabiei, JOM 58(7), 56 (2006)
B.V. Krishna, S. Bose, A. Bandyopadhyay, Metall. Mater. Trans. A 38A, 1096 (2007)
C. Greiner, S.M. Oppenheimer, D.C. Dunand, Acta Biomater. 1, 705 (2005)
B.V. Krishna, W. Xue, S. Bose, A. Bandyopadhyay, Acta Biomater. 3, 697 (2008)
A. Chiba, K. Kumagai, N. Nomura, S. Miyakawa, Acta Mater. 55, 1309 (2007)
Acknowledgments
Authors would like to acknowledge financial support from the Office of Naval Research under the grant number N00014-1-05-0583. We also like to acknowledge financial support from the W. M. Keck Foundation for establishing a Biomedical Materials Research Lab at WSU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bandyopadhyay, A., Krishna, B.V., Xue, W. et al. Application of Laser Engineered Net Shaping (LENS) to manufacture porous and functionally graded structures for load bearing implants. J Mater Sci: Mater Med 20 (Suppl 1), 29–34 (2009). https://doi.org/10.1007/s10856-008-3478-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3478-2