Abstract
Tissue engineering techniques have been proven effective in bone regeneration and repairing load-bearing bone defects. Previous studies, however, have heretofore been limited to the use of slowdegradable or natural biomaterials as scaffolds. There are, however, no reports on using biodegradable, synthetic beta-tricalcium phosphate (β-TCP) as scaffolds to repair weight-bearing bone defects in large animals. In the present study, highly porous β-TCP scaffolds prepared by the polymeric sponge method were used to repair goat tibial defects. Fifteen goats were randomly assigned to one of three groups, and a 26 mm-long defect at the middle part of the right tibia in each goat was created. In Group A (six goats), a porous β-TCP ceramic cylinder that had been loaded with osteogenically induced autologous bone marrow stromal cells (BMSCs) was implanted in the defect of each animal. In Group B (six goats), the same β-TCP ceramic cylinder without any cells loaded was placed in the defect. In Group C (three goats), the defect was left untreated. In Group A, bony union can be observed by gross view, X-ray and micro-computed tomography (Micro-CT) detection, and histological observation at 32 weeks post-implantation. The implanted β-TCP scaffolds were almost completely replaced by tissue-engineered bone. Bone mineral density in the repaired area of Group A was significantly higher (p < 0.05) than that of Group B, in which scant new bone was formed in each defect and the β-TCP hadn’t been completely resorbed at 32 weeks. Moreover, the tissue-engineered bone of Group A had similar biomechanical properties as that of the normal left tibia in terms of bending strength and Young’s modulus (p > 0.05). In Group C, little or no new bone was formed, and non-union occurred, showing that the 26 mm segmental defect of the goat tibia was critical sized at 32 weeks. Thus, it can be concluded that the mechanical properties of the BMSCs/β-TCP composites could be much improved via tissue engineering approach and β-TCP might be used to repair the weight-bearing segmental defects of goat tibias.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Repair of large, weight-bearing bone defects caused by trauma, infection, cancer or congenital malformation is a continuous challenge in orthopaedic surgery. Large defects usually do not heal spontaneously, and surgical intervention is often required. Although autologous bone grafting is still considered the “gold standard” approach for repairing large bone defects at present, this treatment method has certain disadvantages, such as the longer operation time, significant morbidity and lack of sufficient material in some patients [1–3]. Therefore, alternatives to autologous bone grafting are the subject of intensive research.
Tissue engineering approaches have proven very effective in bone regeneration recently, and the successful repair of bone defects has been demonstrated in large animals like canine, goat and sheep [4–9]. An ideal tissue-engineered bone substitute should possess 3 elements: osteoprogenitor cells, osteoinductive factors, and an osteoconductive scaffold, and is considered as a potential substitute for autologous bone transplantation [10].
Screening for or the design of an ideal biomaterial as scaffold still challenges investigators engaged in bone tissue engineering research. From the orthopaedic point of view, the mechanical integrity of the scaffold material can ensure the stability of limbs or sites with bone defects or even replace an internal or external fixation system during treatment [11]. So in previous reports, when repairing weight-bearing bone defects, scaffolds with relatively high biomechanical properties were usually selected, such hydroxyapatite (HA) [5], coral [7, 8], biphasic calcium phosphate ceramics (BCPC) [4, 6] as well as biphasic calcined bone (BCB) [9]. However, as HA, BCPC and BCB (both containing over 60% HA) are not fully resorbable, they will interfere with bone remodeling, theoretically leading to less than-optimal repair results. Coral is a natural, resorbable biomaterial. Its biodegradation rate depends on its pore structure, and cannot be time-controlled as closely as synthetic materials [12]. Thus, besides the basic requirement for biomechanical properties, an optimal scaffold for bone engineering should have a suitable degradation rate that can well match the speed of new bone formation.
Porous β-tricalcium phosphate (β-TCP) is a synthetic and biodegradable ceramic material that has been used clinically in orthopaedic surgery, has attracted attention as a scaffold for bone tissue engineering, due to its good biocompatibility, resorbability, and osteoconductive properties [13–18]. Porous β-TCP could be gradually degraded and replaced by new bone when repairing the bone defect, with a degradation rate matching the new bone formation. However, the compressive strength of porous TCP reaches only 1/20 of cortical bone, and cannot bear strong mechanical forces [18, 19]. As a scaffold material, β-TCP does not combine good mechanical properties with an open porosity, and is thus prone to fragile fracture [8]. Whether it can be used to repair load-bearing bone defect in large animal models is an issue that warrants further investigation.
In our previous study, porous β-TCP scaffold loaded with osteogenically induced bone marrow stromal cells (BMSCs) repaired the critical-sized mandibular defects in canine model. The new bone formation started around 4 weeks post-operation with β-TCP degradation started at same time. Bony-union was achieved with almost complete degradation of β-TCP after 32 weeks, indicating that the BMSCs/β-TCP composites had good osteogenic activity and enhanced biomechanical properties through tissue-engineering [20]. The mandible is a load-bearing bone, but does not bear weight. Whether the porous β-TCP scaffolds could also be potentially used for the repair of fully weight-bearing bone defects via the tissue engineering method remains to be elucidated.
The goal of this study was to determine whether porous β-TCP could be used as a promising scaffold for bone tissue engineering to repair critical-sized, fully load-bearing bone defect in large animals. Elucidation of this point will be of great impact on the potential application of bone tissue engineering to repair large, weight-bearing bone defects.
2 Materials and methods
2.1 Experimental design
Fifteen skeletally mature goats were used in the present study, and all procedures were performed at the facility accredited by the Animal Care and Experiment Committee of Shanghai JiaoTong University School of Medicine. The mean weight of the animals was 22.3 ± 4.1 kilograms. All of the animals had a resection of a 26-mm-long osteoperiosteal segment at the middle part of the right tibial diaphysis, as will be described. The unique identification number for each goat was input into a computer with use of software that randomly assigned each goat to one of three groups. In Group A (six goats) the defect was filled with a porous β-TCP cylinder that had been loaded with in vitro cultured, osteogenically induced autologous BMSCs. In Group B (six goats) the defect was filled with the same β-TCP cylinder but with no cells, and in Group C (three goats) the defect was left untreated (no β-TCP cylinder was implanted). The goats were housed in individual pens with food and water ad libitum, and were allowed unrestricted weight-bearing and activity as tolerated postoperatively. Radiographs were taken post-operatively and at 16-week intervals. At 32 weeks, the animals were euthanized and the involved tibias were removed for micro-computed tomography (Micro-CT) and biomechanical analysis. Specimens were subsequently processed for histological evaluation.
2.2 Isolation and cultivation of goat BMSCs
Goat BMSCs were isolated and expanded using methods reported previously [7]. After intravenous anesthesia with 5% sodium pentobarbital (0.5 mL/kg), 5 mL of bone marrow aspirates were harvested from the iliac crests of each goat and transferred into a pre-heparinized centrifuge tube. Mononuclear cells were separated by Percoll (1.073 g/mL, Sigma, St Louise, MO) gradient centrifugation [20], and were plated in 100-mm dishes (Falcon) at a density of 1 × 105 cells/cm2. Cells were cultured in osteogenic induction media at 37 °C with 5% CO2. The osteogenic induction medium consisted of low-glucose DMEM (Dulbecco’s Modified Eagle Medium, Gibco, Grand Island, NY), supplemented with 10% fetal bovine serum (FBS, Gibco), 10−8 mol/L dexamethasone, 10 mmol/L β-phosphoglycerol and 50 μmol/L L-2-ascorbic acid (all from Sigma). BMSCs were selected on the basis of adhesion and proliferation on the plastic substrate. The culture medium was changed after 48 h and the non-adherent cells were then removed. Subsequent media changes were performed every three days. Once BMSCs reached 80–90% confluence, cells were detached with 0.25% trypsin/EDTA (Gibco) and subcultured at a density of 5 × 105 cells/dish in a 100-mm dish.
2.3 Preparation of the β-TCP cylinders
The β-TCP powder was synthesized by a chemical coprecipitation method [21], while the polymeric sponge method was used to produce the porous β-TCP ceramic cylinders (26 mm high, 15 mm in diameter) [22]. The volume, density, pore size as well as the porosity of the cylinders were measured by a micro-CT system (μCT-80, Scanco Medical, Bassersdorf, Switzerland). Compression testing was also performed to evaluate their biomechanical properties in a universal mechanical tester (Shimadzu AG-5KN, Kyoto, Japan). All the β-TCP cylinders were sterilized by 60Co irradiation before use.
2.4 Cell seeding
BMSCs were seeded onto the β-TCP cylinders using a method modified from that reported previously [20]. Briefly, osteogenically induced autologous BMSCs of passage 3 were detached from culture dishes, centrifuged to remove supernatant, and then resuspended in the culture media at a density of 2 × 107 cells/mL. The medium with suspended cells was slowly injected into the β-TCP cylinder using a syringe (2 mL per cylinder). After being incubated for 4 hours to allow cell attachment, 30 mL of osteogenic induction medium was then added to cover the constructs. The BMSCs/β-TCP constructs were subsequently cultured for 7 days in vitro before implantation, with medium changed every three days.
2.5 Operative procedure
The surgical procedure and stabilization technique were performed according to methods previously reported [9]. The goats were sedated with the intravenous administration of butorphanol (0.2 milligram per kilogram of body weight), acetylpromazine (0.05 mg/kg of body weight), and glycopyrrolate (0.01 mg/kg of body weight). Anesthesia then was achieved through the administration of thiobarbital (eight milligrams per kilogram of body weight to effect) and was maintained with the use of isoflurane in oxygen, administered through an endotracheal tube. The right tibia to be operated on was shaved and prepared. The aspect of the right tibial shaft was exposed through a 7 cm longitudinal incision using a standard lateral approach to the tibia. A 26 mm-long osteoperiosteal segmental cortical defect was made at the mid-portion of the diaphysis with an oscillating bone saw that was cooled continuously through saline irrigation (Fig. 1a). The bone defect was stabilized with a circular external fixator. The defects were left untreated (Group C), or filled with β-TCP cylinder alone (Group B) or filled with BMSCs/β-TCP constructs (Group A) (Fig. 1b). Implants of neither group B nor group A were soaked with autologous blood of the animal. After that, the wound was closed. Each of the animals was then housed in a 16 m2 pen and fed a maintenance ration of hay plus free access to water for the duration of the study.
2.6 Radiographic analysis
High-resolution radiographs of the tibias were carried out immediately after implantation, as well as 16 and 32 weeks postoperatively. All of the radiographs were evaluated with regard to the presence of osseous union at each host bone-implant interface, the presence of osseous callus overlying the implant, and the presence of new bone spanning the entire defect from the distal to the proximal host bone-implant interface.
2.7 Micro-CT measurement
At 32 weeks post-operation, all the animals were sacrificed and the central region of each right tibia, containing the implant and both host bone-implant interfaces, was excised. The defect site of the right tibia was assessed by gross observation, and then a micro-CT system (μCT-80, Scanco Medical, Bassersdorf, Switzerland) was used to visualize axial images of the removed tibial specimens. The CT settings were used as follows: pixel matrix, 1024*1024; voxel size, 36 μm; slice thickness, 36 μm. The three-dimensional images were reconstructed, and the local density and remaining volume of the implant were calculated. Middle segments of the contralateral intact tibias were used as normal control. The analysis of data for density was performed by F test. Between-group differences were assessed using Student–Newman–Kewls test, using SAS 6.12 software. Statistically significant values were defined as p < 0.05.
2.8 Biomechanical analysis
After Micro-CT detection, all the right tibias were subjected to biomechanical testing. The left tibia of each animal was also collected as a normal control group. Each tibia was placed in a three-point bending system, providing an unsupported length of 26 mm. Load was applied in the exterior-to-inferior direction to the midpoint of the unsupported length, approximately in the middle of the repaired defect. The measurement was conducted in a universal mechanical tester (Shimadzu AG-5KN, Kyoto, Japan) and stopped following bone fracture. The deformation of the bone was characterized by the change of the height in the perpendicular direction and was measured using the strain gauge at a loading rate of 0.5 mm/min. By assuming that the bone area is rectangular, the bending strength was calculated with the equation: bending strength = 3LF/(2WT2), where L, F, W and T are the test span (mm), the ultimate load (KN), the width (mm), and the thickness of the specimen (mm), respectively. Young’s modulus was achieved from strength-strain curve as the slope in the linear region. [20]. Data from biomechanical examination were analyzed by paired t-test, using SAS 6.12 software. Statistically significant values were defined as p < 0.05.
2.9 Histological examination
After the biomechanical measurement, the fractured tibia sample, containing the implant and both host bone-implant interfaces, was fixed in 10% buffered formalin, and was decalcified in 15% formic acid in PBS from 6 to 12 weeks. The decalcified tissues of the interfacial portion were then embedded in paraffin. Tissue sections in 5-μm thickness were obtained for hematoxylin and eosin (H&E) staining.
3 Results
3.1 β-TCP cylinder scaffolds
A gross view photograph and Micro-CT pictures of the porous β-TCP cylinders are shown in Fig. 2. The volume of the β-TCP scaffold is 5040.44 ± 224.37 mm3, with the average density of 851.34 ± 16.36 mgHA/cm3. The porosity of the β-TCP scaffolds is 81.7 ± 3.3%, and pore size is 227 ± 11 μm in diameter. It has been shown that all the pores were interconnected, and the average compressive strength of these scaffolds is ∼640 kPa.
3.2 Cultivation of BMSCs
Both the primary cultures and the cultures of passaged BMSCs from all of the animals displayed characteristic spindle-shaped morphology. In addition, BMSCs, cultured in osteogenic induction media, have demonstrated osteogenic phenotype as reported previously [7, 23], such as calcium deposition and positive alkaline phosphatase (ALP) staining (data not shown).
3.3 Animal model
All of the goats could tolerate weight-bearing activity one week postoperatively, and they became active in their open pens. All of the wounds healed without infection, and there were no failures of the external fixation.
3.4 Radiographic analysis
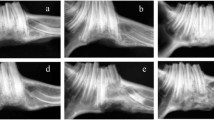
Immediately after implantation, the β-TCP ceramic cylinders with or without BMSCs could be visualized easily because of the radiopacity of the material. In Group A, as new bone formed, the appearance of the implant became smoother and more radiopaque at 16 weeks post-operation. No distinct radiolucent zone at the interface between the implant and the host bone could be visible on the radiographs. The absence of this radiolucent transverse zone was considered as an indication of union between the implant and the host bone. So the external fixators were removed. At 32 weeks post-operation, the volume and radiopacity of newly formed bone were increased, and bony-union was achieved. (Fig. 3a)
Radiographs of tibial defects taken at different time points post-operation. (a) In the BMSCs/β-TCP group, more callus was formed at 16 weeks, and the radiopacity was highly increased and bony-union was achieved at 32 weeks. (b) In the β-TCP group, little callus could be observed at either 16 or 32 weeks. β-TCP scaffold was not completely resorbed, and the radiolucent zone at the cutting ends could be discerned at 32 weeks. (c) In the untreated group, little bone was formed at 16 weeks, and non-union occurred at 32 weeks
On the contrary, in Group B, little callus could be observed both at 16 weeks and at 32 weeks. Distinct radiolucent zones at the interfaces still could be observed at 32 weeks post-operation, suggesting the non-union between the implant and the host bone. Furthermore, the β-TCP ceramic implants hadn’t been completely resorbed at 32 weeks, as the material still could be easily visualized (Fig. 3b).
In Group C, the defects that were left untreated did not heal during the 32-week period of study. Little new bone had formed at the cut ends of the cortices of the host bone by 32 weeks. All of the untreated defects had the radiographic appearance of atrophic non-union (Fig. 3c).
3.5 Micro-CT measurement
To detect the three-dimensional structure of the repaired tibia, micro-CT images were taken at 32 weeks post-operation. In Group A, bony-union was observed and the tibial diaphysis shape was continuous. In seggital plane scans, the interfaces between newly formed bone and host bone could still be observed clearly. Only a few scattered, granular β-TCP fragments with different densities remained embedded in the new bone tissue, indicating that the ceramic scaffolds had been almost completely degraded. Also there was no marrow cavity in the newly regenerated bone (Fig. 4a). In group B which was treated with β-TCP alone, non-union was observed and little osseous callus had developed around the periphery of the implant, but the β-TCP ceramic cylinder still made up the main body of the implant (Fig. 4b). The volume of the left β-TCP in Group B was 1702.57 ± 179.38 mm3, which was about 33.78% of the original volume of β-TCP scaffolds. And in Group C, as non-union had occurred, nearly complete radiolucency was observed at 32 weeks post-operation (Fig. 4c).
3D and 2D micro-CT images of tibial defects at 32 weeks post-implantation. (a) In the BMSCs/β-TCP group, the defect was almost completely repaired by the tissue-engineered bone after 32 weeks of surgery. And in the longitudinal view, boundaries between new bone and host bone could be observed, with some β-TCP remnant in the new bone. (b) In the β-TCP group, non-union and minimal callus were formed. (c) In the untreated group, little new bone was formed, and non-union occurred
To quantify the calcification of repaired tibias, local bone mineral densities (BMD) of implants in Group A and B were measured by micro-CT at 32 weeks post-operation. The tissue-engineered bone in Group A showed relatively high BMD of (573.36 ± 26.35) mgHA/cm3, which was similar to that of the normal control group (604.69 ± 76.66) mgHA/cm3 (p > 0.05). Whereas in Group B, the local BMD of the remnant β-TCP was (312.88 ± 73.62) mgHA/cm3, which was significantly lower than that of Group A (p < 0.01), and also significantly lower than the density of dry β-TCP (p < 0.01) (Fig. 5).
Bone mineral density (BMD) analyses by micro-CT at 32 weeks post-operation. The local BMD of BMSCs/β-TCP implants (n = 6) was similar to that of normal tibias (n = 6, p > 0.05), but significantly higher than that of β-TCP implants (n = 6, p < 0.01). Also the local BMD of β-TCP implants was significantly lower than that of the dry β-TCP scaffolds (n = 3, p < 0.01)
3.6 Biomechanical analysis
To evaluate the biomechanical properties of the repaired tibias, three-point bending tests were performed at 32 weeks post-operation, in which the bone fracture occurred in the middle of the samples in the perpendicular direction. As shown in Table 1, both the bending strength and Young’s modulus in Group A (n = 6) were similar to those of the contralateral normal tibias respectively, indicating the tissue-engineered bone achieved similar mechanical properties to the normal tibia with respects to the biomechanical characteristics tested (p > 0.05). Since there is no bony-union in Group B, which was treated with β-TCP alone, three-point bending test could not be applied in this group.
3.7 Gross and histological examination
In correlation with radiographic and micro-CT analyses, the gross view of the tibias repaired with BMSCs/β-TCP constructs showed solid union at 32 weeks post-operation, though the shape of the repaired area was thinner than that of the normal tibia (Fig. 6a). On the contrary, non-union was observed in Group B with β-TCP alone. The remaining β-TCP scaffold covered by callus and soft tissues was detected in the original defect area in this group (Fig. 6b). In Group C, non-union also occurred. The soft tissue occupied the defect area, and only a trivial amount of new bone formed at both cutting ends (Fig. 6c).
Gross view of the repaired tibias at 32 weeks post-operation. (a) In the BMSCs/β-TCP group, bony-union with some notches was observed. (b) In the β-TCP group, remnant β-TCP scaffold surrounded with soft tissue filled the defect area. (c) In the untreated group, non-union occurred. Arrows indicate the junction sites
To further confirm the gross views and radiographic findings, the histology of the repaired defects at 32 weeks post-operation was analyzed by decalcified tissue section with H&E staining. In Group A, bony-union was observed without clear boundary between newly formed tissue-engineered bone and native bone (Fig. 7a). Irregular osteon formed, in which some remnant β-TCP particles could be found (Fig. 7a1 and a2). However, in Group B treated with β-TCP alone, only minimal new bone formation at the cutting end was observed (Fig. 7b). A large amount of residual β-TCP particles, surrounded by fibrous tissues occupied the defect area. The interface between native bone and the β-TCP implant was also filled with fibrous tissue (Fig. 7b1 and b2). No evident inflammatory response was observed. Since there is no bony-union in Group C, which was left untreated, histological examination was then not applied in this group.
H&E staining of repaired tibias at 32 weeks post-implantation. The macroscopical view of repaired area (dotted line indicated interface between implant and host tibia) in each group was shown: (a) BMSCs/β-TCP group; (b) β-TCP group (bar scales: 1 mm). Detail view of interfacial area (dotted frame, a1, b1) and inside area (solid frame, a2, b2) were taken at high magnification (bar scales: 100 μm). In the BMSCs/β-TCP group, bony-union was observed at the interfacial area (a1), and irregular osteons (arrow head) together with some non-degraded β-TCP particles (arrow) were formed at inside area (a2). In the β-TCP group, fibrous-union occurred with minimal new bone formation (arrow head) at the interfacial area (b1), and non-degraded β-TCP particles (arrow) surrounded by many fibrous tissues were found at inside area (B2)
4 Discussion
In the present study, porous β-TCP ceramics prepared with the polymeric sponge method were used as scaffolds to repair goat segmental tibial defects. It was shown that the critical-sized segmental defect of the goat tibia could be repaired by biodegradable β-TCP scaffolds combined with osteogenically induced autologous BMSCs. Meanwhile, β-TCP scaffolds alone were not sufficient to repair the defect in this model.
Bone tissue engineering has been shown to be an effective approach in bone regeneration, and BMSCs have proved to be a major seed cell source for bone engineering. The osteogenic potential of BMSCs has been demonstrated extensively both in vitro and in vivo, and many studies have succeeded in repairing bone defects using BMSCs in animal models [4–9, 20, 23–25]. Osteogenically induced BMSCs can not only provide an osteogenic cell source for new bone formation, but also secrete growth factors to recruit native cells to migrate into the defect site. In this study, the importance of using BMSCs as seed cells was ascertained again in the application of porous β-TCP scaffolds in bone tissue engineering.
Biodegradable scaffold plays a pivotal role in bone engineering. A premature degradation of scaffold may destroy the tissue structure if new bone has not generated yet, while a lingering degradation of scaffold may also effects the repairing process, such as bone remodeling [20]. Porous β-TCP has shown a good degradation rate that could well match the new bone formation rate, but its weak mechanical properties have limited its application as a bone graft substitute [18]. However, as scaffolds for bone tissue engineering, the biomechanical properties of osteogenically induced BMSCs/β-TCP composites could be significantly improved over dry β-TCP when tissue-engineered bone formed in vivo [18, 20, 22]. So we postulate that fragile β-TCP could be used to repair the load-bearing bone defect via tissue engineering, provided that the rigid external/internal fixation is used at the early stage of bone formation. Via a tissue engineering approach, Xu et al [26] repaired the 21 mm segmental defects in goat tibias using self-secreted extracellular matrix (ECM) of BMSCs as a cell carrier, with the defect stabilized by the same external fixators as ours.
In this study, our result was in consistent with the postulation above. When the β-TCP scaffolds combined with osteogenically induced autologous BMSCs were implanted into the defects of goat tibial defects, porous β-TCP showed a suitable degradation rate that could well match the new bone formation rate in the repairing process. At early stages, β-TCP scaffolds provided a skeletal support for the growth of osteogenic cells with the help of the external fixation, and at the later time point, adequate new bone was generated as they were degraded. When the tissue-engineered bone was able to bear weight, the external fixator was then removed at 16 weeks. Under the complete weight-bearing condition, the newly formed bone tissue continued to remodel and self-organize, and bony-union was achieved with almost complete degradation of β-TCP after 32 weeks. Although the structure of the tissue-engineered bone has not been completely remodelled into the structure of the normal tibia, biomechanical analysis revealed that the regenerated bone was functionally similar to the normal tibia in terms of mechanical properties.
On the other hand, β-TCP alone was ineffective in healing such a critical-sized segmental defect. Bone non-union was observed in all of the defects treated with β-TCP scaffolds alone. For some small bone defects, native osteoblasts could migrate from the sites of the osteotomies into the β-TCP scaffold to generate new bone, and bony-union formed in a repairing process like “creeping substitution” [11, 27]. Osteoconductive scaffolds may therefore achieve bone regeneration in the small bone defects without seeding osteogenic cells. Gao et al [11] used simple TCP cylinders to repair the 16 mm segmental defects of sheep tibias, and the healing rate was 50% (3/6). However, for the large defects in this study, without help of the implanted BMSCs, the native osteoblasts couldn’t migrate into the β-TCP scaffold entirely to bridge the defect. So callus only appeared to arise from the cutting ends of the host bone, and non-union occurred at 32 weeks postimplantation.
It’s known that bone cell differentiation and bone defect regeneration depend on the skeletal location and micromechanical loadings [28]. Studies on the resorption and osteoconduction of β-TCP have always been conducted in load-bearing environments of long bones, and mechanical effects are involved when biomaterial properties are evaluated in load-bearing models [29–32]. Handschel et al [33] reported that β-TCP was hardly resorbed in a non-loading rat calvarial model. Also we found that the current β-TCP scaffold as-prepared showed almost no degradation in a non-loading canine calvarial model 6 months after implantation (data not shown). While in a sheep model of femoral condyle implantation, it has been reported that 55% of the β-TCP implants could be degraded after 24 weeks [34]. The easy degradation of β-TCP in the load-bearing site of long bone indicates that mechanical forces could facilitate this process [29, 35]. In this study, the external fixation was removed at 16 weeks post-operation in the BMSCs/β-TCP group, giving the newly formed bone the complete weight-bearing environment. The mechanical loading stimulated not only the remodeling process of new bone, but also the bioresorption of β-TCP. At 32 weeks, only trivial β-TCP particles were left in the tissue-engineered bone. In the β-TCP group, however, as no bone union was achieved, the external fixator could not be removed during the period observed. Partial loading condition gives rise to the slow degradation of the scaffolds, and still over 33% volume of the β-TCP scaffolds remained at 32 weeks.
By assuming that the bone area is rectangular, and applying the same formula of calculating bending strength to both regenerated bones and control bones, we drew the conclusion that tissue-engineered bone at 32 weeks exhibited similar biomechanical properties to the native bone. However, the regenerated bone seemed to be irregular and had no marrow cavity, while the normal control bones are oblong and have a cavity. Therefore, such an assumption is not exact, and the actual biomechanical strength of the regenerated bone at 32 weeks should be inferior to that of the native bone. This calculating method could only provide an approximate comparison of the biomechanical strength between these two groups, but the results revealed the gradual biomechanical increase of the regenerated bone during osteogenesis. Taking other results into account, BMSCs/β-TCP constructs indeed increased the repair rate of weight-bearing bone defects in this study when compared to β-TCP alone. But the newly regenerated bone may need a longer period of stress stimulation in order to be fully remodelled into cortical bone and achieve the actual mechanical properties of the native bone [7].
In summary, this study demonstrates that porous β-TCP ceramics prepared via the polymeric sponge method could be used as scaffolds to repair the weight-bearing bone defects of goat tibia by means of a bone tissue engineering approach. Therefore, the as-prepared β-TCP may be served as a promising scaffold for bone tissue engineering.
References
E. M. YOUNGER and M. W. CHAPMAN, J. Orthop. Trauma. 3 (1989) 192
R. C. SASSO, J. I. WILLIAMS, N. DIMASI and P. R. Jr. MEYER, J. Bone. Joint. Surg. Am. 80 (1998) 631
D. GROB, Unfallchirurg 89 (1986) 339
S. P. BRUDER, K. H. KRAUS, V. M. GOLDBERG and S. KADIYALA, J. Bone. Joint. Surg. Am. 80 (1998) 985
E. KON, A. MURAGLIA, A. CORSI, P. BIANCO, M. MARCACCI, I. MARTIN, A. BOYDE, I. RUSPANTINI, P. CHISTOLINI, M. ROCCA, R. GIARDINO, R. CANCEDDA and R. QUARTO, J. Biomed. Mater. Res. 49 (2000) 328
T. L. ARINZEH, S. J. PETER, M. P. ARCHAMBAULT, C. van den BOS, S. GORDON, K. KRAUS, A. SMITH and S. KADIYALA, J. Bone. Joint. Surg. Am. 85A (2003) 1927
L. ZHU, W. LIU, L. CUI and Y. L. CAO, Tissue. Eng. 12 (2006) 423
H. PETITE, V. VIATEAU, W. BENSAID, A. MEUNIER, C. de POLLAK, M. BOURGUIGNON, K. OUDINA, L. SEDEL and G. GUILLEMIN, Nat. Biotechnol. 18 (2000) 959
K. R. DAI, X. L. XU, T. T. TANG, Z. A. ZHU, C. F. YU, J. R. LOU and X. ZHANG, Calcif. Tissue. Int. 77 (2005) 55
M. L. JOSEPH, T. EMRE and P. G. B. MATHIAS, Clin. Orthop. 367(Suppl) (1999) 107
T. J. GAO, T. K. TUOMINEN, T. S. LINDHOLM, B. KOMMONEN and T. C. LINDHOLM, Biomaterials 18 (1997) 219
M. ROUDIER, C. BOUCHON, J. L. ROUVILLAIN, J. AMEDEE, R. BAREILLE and F. ROUAIS, J. Biomed. Mater. Res. 29 (1995) 909
K. OHURA, M. BOHNER, P. HARDOUIN, J. LEMAITRE, G. PASQUIER and B. FLAUTRE, J. Biomed. Mater. Res. 30 (1996) 193
K. OHSAWA, M. NEO, H. MATSUOKA, H. AKIYAMA, H. ITO, H. KOHNO and T. NAKAMURA, J. Biomed. Mater. Res. 52 (2000) 460
K. KURASHINA, H. KURITA, Q. WU, A. OHTSUKA and H. KOBAYASHI, Biomaterials 23 (2002) 407
M. SAITO, H. SHIMIZU, M. BEPPU and M. TAKAGI, J. Orthop. Sci. 5 (2000) 275
A. OGOSE, T. HOTTA, H. KAWASHIMA, N. KONDO, W. GU, T. KAMURA and N. ENDO, J. Biomed. Mater. Res. B. 72 (2005) 94
J. DONG, T. UEMURA, Y. SHIRASAKIE and T. TATEISHI, Biomaterials 23 (2002) 4493
M. JARCHO, Clin. Orthop. 157 (1981) 259
J. YUAN, L. CUI, W. J. ZHANG, W. LIU, and Y. L. CAO, Biomaterials 28 (2007) 1005
S. RAYNAUD, E. CHAMPION, D. BERNACHE-ASSOLIANT and P. THOMAS, Biomaterials 23 (2002) 1065
G. LIU, L. ZHAO, L. CUI, W. LIU, and Y. CAO, Biomed. Mater. 2 (2007) 78
Q. SHANG, Z. WANG, W. LIU, Y. SHI, L. CUI and Y. CAO, J. Craniofac. Surg. 12 (2001) 586
A. MURAGLIA, I. MARTIN, R. CANCEDDA and R. QUARTO, Bone 22(5 Suppl) (1998) 131S
C. MANIATOPOULOS, J. SODEK and A. H. MELCHER, Cell. Tissue. Res. 254 (1988) 317
X. L. XU, T. T. TANG, K. R. DAI, Z. A. ZHU, X. E. GUO, C. F. YU and J. R. LOU, Acta. Orthopaedica. 76 (2005) 637
K. D. JOHNSON, K. E. FRIERSON, T. S. KELLER, C. COOK, R. SCHEINBERG, J. ZERWEKH, L. MEYERS and M. F. SCIADINI, J. Orthop. Res. 14 (1996) 351
L. E. LANYON, Bone 18(1 Suppl) (1996) 37S
M. NEO, H. HERBST, C. F. VOIGT and U. M. GROSS, J. Biomed. Mater. Res. 39 (1998) 71
M. M. A. RAMSELAAR, F. C.M. DRIESSENS, W. KALK, J. R. De WIJN and P. J. Van MULLEM, J. Mater. Sci. 2 (1991) 63
M. NEO, C. F. VOIGT, H. HERBST and U. M. GROSS, J. Biomed. Mater. Res. 30 (1996) 485
J. M. SCHMITT, D. C. BUCK, S. P. JOH, S. E. LYNCH and J. O. HOLLINGER, J. Peridontol. 68 (1997) 1043
J. HANDSCHEL, H. P. WIESMANN, U. STRATMANN, J. KLEINHEINZ, U. MEYER and U. JOOS, Biomaterials 23 (2002) 1689
J. LU, M. DESCAMPS, J. DEJOU, G. KOUBI, P. HARDOUIN, J. LEMAITRE and J. P. PROUST, J. Biomed. Mater. Res. 63 (2002) 408
D. BUSER, B. HOFFMANN, J. P. BERNARD, A. LUSSI, D. METTLER and R. K. SCHENK, Clin. Oral. Implants. Res. 9 (1998) 137
Acknowledgements
This work was supported by Major State Basic Research Development Program of China (2005CB522700) and National High Technology Research and Development Program of China (2006AA02A123).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Zhao, L., Zhang, W. et al. Repair of goat tibial defects with bone marrow stromal cells and β-tricalcium phosphate. J Mater Sci: Mater Med 19, 2367–2376 (2008). https://doi.org/10.1007/s10856-007-3348-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3348-3