Abstract
The aim of this study was to investigate in vitro bioactivity of different thermoplastic biodegradable barrier membranes. Three experimental GBR membranes were fabricated using Poly(ε-caprolactone-co-d,l-lactide) P(CL/DL-LA) and particulate bioactive glass S53P4 (BAG; granule size 90–315 μm): (A) composite membrane with 60-wt.% of BAG, (B) membrane coated with BAG; and (C) copolymer membrane without BAG. Membranes were immersed in simulated body fluid (SBF), and their surfaces were characterized with SEM, XRD and EDS after 6 and 12 h and after 1, 3, 5, 7, and 14 days. Calcium phosphate (Ca-P) surface formation was observed on both composite membranes (A and B) but not on the copolymer membrane without bioactive glass (C). The Ca-P precipitation appeared to be initiated on the bioactive glass followed by growth of the layer along the polymer surface. In 6–12 h ion dissolution of the bioactive glass led to formation of the silica rich layer on the surface of the exposed glass granules on composite membrane B whereas only small amounts of silica was observed on the polymer surface of the composite membrane A. At 24 h nucleation of Ca-P precipitation was observed, and by 3–5 days membrane surface was covered with a uniform Ca-P layer transforming from amorphous to low crystalline structure. At 7 days composition and structure of the apatite surface resembled the apatite in bone. Once nucleated, the surface topography seemed to have significant effect on the growth of the apatite layer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Barrier membranes and meshes are frequently used in oral and craniofacial surgery and in periodontology for the fixation of bone grafts and bone substitutes or to support tissue healing by means of guided tissue regeneration (GTR) or guided bone regeneration (GBR). For example, GTR has been successfully applied to gain regeneration of periodontal tissues [1], mucogingival tissue [2] and bone surrounding dental implants [3], and to augment the dentoalveolar ridge [4]. Expanded polytetrafluoroethylene (ePTFE) membrane has shown good results in clinical studies and it is currently most frequently used membrane in periodontal and implant surgery [5, 6]. Titanium has been used as a mesh or in an ePTFE membrane to improve it’s mechanical properties and adjustability. The main disadvantage of these non-degradable membrane materials is the additional surgical procedure needed for the membrane removal. Biodegradability of the membrane may thus be considered beneficial since additional operation can be avoided. Collagen membranes have several favorable properties and they have shown promising results in clinical studies [7]. However, general risks of infection transmission and unfavorable immune reactions have been brought out and these are considered as a disadvantage for the use of xenografts as biomaterials in general. Synthetic biodegradable polymers like polylactide and its copolymer with polyglycolide have been produced into GTR membranes with good clinical performance [8, 9]. However, none of these materials have specific bioactive properties that could promote bone tissue healing or new bone formation.
A composite of thermoplastic biodegradable copolymer Poly(ε-caprolactone-co-d,l-lactide) and bioactive glass was recently developed and evaluated as a moldable bone cavity filler both in vitro and in vivo [10, 11]. Bioactivity, i.e. the formation of a biologically active Ca-P layer on the surfaces of the composites in vitro and their osteoconductive properties in vivo, was found to depend on the weight fraction and granule size range of the bioactive glass used. Composite was further processed into membrane for GTR applications. Aim of this study was to evaluate bioactive glass induced apatite formation on the biodegradable thermoplastic membrane in simulated physiological conditions. Specific aim was to compare apatite formation on membranes where BAG was homogenously distributed within the membrane and polarized membranes where glass was compressed directly on the membrane surface.
2 Materials and methods
Copolymer of ε-caprolactone (Solvay Interox Ltd.) and d,l-lactide (Purac) was polymerised by ring opening polymerisation using Sn(II)octoate (Sigma) as an initiator and glycerol (Rhône-Poulenc) as a co-initiator as previously described [10]. Comonomer molar ratio was 96/4, consisting mainly of ε-caprolactone. The polymer was purified by precipitating it out from a dichloromethane solution with ethanol. Particulate bioactive glass S53P4 (Vivoxid LTD, Finland (former Abmin Technologies)) was used in granule size range 90–315 μm to produce the composites. The composition of the glass is shown in Table 1.
Three different biodegradable membranes were prepared for the study: (A) composite membrane with 60 wt-% of bioactive glass granules, (B) membrane coated with bioactive glass granules, and (C) copolymer membrane without bioactive glass as a reference. Bioactive glass was blended with copolymer in a batch mixer and composites were compression moulded into discoid membranes with thickness of 0.8 mm and a diameter of 12 mm (A), or glass was separately pressed on the surface of the specimen (B). On average there was 0.03 g/cm2 of bioactive glass on case B composites, calculated by the average weights of the samples. Simulated body fluid (SBF) first presented by Kokubo et al. [12] was used for material incubation. Ionic composition of the SBF is shown in Table 2. Three specimens of each type were immersed individually in 10 mL of SBF at 37 °C for 6 and 12 h, 1, 3, 5, 7 and 14 days. Also three native specimens were analyzed. After the immersion period each specimen was rinsed with distilled water, air-dried and stored in a desiccator. Thin film X-ray diffraction spectrometry (XRD) was performed on the specimens to study the changes in the surface characteristics of the materials. For XRD, a thin film Philips X-ray diffractometer (PW3710, Almelo, The Netherlands) was used, using CuKα-radiation (PW3710, 40 KV, 40 mA). Specimens were then carbon coated and examined using a Jeol 6310 scanning electron microscope (SEM) equipped with an energy dispersive X-ray detector (EDS (Voyager)) to study the surface topography and Ca-P precipitation. EDS was used to collect spectrum of all elements of a surface area and Ca:P ratio was calculated on the basis of quantitative analysis of the spectrum.
3 Results
3.1 XRD
XRD analysis of the specimens before the incubation revealed only the crystallinity of the copolymer observed as two separate peaks in 2θ ranges 21–22.5 and 23–24.5 (Fig. 1a) and lower peaks at 30 and 40.5. At 3 days small changes started to appear in the XRD spectra of the specimens with bioactive glass. Low crystalline apatite layer started to appear at 5 days. By 7 days a partially crystalline layer had formed on the composites with several main XRD peaks comparative with XRD of Ha powders (#9–0432) (Fig. 1b). Only minor increase in crystallinity was observed at 14 days compared to that of 7 days. No changes in the XRD pattern of specimens without the bioactive glass or the crystallinity of the copolymer matrix of the composites occurred during the observation period.
3.2 SEM and EDS
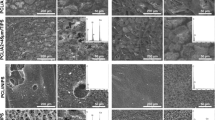
Majority of the glass granules on the composite membrane A were covered by a thin layer of copolymer with only few granules partially exposed on the surface before the incubation (Fig. 2a). On the membrane B all of the granules were partially exposed and they numbered 5–8 granules/mm2 (Fig. 2c and d). The EDS analysis revealed formation of a silica rich layer with low content of calcium and phosphorous on the exposed granules after 6 and 12 h of incubation. Slightly increased amount of silicon was also traced on the polymer matrix surface at close proximity to the granules. Nucleation and growth of the Ca-P layer started to appear at 24 h first in the areas where glass granules were exposed. Nucleation occurred also on the polymer matrix surface on membrane A and it seemed to be strongly affected by the surface topography of the composites.
At day 3 surfaces of the granules on the composite membrane B were covered by a calcium phosphate layer with mean Ca:P ratio of 1.6 whereas on the composite membrane A the Ca-P layer had already grown covering uniform areas on the specimen surface with mean Ca:P ratio being 1.4. At day 5 Ca-P layer covered totally the membrane A and the side of the membrane B that was coated with the bioactive glass granules (Fig. 3).
After 7 and 14 days of immersion Ca-P layer had further grown to cover the whole specimen surface. On the membrane B Ca-P formation occurred only on the side coated with glass granules whereas no changes were observed on the reversed side of the membrane. No changes in the surface topography or elemental composition were observed on copolymer membrane C without bioactive glass during the 14 days immersion.
4 Discussion and conclusions
Composite membranes of Poly(ε-caprolactone-co-d,l-lactide) and bioactive glass granules S53P4 showed induction of apatite formation in simulated physiological conditions. Once nucleated, surface topography seems to have an important role on the growth of the apatite layer. The apatite layer had Ca:P ratio and structure resembling the characteristics of the biological apatite found in bone and tooth enamel.
This study clearly showed that Ca-P surface formation was dictated by the location of glass granules. Uniform Ca-P coverage was seen on the composite membranes where glass granules were homogenously distributed, while polarized glass granule coating resulted in Ca-P surface formation only on the coated surface. These polarized structures may be exploited to produce structures that evoke different biologic response in different areas of the biomaterial-tissue interface, e.g. in fibrous connective tissues and in bone.
As the Ca-P surface transforms from amorphous to partially crystalline its biological behavior may change as well. Protein adsorption and cell response may vary according to material’s surface composition and crystallinity. Immersing the bioactive composite membrane in a solution that supports apatite formation, e.g. SBF, prior the clinical use can be utilized to create specific surface structure when it is considered beneficial for the healing. Membrane with Ca-P surface on one or both sides can then be placed on the recipient site.
However, when used in the treatment of bone defects in vivo apatite formation on the composite surface will also occur as direct biological mineralization at the cement line of bone formation that may be associated with total or partial dissolution of the preformed Ca-P layer followed by precipitation of mineral phase of bone. Both phenomena are generally strongly affected by the adsorption of proteins on the biomaterial surface [13, 14], as is also the case with the bioactive glass [15, 16].
Biomimetic methods utilizing extrinsic ion source have been used to produce Ca-P surfaces on various substrates. However, the composites of bioactive glass provide surrounding tissues with constant bioactivity for a prolonged period of time compared to a resorbable Ca-P surface alone. This intrinsic biomimetic phenomenon may be especially important in the degradable or resorbable biomaterials as it provides favorable conditions for the regenerating tissues while the material degrades. Biodegradable GBR membranes presented in this study may vary in their clinical performance depending on the implantation site. The use of polar structures or the formation of the apatite surface prior the membrane placement may offer ways of controlling the healing process and final clinical outcome.
References
J. GOTTLOW, S. NYMAN, J. LINDHE, T. KARRING and J. WENNSTRÖM, J. Clin. Periodontol. 13 (1986) 604
A. SCABBIA and L. TROMBELLI, J. Clin. Periodontol. 25 (1998) 1041
M. LORENZONI, C. PERTL, R. A. POLANSKY, N. JAKSE and W. A. WEGSCHEIDER, Clin. Oral. Impl. Res. 13 (2002) 274
D. BUSER, U. BRÄGGER, N. P. LANG and S. NYMAN, Clin. Oral. Impl. Res. 1 (1990) 22
E. E. MACHTEI, S. G. GROSSI, R. DUNFORD, J. J. ZAMBON and R. J. GENCO, J. Periodontol. 67 (1996) 523
C. DAHLIN, L. ANDERSSON and A. LINDE, Clin. Oral. Impl. Res. 2 (1991) 159
N. BLUMENTHAL and J. STEINBERG, J. Periodontol. 61 (1990) 319
M. SIMION, U. MISITANO, L. GIONSO and A. SALVATO, Int. J. Oral. Maxillofac. Implants 12 (1997) 159
T. TEPARAT, C. W. SOLT, L. J. CLAMAN and M. BECK, J. Periodontol. 69 (1998) 632
J. RICH, T. JAAKKOLA, T. TIRRI, T. NÄRHI, A. YLI-URPO and J. SEPPÄLÄ, Biomaterials 23 (2002) 2143
T. O. NÄRHI, J. A. JANSEN, T. JAAKKOLA, A. DE RUIJTER, J. RICH, J. SEPPÄLÄ and A. YLI-URPO, Biomaterials 24 (2003) 1697
T. KOKUBO, H. KUSHITANI, S. SAKKA, T. KITSUGI and T. YAMAMURO, J. Biomed. Mater. Res. 24 (1990) 721
G. K. HUNTER, P. V. HAUSCHKA, A. R. POOLE, L. C. ROSENBERG and H. A. GOLDBERG, Biochem. J. 317 (1996) 59
G. DACULSI, P. PILET, M. COTTREL and G. GUICHEUX, J. Biomed. Mater. Res. 47 (1999) 228
T. A. MAHMOOD and J. E. DAVIES, J. Mater. Sci. Mater. Med. 11 (2000) 19
E. A. KAUFMAN, P. DUCHEYNE, S. RADIN, D. A. BONNELL and R. COMPOSTO, J. Biomed. Mater. Res. 52(4) (2000) 825
Acknowledgements
This study was partially conducted under the umbrella of the Center of Excellence of Bio- and Nanopolymers Research Group, which is jointly funded by the Academy of Finland (CoE program 77317), National Technology Agency, TEKES (nr: 40319/02) and the participating institutes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tirri, T., Rich, J., Wolke, J. et al. Bioactive glass induced in vitro apatite formation on composite GBR membranes. J Mater Sci: Mater Med 19, 2919–2923 (2008). https://doi.org/10.1007/s10856-007-3308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3308-y