Abstract
Nano-hydroxyapatite/chitosan/carboxymethyl cellulose (n-HA/CS/CMC) composites with weight ratios of 70/10/20, 70/15/15 and 70/20/10 were prepared through a co-solution method. The properties of the composites were characterized by means of burn-out test, IR, XRD, TEM and universal material testing machine. The degradation and bioactivity were also investigated by in vitro test in a simulated body fluid (SBF) for 8 weeks. The results showed that n-HA particles were dispersed uniformly in organic phase, and strong chemical interactions formed among the three phases. Moreover, the composites were similar to natural bone in morphology and size. In addition, the compressive strength was improved compared with n-HA/CS composite. The biodegradation rate was controllable by altering weight ratio of the CS/CMC. Meanwhile, the composites could induce apatite particles to deposit in SBF. All the above results indicate that the novel composites of n-HA/CS/CMC have a promising prospect used for bone repair materials in view of the good mechanical property, adjustable biodegradation rate and bioactivity in SBF. Additionally, the study would provide a good guide to exploit clinical application of natural cellulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that cellulose is the richest organic resource in the nature. Carboxymethyl cellulose (CMC) is a kind of hydrosoluble cellulose ether derivate, which is obtained from natural cellulose by chemical modification [1, 2]. Usually, people use Na-CMC, a kind of biodegradable anionic polymer by virtue of the solubility. Because of its unique properties such as coherence, thickening, making membrane, suspension and keeping water, CMC has been widely used in the field of foodstuff, medicament, cosmetic, coating material, pain, spinning and weaving, petroleum, paper making, and so on [3, 4]. Additionally, the membrane made of hyaluronic acid and CMC was reported to have good biocompatibility and anti-adhesion effect in peritoneum [5, 6]. However, to date, there is no report of whether CMC can be used in the field of bone repair.
Currently, there is a great difficulty to develop an ideal material for the substitution of damaged bone. As we know, perfect substitution materials for bone repair should have good biocompatibility and a suitable biodegradation rate as well as higher mechanical property to support the ingrowth of new bone tissue. Hydroxyapatite (HA), which is the main inorganic constituent of bone, has been extensively investigated due to its excellent biocompatibility and bioactivity with human tissue [7, 8]. Especially, a nano-scaled HA with extraordinary properties such as high surface area to volume ratio and ultra fine structure similar to that of biological apatite, which is of a great effect on cell-biomaterial interaction [9], has been reported to be used for the treatment of bone defects, and it could bond to living bone in implanted areas [10, 11]. However, the fragility and low mechanical strength of n-HA made it unsuitable to be applied in load-bearing sites [12, 13]. Thus, many scientists turn to the researches on n-HA/organic polymer composites [14–17]. Chitosan (CS), a natural biodegradable cationic polymer, has been proved to be non-cytotoxic and having some biological activities [18]. In recent years, CS-based materials have aroused much interest in biomedical field [19–22]. Accordingly, n-HA/CS composite has been reported to substitute the damaged bone [23–25]. But its compressive strength and biodegradation are not desirable. Therefore, many cross-linking agents are tried such as glutaraldehyde [26]. Whereas, the toxicity of glutaraldehyde is not favorable to the adhesion and proliferation of osteoblasts [27–30]. Fortunately, CMC can interact strongly with CS due to the opposite electric charge and the structural similarity (Fig. 1), thus a compact network of polyelectrolyte complexes could be formed [31, 32], which is expected to improve the mechanical property and adjust the biodegradation rate.
Based on the above thought, in the paper, we prepared three composites of n-HA/CS/CMC with weight ratios of 70/10/20, 70/15/15 and 70/20/10 respectively through a co-solution method. Additionally, the in vitro degradation and bioactivity of these composites were evaluated by soaking them in SBF for different periods. All the results showed that the novel n-HA/CS/CMC composites had good mechanical property, adjustable biodegradation rate and high bioactivity, and it may be a potential candidate as bone repair materials.
Materials and methods
Raw materials
n-HA was prepared in our laboratory [33]. An 80-mesh chitosan powder with a molecular weight of about 250,000 and a N-acetylation degree of 80% was purchased from Haidebei Bioengineering Co. Ltd, Jinan, China. Sodium carboxymethyl cellulose was purchased from Kelong Chemical Agent Factory, Chengdu, China, with a molecular weight of about 4.2 × 108 and a substitution degree of 0.7. All other reagents used here were of analytical grade.
Preparation of n-HA/CS/CMC composites
The n-HA/CS/CMC composites were synthesized by the following procedure. First, 2 wt% CS solution was obtained by dissolving CS in 2 wt% acetic acid solution. Second, some amount of n-HA was added into CS solution. Then 2 wt% CMC solution was added in the above mixed solution with constant stirring in ambient condition. The dropping speed was about 4 mL/min. After titration, the stirring was kept for 8 h, and the pH value was kept at 5.5. Finally, the obtained slurry was aged for 24 h, the precipitate was filtered, washed with deionized water and dried in a vacuum oven at 60 °C.
Characterization of n-HA/CS/CMC composites
The uniformity of these composites was measured by burn-out test at 800 °C. The crystal structure of n-HA, CS, CMC and their interactions were determined by X-ray diffractometry (XRD) and infrared spectroscopy (IR). The microstructures of n-HA and n-HA/CS/CMC composites were observed with a transmission electron microscope (TEM) (JME-100CX, Seike Instruments) on a 200 kV. TEM samples were prepared by ultrasonication dispersion method using deionized water.
Additionally, the n-HA/CS/CMC composites were adjusted into paste with a mixed solution containing citric acid, acetic acid, gelatin, CaCl2, K2HPO4, and deionized water, then placed into a particular injector and pressed into cylindrical blocks with a size of Φ6 × 12 mm and dried at 60 °C for compressive strength testing, and n-HA/CS with a weight ratio of 70/30 was also prepared by the same method for comparison.
In vitro test
The n-HA/CS/CMC specimens were dried and weighted, noted as W0. The SBF solution was prepared by dissolving reagent chemicals of NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2, and Na2SO4 into deionized water. The ion concentrations of the used SBF resembling human blood plasma were shown in Table 1 [34]. The fluid was buffered at physiological pH 7.40 at 37 °C with tri-(hydroxymethyl) aminomethane and hydrochloric acid. The specimens were immersed in 10 mL of SBF for 1, 2, 3, 4, 6 and 8 weeks. All the test tubes were placed in a rocking water bath with a constant temperature (37 ± 0.5 °C). After soaking, the specimens were removed from the fluid, gently rinsed with deionized water for several times, and cleaned with filter paper to get rid of water on the surface. These specimens were weighed and noted as W1, then they were weighed again after being dried, and noted as W2. The rate of weight lose (WL) was calculated based on the formula of WL = (W0−W2) /W0 × 100%, and the rate of water absorption (WA) was determined by the formula of WA = (W1−W2)/W2 × 100%. The surface microstructure of n-HA/CS/CMC specimens after soaking was observed by scanning electron microscope (SEM) (JSM -5900LV, Japan). The specimens were removed from SBF, gently rinsed with deionized water and dried. After the dried samples were coated with gold, the examination was carried out at an accelerating voltage of 20 kV.
Results
Characterization of n-HA/CS/CMC composites
Burn-out test
Three different parts of the same composite were sintered in air at 800 °C for 4 h. Table 2 lists the theoretical and experimental compositions of the composites. It can be seen that the actual compositions were very close to the theoretical ones. Moreover, different parts of the same composite had almost identical composition, which indicates that the three phases disperse into each other uniformly.
IR analysis
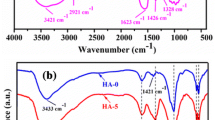
IR spectra of pure n-HA, CS, CMC and n-HA/CS/CMC composites are given in Fig. 2. In Fig. 2a the characteristic PO 3−4 and OH− derived bands as well as adsorbed water bands of pure n-HA were observed. In Fig. 2e, the bands of at 1,655 cm−1 and 1599 cm−1 were assigned to amide I (C = O) and amino (NH −2 ) of pure CS. The spectrum in Fig. 2f of pure CMC displayed two characteristic absorption bands at 1,607 cm−1 and 1,420 cm−1, which represents symmetry stretching and asymmetry stretching of COO− group respectively. However, comparing with these IR spectra, it can be seen that the specific peaks of pure n-HA, CMC and CS all appeared in the spectra of n-HA/CS/CMC composites (as shown in Fig. 2b–d) except for slight band-shifts. An absorption at 1655 cm−1 in CS shifted to 1,612∼ 1,644 cm−1 in the three n-HA/CS/CMC composites, which might be the result of the formation of NH +3 . Additionally, the peak of asymmetry stretching of COO− was still found at ∼1,420 cm−1. Clearly, the ionic cross-link of COO− and NH +3 could be formed because of the strong static electric interaction. At the same time, –OH in composites had a slight band-shifts, which suggests that they have formed inter- or intra- hydrogen bonds among the three phases of n-HA/CS/CMC composites.
XRD analysis
Figure 3 shows the X-ray diffraction patterns of pure n-HA, CS, CMC and n-HA/CS/CMC composites. The characteristic peaks of n-HA are shown in Fig. 3a. In Fig. 3e, two main diffraction peaks of CS at 2θ = 10° and 20° were observed. In Fig. 3f, we can find two main diffraction peaks of CMC at 2θ = 32°and 46°. The XRD patterns of the n-HA/CS/CMC composites shown in Fig. 3b–d were characterized by specific diffraction peaks arising from n-HA and CMC. However, the specific peaks of CS disappeared evidently in three composites, which might be the result of the interaction of CS and CMC. In conclusion, it can be inferred the interaction mode of the three phases of n-HA, CS and CMC, as shown in Fig. 4.
TEM observation
TEM photographs of n-HA and n-HA/CS/CMC are shown in Fig. 5. Figure 5a is for n-HA and Fig. 5b is for n-HA/CS/CMC composite whose weight ratio is 70/15/15. It can be seen that the n-HA exhibited nanometer short rod crystals with a mean size of about 50 nm in length and 10 nm in width, and these n-HA crystals had a good dispersive property and displayed a relatively uniform morphology. With the addition of CS and CMC, the composite particles showed longer and wider with an average size of about 80 nm in length and 30 nm in width, and it was still in the range of nanometer grade.
Compressive strength
Table 3 illustrates the values of the compressive strength of three n-HA/CS/CMC composites and n-HA/CS composite. From the data, it can be seen that the values of three n-HA/CS/CMC composites were higher than that of n-HA/CS, and the n-HA/CS/CMC composite with a weight ratio of 70/15/15 had the highest compressive strength.
In vitro test
The rates of weight loss and water absorption
The rates of weight loss and water absorption of three n-HA/CS/CMC composites as a function of soaking time in SBF solution are shown in Fig. 6. It can be seen that the rate of weight loss of three n-HA/CS/CMC composites all increased during the soaking time, and the rate of weight loss was more significant when the content of CMC in n-HA/CS/CMC composites was higher. The rate of weight loss of the n-HA/CS/CMC composite with 70/10/20 weight ratio was up to 50.12% after it was soaked at 8 weeks. However, the rate of weight loss of the n-HA/CS/CMC composite whose weight ratio is 70/15/15 had little change tendency.
According to the change tendency of their rates of water absorption, we can find the rates of water absorption of three n-HA/CS/CMC composites increased at beginning and then decreased with the soaking time. Likely, the rate of water absorption of the n-HA/CS/CMC composite whose weight ratio is 70/15/15 had little change tendency.
SEM observation
Figure 7 shows the SEM microstructures of the n-HA/CS/CMC specimens whose weight ratio is 70/10/20 before and after soaking in SBF solution at 37 ± 0.5 °C for 4 and 8 weeks respectively. From the SEM photos, we find that the surface of the specimen was coarse before soaking, in which n-HA particles imbedded uniformly in the organic polymer of CS/CMC with no obvious agglomeration. After 4 weeks soaking, a few apatite particles could be seen to deposit on the surface, and there were a few micropores on the surface, as shown in Fig. 7(b). After 8 weeks soaking, more and more apatite particles were present on the surface.
Discussion
Bone is mainly composed of n-HA and collagen. According to bionic principle, it is an ideal approach to design n-HA/organic composites materials with good biodegradability and bioactivity used for bone repair. However, the problem of interface is a great obstacle to develop ideal composite. To improve the interface, in this study we chosed CMC, a natural-based biodegradable material with good biocompatibility, to act as raw material and ionic cross-linking agent. The reason is that CMC has opposite electric charge with CS and it is very similar to CS in structure, so that they can interact easily in solution and form a compact network structure of polyelectrolyte under ambient condition, which will be conduce to combine organic and inorganic phases. It is no double that it will improve the mechanical property compared with the simple CS, and the compressive strength of the n-HA/CS/CMC composite with a weight ratio of 70/15/15 could reach the highest because of the strongest cross-linking interaction of CS and CMC. Moreover, the IR and XRD analyses also showed that strong chemical interactions such as ionic bonds and hydrogen bonds were formed among the three phases, and the interaction mode was supposed in Fig. 4. Besides, burn-out test is a simple method to calculate the actual composition of organic/inorganic composites and determine the homogeneity of these composites. Here, we found that the n-HA particles dispersed uniformly in organic phase of CS and CMC, which is also profit to improve the mechanical and biological properties of bone repair materials. In addition, the co-solution for n-HA/CS/CMC composites can avoid high temperature and pressure, so the basic properties of the three components would still be retained in these composites. For example, we found n-HA still belonged to nanometer level after compounding. It is reported that natural bone apatite has a needle crystal size of φ5∼10 nm × 20∼50 nm [35], so we can conclude that the n-HA crystals here were very mimetic to bone apatite in composition, morphology and size, which is benefit to form bone bonding in osseous areas [36].
On the other hand, it is very essential to evaluate degradation and bioactivity of biomaterials [37]. As we known, the ion concentrations of SBF resembles that of human blood plasma, so it is a significant means to soak the composites in SBF to evaluate in vitro properties. From the rate of weight loss and water absorption, we can conclude that the n-HA/CS/CMC composites degraded in SBF with the soaking time. The rate of weight loss of the n-HA/CS/CMC composite with a weight ratio of 70/10/20 can reach 50.12% at 8 weeks. Obviously, comparing with the n-HA/CS composite with the weight ratio of 70/30 whose weight loss is about 12% at 8 weeks [38], the n-HA/CS/CMC composites can accelerate the degradation of specimens. The reason may be that CMC degrades quicker than CS. However, the rate of weight loss for the n-HA/CS/CMC composite with the weight ratio of 70/15/15 was the least among the three n-HA/CS/CMC composites, only 18.2% at 8 weeks. The fact suggested that the n-HA/CS/CMC composites had an adjustable degradation rate, which is useful to develop new materials for bone repair. Additionally, the degradation of n-HA/CS/CMC composites resulted in more micropores on the surface, which can hold more water. Meanwhile, more apatite particles deposited and covered these pores, so water absorption increased at initial weeks and then decreased. Similarly, the degradation rate of the n-HA/CS/CMC composite with weight ratio of 70/15/15 was almost equal to that of apatite particles deposited on the surface, therefore, its water absorption rate had little change tendency during soaking in SBF. Furthermore, it is a critical factor to have good bioactivity for the success of bone repair materials in general [39]. According to the SEM microphotograph, we can find that more apatite particles deposited on the surface at 8 weeks, suggesting that the n-HA/CS/CMC composites had high bioactivity, which might result from the bioactivity of the raw materials and no other poisonous agents present during the preparing process.
Conclusion
In the paper, three n-HA/CS/CMC composites with different weight ratios were fabricated by a co-solution method, and the properties were also investigated. Based on the above analyses and discussion, we can make a conclusion that a novel bone repair material can be obtained by incorporating CMC into n-HA/CS system. Not only did it compound uniformity by chemical interactions and resembled natural bone apatite in composition, morphology and size, but also it improved the compressive strength compared with n-HA/CS composite and had controllable degradation rate via adjusting the CS/CMC weight ratio. Importantly, the method of preparing and molding was simple without introducing any toxic agents, so as to retain good bioactivity in products. Therefore, we can infer that the n-HA/CS/CMC composites prepared in this paper would be a novel and desirable bionic bone repair material in view of its good mechanical property, adjustable biodegradation rate and high bioactivity, which showed a promising prospect in the field of biomedicine. Additionally, the study also provided a good guide to exploit clinical application of natural cellulose in future.
References
D. R. BISWAL and R. P. SINGH, Carbohydr. Polym. 57 (2004) 379
C. H. N. SIEGER, A. G. M. KROON, J. G. BATELAAN and C. G. Van GINKEL, Carbohydr. Polym. 27 (1995) 137
E. K. JUST and T. G. MAJEWICZ, Encyclopedia of Polymer Science and Technology, 2nd Ed., Vol. 3, Wiley, New York, 1989, pp 226
D. L. HE, L. L. BAO, Y. M. LONG, W. Z. WEI and S. Z. YAO, Talanta 50 (2000) 1267
J. W. BURNS, M. J. COLT, L. S. BURGESS and K. C. SKINNER, Eur. J. Surg. 577 (Suppl) (1997) 40
D. E. BECK, Eur. J. Surg. 577 (Suppl) (1997) 49
K. KURASHINA, H. KURITA, H. TAKEUCHI, M. HIRANO, C. KLEIN and K. De GROOT, Biomaterials 16 (1995) 119
W. SUCHANEK and M. YOSHIMURA, J. Mater. Res. 13 (1998) 94
T. J. WEBSTER, R. W. SIEGEL and R. BIZIOS, Biomaterials 21 (2000) 1803
L. G. GUTWEIN and T. J. WEBSTER, Biomaterials 18 (2004) 4175
W. TAN, R. KRISHNARAJ and T. A. DESAI, Tissue Eng. 2 (2001) 203
S. ITOH, M. KIKUCHI, K. TAKAKUDA, Y. KOYAMA, H. N. MATSUMOTO, S. ICHINOSE, J. TANAKA, T. KAWAUCHI and K. SHINOMIYA, J. Biomed. Mater. Res. Part A 54 (2001) 445
Y. WATANABLE, H. ERYU and K. MATSUURA, Acta Mater. 5 (2001) 775
Z. K. HONG, X. Y. QIU, S. JINGRU, M. X. DENG, X. S. CHEN and X. B. JING, Polymer 45 (2004) 6699
H. S. ITO, K. SHINOMYA and T. KAWAUCHI, J. Biomed. Mater. Res. 54 (2001) 445
M. HUANG, J. Q. FENG, J. X. WANG, X. D. ZHANG, Y. B. LI and Y. G. YAN, J. Mater. Sci. Mater. Med. 14 (2003) 655
M. KIKUCHI, S. ITIH, S. ICHINOSE, K. SHINOMIYA and J. TANAKA, Biomaterials 22 (2001) 1705
T. CHANDY and C. P. SHARMA, Biomater. Artif. Cells Artif. Organs 18 (1990) 1
R. MURUGAN and S. RAMAKRISHNA, Biomaterials 25 (2004) 3829
B. S. CHUNG , C. K. LEE , K. S. HONG, H. J. YOUN, H. S. RYU, S. S. CHUNG and K. W. PARK, Biomaterial 21 (2000) 1291
A. LAHIJI and A. SOHRABI, J. Biomed. Mate.r Res. 49 (2000) 301
I. ADEKOGBE and A. GHANEM, Biomaterials 26 (2005) 7241
R. MURUGAN and S. RAMAKRISHNA, Biomaterials 17 (2004) 3829
K. H. WON, C. JONTHAN and K. H. EE, J .Biomed. Mater.Res. 72 (2005) 136
L. J. KONG, Y. GAO, W. L. CAO., Y. D. GONG, N. M. ZHAO and X. F. ZHANG, J. Biomed. Mater. Res, Part A 75 (2005) 275
Y. F. MI, H. L. TAN and H. SUNG, Biomaterials 23 (2002) 181
A. MORITZ, M. GRIMM, E. EYBL, M. GRABENWOGER, F. GRABENWOGER, P. BOCK and E. WOLNER, Eur.J.Cardiothorac. Surg. 5 (1991) 155
F. J. SCHOEN, J. W. TSAO and R. J. LEVY, Am. J. Pathol. 123 (1986) 134
G. GOLOMB, F. J. SCHOEN, M. S. SMITH, J. LINDEN, M. NIXON and R. J. LEVY, Am. J. Pathol. 127 (1987) 122
E. EYBL, A. GRIESMACHER, M. GRIMM and E. WOLNOR, J. Biomed. Mater. Res. 23 (1989) 1355
W. ARGÜELLES-MONAL and C. PENICHE-COVAS, Makromol. Chem. Rapid Commun. 9 (1998) 693
H. FUKUDA and Y. KIKUCHI, Makromol. Chem. 180 (1979) 163
L. ZHANG, Y. B. LI, X. J. WANG, X. L. PENG, Y. ZUO and J. M. HAN, High Tech.Lett. 10 (2004) 48
T. KOKUBO, H. KUSHITANI, S. SAKKA, T. KITSUGI and T. YAMAMURO, J Biomed. Mater. Res. 24 (1990) 723
L. WU, Y. B. LI, Y. ZUO, L. ZHANG, W. H. YANG and Y. H. MU, Mater. Sci. Forum 938 (2006) 510
M. TANAHASHI, T. KOKUBO, T. NAKAMURA, Y. KATSURA and M. NAGANO, Biomaterials 17 (1996) 47
Q. Q. QIU, P. DUCHEYNE and P. S. AYYASWAMY, Biomaterials 52 (2000) 66
L. ZHANG, Y. B. LI, A. P. YANG, X. L. PENG, X. J. WANG and X. ZHANG, J. Mater. Sci. Mater. Med. 16 (2005) 213
M. MARCOLONGO, P. DUCHEYNE, J. GARINO and E. SCHEPERS, J. Mater. Sci. Mater. Med. 39 (1998) 61
Acknowledgement
This study was funded by China-Netherlands programme strategic alliances (China 973 found: 2004CB720604).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liuyun, J., Yubao, L., Li, Z. et al. Preparation and properties of a novel bone repair composite: nano-hydroxyapatite/chitosan/carboxymethyl cellulose. J Mater Sci: Mater Med 19, 981–987 (2008). https://doi.org/10.1007/s10856-007-3208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3208-1