Abstract
Gelatin, chitosan and hyaluronan with a weight ratio of 82.6%, 16.5% and 0.1% were chosen as a scaffold material to mimic the composition of natural cartilage matrix for cartilage tissue engineering. Water soluble carbodiimide was added into the biomacromolecule solution with a concentration of 5% to crosslink the complex. Following a freeze-drying procedure, a porous scaffold (control) was then prepared. To enhance chondrogenesis, heparin was covalently immobilized onto the scaffold by carbodiimide chemistry, through which basic fibroblast growth factor (bFGF) was further incorporated by a bioaffinity force. Incubation in phosphate buffered saline (PBS, pH 7.4) at 37 °C caused the weight loss of all kinds of the scaffolds, which could be brought by both the degradation and dissolution of the biomacromolecules. Compared with the control, however, the heparinized scaffold showed stronger ability to resist the weight loss, implying that a higher crosslinking degree was achieved by incorporation of the heparin. Rabbit auricular chondrocytes were seeded onto the ternary complex scaffold containing bFGF to assess cell response. Chondrocytes could adhere and proliferate in all kinds of the scaffold, regardless of the existence of bFGF. No significant difference on glycosaminoglycan (GAG) secretion was recorded between these scaffolds after cultured for 7 and 21 days too, although the absolute value from the Scaffold-heparin-bFGF was somewhat higher. However, chondrocytes seeded in the Scaffold-heparin-bFGF indeed showed significant higher viability than that on the control scaffold. These results reveal that the ternary complex scaffolds, in particular the one containing bFGF, are a potential candidate for cartilage tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural articular cartilage lacks blood supply to support its repair and remodeling. Minor injury to articular cartilage may lead to progressive damage and degeneration because of the limited capacity for spontaneous repair. Recently, tissue engineering involving the combination of cells, scaffold, and bioactive agents has emerged as a new method to repair cartilage defects and to restore cartilage function. In tissue engineering, scaffolds provide the initial structural support and retain cells in the defective area for cell growth, metabolism and matrix production [1], thus playing an important role during the development of engineered tissues [2]. The scaffolds are readily degradable when the cells secrete their own matrix and act as a delivery system for bioactive agents, such as cell growth factors [3].
Currently, many kinds of polymer materials, both synthetic and natural, are employed as the scaffolds for cartilage tissue engineering. The synthetic polymer-based biomaterials include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymers (PLGA) [4–8]. These synthetic materials have certain shortcomings such as hydrophobicity and lack of informational structure for cell attachment. They undergo degradation mainly through hydrolysis. Their degradation products, e.g. glycolic acid or/and lactic acid, are acidic and toxic to implanted cells, probably causing severe inflammation. By contrast, the natural biomaterials such as collagen, gelatin, chondroitin, chitosan, fibrin and hyaluronic acid [3, 9–18] exhibit multiple bioactivities because they are either the components of extracellular matrix (ECM) or originated from organisms, thus containing cell-specific domains such as RGD (Arg-Gly-Asp) sequence. They are also biosorbable or biodegradable. However, these materials often lose their shapes and sizes because of the rapid degradation upon contacting with body fluid or cell-culture medium.
An ideal cartilage scaffold should be able to provide a stable three-dimensional microenvironment and maximally mimic the natural environment of the cartilage matrix, both in terms of chemical composition and physical structure. Cartilage-specific ECM components such as type II collagen and glycosaminoglycans (GAGs) play a critical role in regulating the expression of chondrocyte phenotype and in supporting chondrogenesis both in vitro and in vivo. In hyaline cartilage, type II collagen, chondroitin sulfate and hyaluronan are the main ECM components, which occupy 15–20%, 5–10% and 0.05–0.25%, respectively [19]. Therefore, those scaffolds with an informational function for promoting cell adhesion, differentiation and proliferation are more promising [20].
In this study, gelatin, chitosan and hyaluronan are chosen to construct a ternary complex scaffold to mimic the main components of cartilage matrix. Heparin is grafted to the scaffold, through which basic fibroblast growth factor (bFGF) is further introduced by a bioaffinity interaction. The scaffold is expected to provide the necessary information for cell attachment to meet the requirements for cartilage tissue engineering.
Gelatin is a partial derivative of collagen, formed by breaking the natural triple-helix structure of collagen into single-strand molecules by hydrolysis. Gelatin is nonimmunogenic compared to its precursor and presumably retains informational signals such as RGD sequence, thus can promote cell adhesion, migration, differentiation and proliferation [3]. Chitosan, a partially deacetylated derivative of chitin, has glucosamine and N-acetylglucosamine in its molecule, thus is structurally similar to GAG. Chitosan and its degradative products are nontoxic in vivo. Chitosan has been shown a promising biomaterial because of its biocompatibility, biodegradability, low immunogenicity and cationic nature [13–15]. Since chitosan shows excellent cell supporting property, composites of chitosan and GAG or collagen are able to create suitable biomimetic microenvironment for cell implantation [10, 21–23]. Hyaluronic acid or hyaluronan (HA), is a water soluble polysaccharide that is widely distributed throughout the ECM of all connective tissues in human and other animals. It is a non-sulfated glycosaminoglycan consisting of multiple disaccharide units of glucuronic acid and N-acetylglucosamine. Hyaluronan plays an essential role in maintenance of the normal ECM and is a chondroinductive agent for modulating the pericellular matrix during embryonic cartilage development. As a consequence, hyaluronan and its derivatives have been widely explored as materials for cartilage tissue engineering [17–18].
To further enhance chondrogenesis, scaffold that can slowly release trophic growth factors is more promising in cartilage tissue regeneration. bFGF, an 18-kDa polypeptide, is a primary promoter of proliferation for a variety of cell types including chondrocyte. Binding of bFGF to heparin or heparan sulfate can facilitate the bFGF to maintain its biological activity, resulting in an increased resistance against thermal denaturation and enzymatic degradation [24–27].
Therefore, a gelatin/chitosan/hyaluronan ternary complex scaffold is fabricated by a freezing and lyophilizing technique. Chondrocyte culture is performed to determine the applicability of the ternary complex scaffold on cartilage tissue engineering.
Materials and methods
Materials
Chitosan (percentage of deacetylation 85%, Mη 6.2 × 105) and gelatin were obtained from Haidebei Bioengineering Co. and Shanghai Chemical Industries Co. Ltd. (China), respectively. Sodium hyaluronate, chondroitin sulfate, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDAC), heparin, Alcain blue 8GX and fluorescein diacetate (FDA) were purchased from Sigma. Recombinant bovine basic fibroblast growth factor (bFGF) was obtained from Changchun Changsheng Genetic Pharmaceutic Co. Ltd. (China). All other reagents and solvents were of analytical grade and used as received.
Preparation of the ternary complex scaffold
The scaffold was prepared by a freeze-drying technique. In brief, 0.5 g gelatin powder and 5 mg sodium hyaluronate were dissolved in 10 mL triple-distilled water. After agitation for 2 h at room temperature, 2 mL 5% (w/v) chitosan/0.5 M acetic acid solution was added. After continuous agitation for 1 h, the gelatin/chitosan/hyaluronan solution was crosslinked by 2 mL 1% EDAC solution for 5 min at 37 °C. The pH value of the solution was controlled at 5–6. The solution was then frozen at −20 °C for 2 h, and lyophilized at −50 °C for 48 h. The dried scaffold was cut into 6 × 6 × 3 mm pieces, which were further crosslinked by 0.3% EDAC at 37 °C for 1 h, either in the presence of heparin or not, then were lyophilized for 48 h.
Structure observation
The scaffold was dehydrated by treatment with a series of graded ethanol solutions (75%, 85%, 95% and 100%, each for 1 h), then placed in a vacuum oven at 50 °C overnight. The scaffold was cut into pieces with a razor blade before coating with a thin gold layer for scanning electron microscopy (SIRION 100, FEI) examination.
Quantification of grafted heparin
The amount of grafted heparin was quantified by Alcian blue examination. The heparinized scaffold was dissolved in 2 mL 1% papain solution supplemented with 0.09% EDTA and 0.04% cysteine and maintained at 37 °C for 24 h. 2 mL Alcain blue 8GX (about 1.4 mg/mL, dissolved in 0.1 mol/L HCl) was then added into the mixture. After 10 min, the absorbance at 490 nm was recorded by a microplate reader (Bio-Rad 550). The concentration was found by referring to a calibration curve constructed from known concentrations of heparin solutions.
PBS uptake
The uptake capacity of the gelatin/chitosan/hyaluronan scaffolds was determined by incubation in phosphate buffered saline (PBS, pH7.4) at 37 °C. A known weight (W 0) of the scaffold was placed in PBS for 1 h. The wet weight (W 1) of the scaffolds was determined immediately after the surface-adsorbed water was removed by filter papers. The PBS uptake ratio (P a) was thus calculated:
The value is expressed as mean ± standard deviation (n = 3).
Weight loss
The weight loss of the scaffolds was followed as a function of incubation time in PBS at 37 °C. The scaffold was taken from the PBS at a given time interval, washed with distilled water, lyophilized and weighted (W t). The weight loss ratio (P loss) was thus calculated:
Each experiment was repeated for three times, and the average value is expressed as mean ± standard deviation (n = 3).
Release of gelatin and polysaccharides
The stability of the ternary complex scaffold was evaluated by examining the release of gelatin and polysaccharides. After incubated in PBS at 37 °C for a given time, the remaining scaffold was taken from the medium and dried in a vacuum oven at 50 °C. Then it was completely hydrolyzed in 6 M HCl at 120 °C for 12 h. The amount of hydroxyproline released from the gelatin molecules was quantified by ultraviolet spectroscopy (UV-Probe 2550, Shimadzu) by referring to a calibration curve [28]. Taking into account the feeding amount of each component, the weight loss and the amount of gelatin in the remained scaffold, the release of gelatin and polysaccharides can be obtained.
Cell culture
Three samples were evaluated. A known weight of EDAC-crosslinked scaffold (control sample) and heparinized scaffold (heparinization time 60 min) were sterilized by 75% ethanol solution. After washed with PBS, they were treated in 900 U/mL bFGF solution at room temperature for 1 h to incorporate bFGF, obtaining the samples of Scaffold-bFGF and Scaffold-heparin-bFGF, respectively. Chondrocytes were isolated from rabbit ears and cultured as described previously [29]. Before cell seeding, all the scaffolds were placed on the bottoms of a 24-well tissue culture plate. The chondrocyte suspension with a cell density of 500 × 104 cells/mL was injected into the scaffolds. The cell-scaffold constructs were cultured statically in F-12 culture medium for a given time.
Cell viability was measured by MTT (3-(4,5-dimethyl) thiazol-2-yl-2,5-dimethyl tetrazolium bromide) assay. 200 μL MTT solution (5 mg/mL) was added into each well. After continuous culture for 5 h, the formed formazan pigment was dissolved by 800 μL dimethyl sulphoxide (DMSO). The absorbance at 490 nm was recorded by a microplate reader (Bio-Rad 550) in a 96-well TCPS containg 200 μL formazan/DMSO solution.
Chondrocytes in the scaffolds were observed under SEM (Stereoscan 260, Cambridge) after fixed with 2.5% glutaraldehyde at 4 °C for 24 h followed by graded ethanol dehydration and critical point drying, and under confocal laser scanning microscopy (CLSM, Bio-Rad Radiance 2100) after fluorescein diacetate (FDA) staining [30, 31]. FDA (no fluorescence) could penetrate through cell membranes and was hydrolyzed into fluorescein by viable cells, which was then excited at 488 nm.
The amount of GAG secreted by the cultured chondrocytes was determined by the specific binding of Alcian blue to GAG under low pH as described above in heparin quantification. The content of GAG secreted by the chondrocytes in the scaffold was quantified by referring to a calibration curve of chondroitin sulfate at the same conditions.
Statistical analysis
Experimental data were analyzed using ANOVA. Results were reported as mean ± standard deviation. The significant level was set as p < 0.05.
Results and discussion
Scaffold morphology
At the fabricating conditions, porous scaffold with an average pore diameter of 100 μm was obtained (Fig. 1a). Pores are evenly distributed through the whole scaffold. After grafting of heparin and incorporating of bFGF, the porous structure was preserved with an enlarged pore diameter (120 μm) (Fig. 2b). The porosity of both scaffolds is around 90%, which is slightly smaller than that of the theoretical value according to the solid content (∼95%). The slight enlargement of the average pores should be the result of rearrangement of the biomacromolecules during the hydration processes, as has been observed before [10]. This would mean that, in spite of the EDAC crosslinking, the biomacromolecules involved in the scaffolds still preserve some extent of mobility to reorganize their structures. An ideal scaffold should create a stable three-dimensional microenvironment and has enough large porosity for cell infiltration and differentiation. In spite of the minor change of the pore size, Fig. 1 shows that the overall porous structure of the scaffolds can be stably preserved even in wet state.
SEM photomicrographs of (a) the gelatin/chitosan/hyaluronan (82.6:16.5:0.1 wt) ternary complex scaffold showing a porous structure with a uniform pore size of about 100 μm, and (b) after immobilization of heparin showing a pore size of 120 μm. The total concentration of the biomacromolecules was controlled as 5%, and the EDAC amount was 3% of the biomacromolecules
Grafting of heparin on the scaffold
Since heparin can recognize and bind bFGF via a bioaffinity interaction, coupling of heparin onto the scaffold is beneficial of the stable conjugation of bFGF at mild conditions. This may in turn help to preserve the bioactivity of the cell growth factor. Figure 2 shows that at a constant feeding ratio of heparin/scaffold (5 mg/100 mg) the grafting efficiency of heparin increased gradually from 4% at 20 min to 23% at 60 min. After that, the grafting percentage increased very slowly. It is expected that the incorporated amount of bFGF in the scaffold should correlate with the amount of the grafted heparin. Therefore, the reaction time of 60 min was chosen for all the next studies.
Weight loss
Scaffold used for cartilage tissue engineering should be biocompatible and biodegradable. The degradation behavior of a scaffold has a crucial impact on the long-term performance of a cells/scaffold construct. Figure 3 presents the weight loss of the scaffolds in PBS at 37 °C as a function of time. Both scaffolds lost their weight steadily up to 14 days checked so far. However, the heparinized scaffold showed significantly slower losing rate than the control (p < 0.05 except for day 14). At day 14, their weight losses were about 23% and 30%, respectively. During this in vitro incubation process, the biomacromolecules can be either degraded into small pieces or simply released or the both. At present we cannot distinguish which factor dominates the weight loss.
The control scaffold is composed of 82.6% gelatin, 16.5% chitosan and 0.1% hyaluronan, while the heparinized scaffold contains another 1% heparin. These biomacromolecules can be classified into protein and polysaccharides. The weight losses should thus be contributed by both kinds of materials. Figure 4 shows that the gelatin in both the control and the heparinized scaffolds were released linearly along with the incubation time, while the polysaccharides were released linearly up to 9 days, then more rapidly at day 12. From the heparinized scaffold both the gelatin and the polysaccharides were released by a significant slow rate (p < 0.05 except for day 3), which is consistent with the overall weight losses shown in Fig. 3. Since more polysaccharides were released, the gelatin amount in the remained scaffolds at day 12 increased to 87.3%, with no statistical difference between both kinds of the scaffolds. All these results demonstrate that the heparinized scaffold has better stability than the control, which should be brought by incorporation of heparin. Heparin is full of carboxylic and sulphonate groups in its molecule, which is negatively charged at neutral pH. It can thus interact with other components, in particular the chitosan having plenty of amino groups, in the scaffold via both covalent bonding (between the –COOH and –NH2 groups) and electrostatic force. In this way it can be regarded as a crosslinker to stabilize the scaffold structure although its amount is very small.
PBS uptake
It is crucial that the nutrition can penetrate into the scaffold. Therefore, the ability of the scaffold to adsorb water is one of the important factors in determining its biological activity. Here we used PBS to evaluate the uptake ability (at 37 °C) to mimic the cell culture medium and conditions. Figure 5 shows that both the scaffolds could adsorb high amount of PBS initially, with values of 18 and 16 times for the control and the heparinized scaffolds, respectively. Along with the incubation, these values decreased rapidly to 11 at day 6, and then to nine at day 14. No significant difference (p > 0.05) between these two samples was found since day 2. The uptake ability depends on both the hydrophilicity and the three-dimensional structure of the scaffolds. In general, the uptake ability will be weakened at a higher cross-linking degree because of reduction of the hydrophilic groups. This is exactly the case at the very initial stage, since higher crosslinking degree for the heparinized scaffold is expected. Along with the leaching or degradation of the soluble and swollen components from the scaffolds, the overall crosslinking degree of the remained residues should be considerably improved. Meanwhile, the hydrophilicity should be decreased too. Moreover, it has been identified that the scaffolds may collapse their structures as a result of degradation to reduce the water-maintenance capability. Consequently, the uptake ability is decreased. This can be partly evidenced by the fact that the remained scaffolds gradually became harder and lost their elasticity. Nonetheless, the ultimate uptake ability was still high enough to sustain the necessary biological functions for nutrient transportation.
Chondrocyte response to the scaffolds
bFGF is a primary promoter of cell proliferation, which stimulates, inter alia, the proliferation and migration of chondrocytes. At a normal physiological pH and temperature, bFGF diffuses rapidly when delivered without stabilization, undergoes proteolysis, and consequently loses its bioactivity [20]. bFGF is known as heparin-binding growth factor because of its high affinity for heparin and heparan sulfate. Binding with heparin can protect it from proteolytic degradation and maintain a capacity of long-term biological functions. To evaluate the cell response to the heparinized scaffold loaded with bFGF (Scaffold-heparin-bFGF), in vitro chondrocyte culture was performed, using the ternary complex scaffold (control) and the Scaffold-bFGF as comparisons.
Figure 6 presents the SEM images of the scaffolds seeded with chondrocytes and cultured for 2 weeks. A number of chondrocytes on all the scaffolds can be identified as indicated by the arrows. The porous structure of the scaffolds was basically preserved too. After stained with FDA, the viable cells in the scaffolds were imaged by CLSM (Fig. 7). In all the scaffolds the chondrocytes spread well and homogenously distributed throughout the entire matrixes. A homogenous distribution of cells is always important for the development of engineered tissues. Though no significant difference in cell morphology could be concluded, one can still find that more cells were in round shape in the Scaffold-heparin-bFGF than others. The round shape of chondrocytes is an indicator of phenotype retention and is essential for matrix formation.
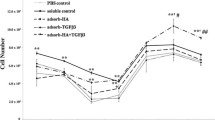
Measured by MTT, the cell viability in the Scaffold-heparin-bFGF was significantly higher (p < 0.05) than that of the control from day 3 to day 9, while no significant difference (p > 0.05) was found between the Scaffold-bFGF and the control except for day 6 (p < 0.05) (Fig. 8). At still longer culture time (12 days), all the scaffolds had no significant difference (p > 0.05). This has demonstrated that incorporation of bFGF through binding with heparin shows higher efficiency to promote chondrocyte proliferation, in particular at the initial culture period [25–27].
GAG is a kind of polysaccharides secreted by chondrocytes, together with collagen type II forming the main components of cartilage matrix. Secretion of GAG by the in vitro cultured chondrocytes can be regarded as a sign of maintenance of cell phenotype. Figure 9 shows that all the chondrocytes could normally secret abundant GAG when cultured for 7 and 21 days. At each culture interval, no significant difference (p > 0.05) was found between the three scaffolds, although the absolute values of the Scaffold-heparin-bFGF were somewhat larger. Compared with the values of 7 days, cells cultured for 21 days secreted significantly higher amount of GAG (p < 0.05) when seeded in the control and the Scaffold-bFGF, while no difference was found for cells seeded in the Scaffold-heparin-bFGF. These results prove that the bFGF incorporated by heparin binding is helpful to improve the cell viability, which is consistent with previous reports [32, 33]. However, the ability to enhance GAG secretion is not prominent, implying that the effect of bFGF on maintaining chondrocytic phenotype is rather limited. It has to mention that the amount of synthesized matrix was not enough to form a cartilage analogue yet. Further studies are underway to culture the construct for a longer period and culture in vivo.
Conclusion
A porous scaffold (control) composed of gelatin/chitosan/hyaluronan ternary complex, with a structure mimicking the composition and microenvironment of natural cartilage matrix, has been fabricated by a freeze-drying technique. After further covalent immobilization of heparin with EDAC chemistry, bFGF is finally incorporated into the scaffold through bioaffinity with heparin (Scaffold-heparin-bFGF). Weight losses of both the control and the heparinized scaffold occurs when incubated in PBS at 37 °C, showing almost linear increase along with the time. Both degradation and dissolution of the components may contribute to the weight loss. Nonetheless, the heparinized scaffold always displays a smaller weight loss, demonstrating the relatively stable structure brought by the heparin incorporation. Moreover, the PBS uptake ability is still preserved at a high enough level, although it decreases as a function of incubation time. In vitro cell culture finds that the chondrocytes can evenly distribute throughout all kinds of the scaffolds and secrete new GAG. Though no significant difference has been found with respect to GAG secretion, chondrocytes seeded in the Scaffold-heparin-bFGF actually show significant higher viability during a culture period from 3 to 9 days. Together with its biocompatibility and biodegradability, the scaffold can be a good candidate for cartilage tissue engineering. Further studies are underway to culture the cells/scaffold construct for a longer period and to explore the physical and mechanical properties of the engineered cartilage thereby.
References
L. A. SOLCHAGA, V. M. GOLDBERG and A. I. CAPLAN, Clin. Orthop. 391S (2001) 161
E. B. HUNZIKER, Osteoarthritis Cartilage 10 (2002) 432
C. H. CHANG, H. C. LIU, C. C. LIN et al. Biomaterials 24 (2003) 4853
Z. W. MA, C. Y. GAO, J. JI and C. SHEN, Euro. Polym. J. 38 (2002) 2279
Z. W. MA, C. Y. GAO, Y. H. GONG and J. C. SHEN, Biomaterials 26 (2005) 1253
Y. CAO, J. P. VACANTI, K. T. PAIGE et al. Plast. Reconstr. Surg. 100 (1997) 297
L. E. FREED, J. C. MARQUIS, A. NOHRIA et al. J. Biomed. Mater. Res. 27 (1993) 11
L. LU, G. N. STAMATAS and A. G. MIKOS, J. Biomed. Mater. Res. 50 (2000) 440
C. Y. GAO, D. Y. WANG and J. C. SHEN, Polym. Adv. Technol. 14 (2003) 373
L. MA, C. Y. GAO, Z. W. MAO, J. ZHOU and J. C. SHEN, Biomaterials 25 (2004) 2997
J. L. C. van SUNSANTE, J. PIEPER, P. BUMA et al. Biomaterials 22 (2001) 2359
T. USHIDA, K. FURUKAWA, K. TOITA and T. TATEISHI, Cell Transplant 11 (2002) 489
W. Y. XIA, W. LIU, L. CUI et al. J. Biomed. Mater. Res. Part B: Appl. Biomater. 71B (2004) 373
S. E. KIM, J. H. PARK, Y. W. CHO and H. CHUNG, J. Control. Rel.. 91 (2003) 365
J. K. F. SUH and H. W. T. MATTHEW, Biomaterials, 21 (2000) 2589
C. PERKA, U. ARNOLD, R. S. SPITZER and K. LINDENHAYN, Tissue Eng. 7 (2001) 359
G. LISIGNOLI, S. CRISTINO, A. PIACENTINI and S. TONEGUZZI, Biomaterials 26 (2005) 5677
M. RADICE, P. BRUN, R. CORTIVO et al. J. Biomed. Mater. Res. 50 (2000) 101
A. MAROUDAS, Physiochemical properties of articular cartilage in adult articular cartilage, 1st edn (Pitman Medical Publishing Co., Kent, UK 1979) 215
E. RUOSLAHTI and M. D. PIERSCHBACHER, Cell 44 (1986) 517
L. MA, C. Y. GAO, Z. W. MAO et al. Biomaterials 24 (2003) 4833
L. MA, C. Y. GAO, Z. W. MAO et al. J. Biomater. Sci.: Polymer Edition 14 (2003) 861
S. T. BOYCE, D. J. CHRISTIANSON and J. F. HANSBROUGH, J. Biomed. Master. Res. 22 (1988) 939
S. S. CAI, Y. C. LIU, X. Z. SHU and D. GLENN, Biomaterials 26 (2005) 6054
M. J. B. WISSINK, R. BEERNINK, A. A. POOT et al. J. Control. Rel. 64 (2000) 103
M. MARIA, L. FOTINI and K. K. NIKOS, J. Pharm. Biomed. Analy. 32 (2003) 823
E. S. SAKIYAMA and J. HUBBELL, J. Control. Rel. 65 (2000) 389
D. T. CHEUNG, N. PERELMAN, E. C. KO and M. E. NIMNI, Connect Tissue Res. 13 (1985) 109
Z. W. MA, C. Y. GAO, Y. H. GONG, J. JI and J. C. SHEN, J. Biomed. Mater. Res. Part B: Appl. Biomater. 63 (2002) 838
S. BANCEL and W. S. HU, Biotechnol. Prog. 12 (1996) 398
Y. HONG, C. Y. GAO, Y. XIE, Y. H. GONG and J. C. SHEN, Biomaterials 26 (2005) 6305
M. MATSUSAKI and M. OCHI, Gen. Pharmac. 31 (1998) 759
T. FUJISATO and T. SAJIKI, Biomaterials 17 (1996) 155
Acknowledgements
This study is financially supported by the the Major State Basic Research Program of China (2005CB623902), the Natural Science Foundation of China (No. 20434030) and the National Science Fund for Distinguished Young Scholars of China (No. 50425311).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, H., Gong, Y., Lao, L. et al. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. J Mater Sci: Mater Med 18, 1961–1968 (2007). https://doi.org/10.1007/s10856-007-3095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3095-5