Abstract
Various zeolites were kept in simulated body fluid (SBF) for different periods of time. Possible changes that may occur in the crystalline structures of zeolites and the chemical composition of SBF were determined by various analysis techniques after this treatment. The possible effects of two different zeolites on the morphology and viability of chronic myelogeneous leukemia and swiss albino fibroblast culture cells were also investigated. It was determined that when different types of zeolites were kept in the SBF for up to 14 days, their crystal structures were not affected. Observable amounts of Si were detected in the SBF samples after their treatment with all the zeolites investigated. Another variation in the chemical composition of SBF, worth to mention, was the increase of about 10% in its K content after the treatment carried out by using clinoptilolite. The zeolites KA and silicalite, which allowed the lowest and highest amount of silicon transfer into the SBF, respectively, were observed not to have any significant biological effect on the two different cell generations investigated under the conditions used in this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zeolites are hydrated microporous crystalline aluminosilicates that may occur naturally or be synthesized in laboratory conditions. The zeolite framework consists of an assemblage of SiO4 and AlO4 tetrahedra, joined together in various regular arrangements through shared oxygen atoms, to form an open crystal lattice. The micropore structure is determined by the crystal lattice, which contains pores of molecular dimensions into which guest molecules can penetrate. The unique properties of zeolites, such as ion-exchange, discrimination between molecules of different size and shape, existence of strong acidic sites and active hosts for metal catalyzed reactions, enable them to be used in diverse processes such as ion exchange, adsorption and catalysis. It seems inevitable that applications related to medicine may also benefit from all these favorable properties.
The use of zeolites in medicine is a relatively recent subject of interest that is emerging at a sound pace. Especially, the natural zeolite clinoptilolite has been the main subject of interest for these applications due to its abundance and the variety of the ions in its structure. The inclusion of zeolite into animal diets has proven to be useful against the toxic effects of aflatoxins [1] and mycotoxins [2]. Zeolites may be used as antibacterial agents [3] when especially Ag is incorporated into these materials by ion-exchange. The encapsulation of different ions and molecules in the zeolite and then the slow release of these materials provide the opportunity of zeolites to be used in drug complexation. Zeolite Y has been shown to be suitable for the slow release of some anthelmintics [4]. Zeolites may also be utilized as biosensors. Some types of zeolites may coimmobilize enzyme and mediator for preparing a biosensor [5]. The prevention of diarrhea seems possible by using drugs based on clinoptilolite [6]. Additionally, improvements have been reported after treating various types of tumors with clinoptilolite [7]. One study has been performed to determine the effects of clinoptilolite on serum chemistry and hematopoiesis in mice [8]. Silicon has been suggested to play a physiological role in the bone calcification process. Owing to the significant Si content of zeolites, these materials may be useful in enhancing bone health. Zeolite A has been shown to induce osteoblast proliferation in vitro [9]. The bioavailability of silicon and aluminum from zeolite A in dogs has also been investigated [10].
In spite of the increased amount of research on potential applications of zeolites in medicine, the investigations carried out to determine the biologic effects of these materials are still insufficient. In the scope of this study, firstly, experiments were carried out to determine the effects of various zeolite types on the chemical composition of simulated body fluid (SBF), which contained inorganic elements similar to those present in human serum. The maintenance of the crystalline structure of the zeolites kept in SBF was also checked. The second part of the study involved the determination of possible effects of zeolites on the morphology and viability of two different kinds of cells, namely, chronic myelogeneous leukemia and swiss albino fibroblast.
Materials and methods
Five different types of zeolites, namely, clinoptilolite, silicalite, KA, NaX and SAPO-11, were utilized in the first part of the experiments carried out to determine the effects of these materials on the chemical composition of SBF. The natural zeolite clinoptilolite originated from a deposit near Bigadiç, Turkey. The ground zeolite particles were sieved to have a particle size smaller than 20 μm. The chemical composition of clinoptilolite mainly consisted of (in wt%) 66.17 SiO2: 9.72 Al2O3: 0.134 Na+: 1.50 K+: 2.51 Ca2+: 0.61 Mg2+: 3.19 Fe3+: 14.47 H2O. Commercial (Aldrich) silicalite, NaX and NaA zeolite samples were used in the experiments. The NaA zeolite was repeatedly ion-exchanged to obtain its potassium form KA (about 80% K, 20% Na). SAPO-11, a silicoaluminophosphate, containing phosphorus in addition to the silicon and aluminum of a classical type of zeolite, was obtained according to a synthesis method in the literature [11].
The SBF was prepared by taking into consideration the inorganic contents of human plasma. The composition of SBF employed was (in mmol/L): 142 Na: 5 K: 2.5 Ca: 1.5 Mg: 147.8 C: 4.2 HCO3: 1 HPO4: 0.5 SO4. In the experiments, the zeolites were stored in SBF for different periods of time ranging between 1 and 24 days in an oven kept at 37 °C. The zeolite samples were saturated in a controlled humidity atmosphere (20% NH4Cl) prior to the experiments. Two different SBF volume/zeolite mass ratios, namely, 3.0 mL/mg and 0.27 mL/mg, were used in these experiments. A membrane filter was used for separating the solid zeolite from the solution phase after the finalization of the experiments. The solid phase obtained after filtration, contained particles larger than 100 nm. The solution phase remaining after the removal of the solid zeolite was analyzed by using different techniques. The Na and K contents were measured by a flame photometer (Corning-EEL) while the Ca and P contents were determined by a spectral photometer (Dr. Lange LP 300). The Si and Al contents of the treated SBF samples were determined by using atomic absorption spectroscopy (Perkin-Elmer 1100B), the detection range of which is expressed in ppm. Repeated measurements were performed and the average error in the analyses was determined to be equal to 3.4%. The phase identification and purity of the solid zeolite samples treated with SBF were accomplished by X-ray diffraction (XRD) analyses. XRD (Shimadzu XRD-6000Model) was run with Cu Kα1 radiation in the 2θ range of 0°–70°. Scanning electron microscopy (SEM) analysis (JEOL 5410) was applied for the determination of the crystal morphology of zeolites before and after treatment with SBF.
In the second part of this study, the possible effects of two types of zeolites on the viability and morphology of culture cells were investigated. In this section, K562 (Chronic myelogeneous leukemia) and 3T3 (swiss albino fibroblast) cells were cultured in DMEM supplemented with 10% FCS, 100 IU/mL penicillin and streptomycin and kept in a controlled atmosphere (5% CO2) incubator at 37 °C. Approximately, 105 cells were plated per well in 6-well plates in 1 mL medium.
1 mM solutions of zeolites KA and silicalite in SBF were prepared. The zeolite particles precipitated at the bottom of the solution. Before the experiments, the solutions were shaken to obtain a relatively homogeneous distribution of the particles, and were then introduced into an environment in which the cells were plated 24 h earlier, in a manner to obtain different concentrations of 100, 0.5 and 0 μM. Supports of nitrocellulose membranes (Sigma Catalog No: Z35, 308-6) were placed in the wells to prevent the zeolite particles from hindering the imaging and counting of the cells. The cells treated in this manner with the zeolites KA and silicalite, were monitored microscopically for 3 days.
The degree of the cell viability was determined by staining the cells with trypan blue. At the end of the zeolite treatment period, cells were harvested and washed twice with PBS (Phosphate Buffered Saline). The cell pellets were resuspended in 0.25 mL DMEM plus 0.05 mL of 1.2% trypan blue in 140 mM NaCl. Stained cells were observed 5 min after the addition of trypan blue by phase-contrast in an inverted light microscope at ×100 magnification. Two fields of 100 cells were counted in cell preparates. The degree of viability is the mean percentage of unstained cells. The experiments were repeated three times.
Results and discussion
Structural analysis of zeolites kept in SBF
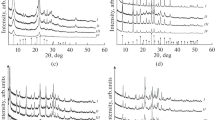
The XRD and SEM analyses showed that the crystalline structures of zeolites KA, NaX, SAPO-11, clinoptilolite and silicalite remained unaffected after their treatment with SBF in the time range investigated (up to 14 days at 37 °C). A SBF volume/zeolite mass ratio of 0.27 mL/mg was used for these samples. No phase other than the original zeolite could be observed in the samples according to the XRD analyses. The X-ray diffractograms of the zeolite KA samples kept in SBF were also investigated as a function of the treatment time and the results were compared to the diffractogram of the original zeolite KA. The X-ray diffractograms of the original KA zeolite and those pertaining to KA kept in SBF for 1, 4 and 14 days may be seen in Fig. 1. It may be observed that no loss in crystallinity occurred for the zeolite KA samples kept in SBF for different periods of time. In other words, no indication of a partial collapse of the zeolite A structure was present. The SEM analyses exhibited that there was no significant change in the morphology of the zeolite crystals after their treatment with SBF. Figure 2a and b represent the SEM pictures of the original zeolite KA crystals and the pictures of KA crystals kept in SBF for 14 days, respectively. It may be observed from the SEM pictures that the characteristic cubic morphology of zeolite A crystals still prevailed after their treatment with SBF.
Chemical analysis of the effects of zeolites on SBF
The Na, K, Ca, P, Si and Al contents of the SBF samples treated with different types of zeolites were measured. In general, only small changes, which were in the range of experimental error, and that could also be related to the adsorption of some water molecules in the zeolites, were observed in the concentrations of Na, K, Ca and P in SBF. The only exception seemed to be the increase of about 10% in the K concentration of the original SBF when clinoptilolite was used for the treatment.
The most interesting observation was the detection of notable amounts of silicon in the SBF samples after their treatment with different types of zeolites. The amount of silicon in the samples depended on the type of zeolite used for treatment. Meanwhile, it was observed that for the use of all the zeolites investigated, no detectable amount of Al was transferred into SBF. Table 1 shows the Si content of SBF after its treatment with different zeolites for 14 days. The ratios of the amounts of Si detected in solution to the Si contents of the original zeolites were also indicated in the table in parentheses (in %). Two different SBF volume/zeolite mass ratios were utilized.
It may be observed from the table that the highest amount of Si loss by the zeolites was obtained for silicalite. This may be partly explained by silicalite having the highest Si/Al ratio, as mentioned before. Si loss was lowest for KA zeolite. The Si/Al ratio of this zeolite is equal to 1, the lowest possible Si/Al ratio that zeolites can have. Actually, the amount of Si lost by the zeolites may still be regarded to be moderate when compared to the original Si contents of the zeolites, except for SAPO-11, which lost about a quarter of its original Si atoms. Due to the relatively small amount of silicon in SAPO-11, the amount of Si transferred into SBF remained smaller in magnitude when compared to the amount lost by silicalite. The lowest ratio of the amount of Si lost by the zeolite to the total amount of Si in the original zeolite was obtained for clinoptilolite, followed close by zeolite KA. Clinoptilolite probably owes its stability to the fact that it is a natural zeolite.
An interesting observation was that Si atoms were transferred from the zeolites into the SBF while Al atoms did not act likewise. A possible explanation may be that a very small part of the zeolitic structure was lost. It is difficult to detect such a small amount of structure collapse from XRD analyses. The Si atoms may have been transferred into the solution while Al atoms in the related part of the zeolite framework became extraframework atoms.
Figure 3 shows the variation of the Si content of SBF, treated with zeolite KA, with respect to the treatment time. The SBF volume/zeolite mass ratio used in these experiments was equal to 0.27 mL/mg. It may be observed that during the first day of experiment, almost no Si was transferred into SBF. Significant amounts of Si transfer occurred between the second and fourth days, after which only very small enhancements could be observed in the Si content of treated SBF. These results give an indication for the time range during which Si transfer occurs from the zeolite to SBF.
Analysis of the effects of zeolites on culture cells
The first step in this part of the study was the selection of the zeolites to be used in the investigations carried out to determine the possible effects of these materials on cell morphology and viability. Zeolites KA and silicalite have quite different characteristics that may be regarded to be on opposite ends. For example, KA is hydrophilic and has a low Si/Al ratio, while silicalite is hydrophobic and has a very high Si/Al ratio. The results of the chemical analyses showed that the use of zeolites A and silicalite allowed the lowest and highest amount of silicon transfer into SBF, respectively. Thus, the zeolites KA and silicalite were employed in this part of the study. The possible effects of these zeolites on K562 (chronic myelogeneous leukemia) and 3T3 (swiss albino fibroblast) culture cells were investigated.
As a result of the microscopic observations performed, the morphologies of K562 and 3T3 culture cells were determined to remain unaffected from the presence of zeolite-treated SBF in the growth environment. There was no disturbance in the integrity of the whole membrane. A swelling or shrinkage of the membrane was not observed, either. The cells were randomly distributed and did not form discrete colonies. The pictures of the 3T3 cells kept in SBF and in SBF treated with silicalite are given in Fig. 4a and b, respectively. The pictures of the K562 cells kept in SBF and in SBF treated with silicalite are shown in Fig. 5a and b, respectively. Similar results were obtained for the set of experiments utilizing SBF treated with zeolite KA. The effects of the SBF samples treated with zeolites KA and silicalite on the viability of K562 and 3T3 cells may be seen in Tables 2 and 3, respectively. No significant difference could be observed between the treated and control cell samples, regarding the viability of the cells. The SBF samples treated with the zeolites KA and silicalite did not affect the cell division in any (+ or −) direction. The number of the cultured cells, which was equal to 105 per mL at the beginning of the experiments, increased to about 7 × 105 both for the treated and control samples after 4 days.
In various studies, it has been reported that zeolites A and clinoptilolite may exhibit significant effects on the properties and growth of various cells [7, 9]. The contradicting results obtained in this study may indicate that these effects are strongly dependent on the experimental conditions used. Different types of zeolites may have very different characteristics and it does not seem very reasonable to expect that all zeolites should exhibit the same kind of effect on every type of cell. It should also be mentioned that it is important to know the reason, means and conditions that zeolites come into contact with live organisms for being able to give a definite decision about the effects of these materials. As an example, the highest zeolite concentration for the SBF utilized in our experiments was equal to 100 μM. The possible effects of using higher concentrations may also be investigated.
Conclusions
The crystal structures of the zeolites clinoptilolite, silicalite, KA, NaX and SAPO-11 were not affected from their treatment with SBF. These zeolites may be potentially useful for the controlled release of antibiotics, especially for the local treatment of osteomyelitis. Notable amount of silicon was detected in SBF after its treatment with all the zeolites investigated in this study. The amount of silicon transferred into SBF depended on the Si/Al ratio of the zeolite. In case this behavior also holds true when human serum is used, it may be possible to use zeolites orally to improve bone health. It was determined that zeolites KA and silicalite were not toxic and did not affect the morphology and viability of the chronic myelogeneous leukemia and swiss albino fibroblast culture cells. It should be reminded that the effects of zeolites on cells might depend strongly on the experimental conditions used. In the second part of our research, the effects of implanting zeolite in animal bones and the ability of these materials to influence osteoblast cells will be investigated in-vivo. These experiments are expected to illuminate the usefullness of these materials in orthopedics.
References
S. S. PARLAT, A. O. YILDIZ and H. OGUZ, Br. Poultry J. Sci. 40 (1999) 495
S. C. KYRIAKIS, D. S. PAPAIOANNOU, C. ALEXOPOULOS, Z. POLIZOPOULOU, E. D. TZIKA and C. S. KYRIAKIS, Microporous Mesoporous Mater. 51 (2002) 65
M. HOTTA, H. NAKAYIMA, K. YAMAMOTO and M. AONO, J. Oral Rehab. 25 (1998) 485
A. DYER, S. MORGAN, P. WELLS and C. J. WILLIAMS, J. Helmintol. 74 (2000) 137
B. LIU, F. YAN, J. KONG and J. DENG, Anal. Chim. Acta 386 (1999) 31
G. RODRIGUEZ-FUENTES, M. A. BARRIOS, A. IRAZIOZ, I. PERDOMO and B. CEDRE, Zeolites 19 (1997) 441
K. PAVELIC, M. HADZIJA, L. BEDRICA, J. PAVELIC, I. DIKIC, M. KATIC, M. KRALJ, M. H. BOSNAR, S. KAPITANOVIC, M. POLJAK-BLAZI, S. KRIZANAC, R. STOJKOVIC, M. JURIN, B. SUBOTIC and M. COLIC, J. Mol. Med. 78 (2001) 708
I. MARTIN-KLEINER, Z. FLEGAR-MESTRIC, R. ZADRO, D. BRELJAK, S. S. JANDA, R. STOJKOVIC, M. MARUSIC, M. RADACIC and M. BORANIC, Food Chem. Toxic. 39 (2001) 717
P. E. KEETING, M. J. OURSLER, K. E. WIEGAND, S. K. BOUDE, T. C. SPELSBERG and B. L. RIGGS, J. Bone Miner. Res. 7 (1992) 1281
E. A. CEFALI, J. C. NOLAN, W. R. MCCONNELL and D. L. WALTERS, Int. J. Pharm. 127 (1996) 147
S. T. Wilson, S. Oak, B. M. Lok and E. M. Flanigen, US Patent, 4,310,440 (1982)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceyhan, T., Tatlier, M. & Akçakaya, H. In vitro evaluation of the use of zeolites as biomaterials: effects on simulated body fluid and two types of cells. J Mater Sci: Mater Med 18, 1557–1562 (2007). https://doi.org/10.1007/s10856-007-3049-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3049-y