Abstract
Films and sponges of chitosan (CHI), chitosan/hyaluronic acid (CHI–HA) and chitosan/chondroitin sulphate (CHI–CHOS), were prepared by film deposition or lyophilization (sponges), avoiding the formation of interpolyelectrolyte complexes. The biological behaviour of the systems was analysed by studying the cell behaviour using a fibroblast cell line and standard biological MTT and Alamar Blue tests. The morphology of films, sponges and cell seeded samples was analysed by ESEM. The results obtained indicate that all the systems can be considered as good supports for cell adhesion and proliferation, but there is specific activation of the proliferative process in the presence of hyaluronic acid and chondroitin sulphate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
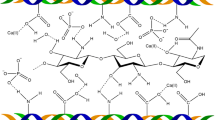
Chitosan (CHI) [(1→4)-2-amino-2-deoxy-β-d-glucan] is a cationic linear polysaccharide (Fig. 1a) obtained by extensive deacetylation of chitin, a structural polysaccharide commonly found in nature [1]. It is a biocompatible, biodegradable, non-toxic and mucoadhesive polymer, which makes it attractive for applications in medicine and pharmacy [2–4]. It has been established that CHI could achieve haemostasis and allow the promotion of normal tissue regeneration [5, 6]. For this reasons CHI has attracted attention for its potential use as scaffold in tissue engineering.
Glycosaminoglycans (GAGs) are anionic polysaccharides (chondroitin 4-sulphate, chondroitin 6-sulphate, dermatan sulphate, keratan-sulphate, heparan sulphate and hyaluronic acid) which are natural components of the extracellular matrix [7].
Hyaluronic acid (HA) (Fig. 1b) is present in all soft tissues of higher organisms and in particularly high concentrations in the synovial fluid and vitreous humor of the eye. It plays a vital role in many biological processes such as tissue hydration, proteoglycan organization, cell differentiation, angiogenesis, and acts as a protective coating around the cell membrane [8, 9]. It is suspected of playing a vital role in cell motility and cell–cell interactions [10, 11].
Chondroitin 4-sulphate (Fig. 1c) and chondroitin 6-sulphate are the most abundant mucopolysaccharides in the body and occur both in skeletal and soft connective tissue. Chondroitin sulphate has shown in vivo anti-inflammatory properties in animal models and in vitro regulation of chondrocyte metabolism, such as stimulation of proteoglycan and collagen synthesis, and inhibition of the production of cytokines involved in cartilage degradation [12].
The structural similarities between CHI, chondroitin 4-sulphate (CHOS) and hyaluronate (HA) can be appreciated in Fig. 1. Together with these GAGs, CHI has been used as a mayor component in the preparation of biodegradable scaffolds in tissue engineering. The general purpose has been to attain a synergistic effect by combining these biopolymers. Considering that the composition and the preparation method could control (to some extent) the swelling, mechanical properties as well as the biodegradation rate of the scaffold, which can be tailored to mimic the properties of native tissue.
In this sense, Lee et al. [13] have considered the wound-healing characteristics of polyelectrolyte complex sponges composed of hyaluronic acid and CHI with or without an antimicrobial agent (silver sulfadiazine) by their application to a full-skin defect of a Wistar rat in vivo. Histological studies confirmed a greater proliferation of fibroblasts in the wound bed and a distinct reduction in infectious agents as compared with the traditional gauze. More recently CHI–gelatin-hyaluronic acid scaffolds have also been proposed as skin substitutes [14].
The biological properties of polyelectrolyte complexes PECs of CHI with hyaluronic acid or chondroitin sulphate were studied by Denueziere et al. [15]. All materials proved to be cytocompatible, but best results were always obtained with pure CHI. In other words, they didn’t find the expected synergy when using the complexes. This was explained by taking into account that complex formation removes the individual charges of the polymers, modifying to some extent the chemical structure necessary for cell recognition. In vitro experiments revealed a superior cell adhesion for CHI and PEC materials in comparison with that of the blank. However, cell proliferation was lower than the blank, even for pure CHI.
In the present paper we report the preparation of novel chitosan-hyaluronic acid and chitosan-chondroitin sulphate films and sponges. These materials were prepared avoiding complexation between the polyelectrolytes, in order to preserve their functionality for cell recognition. The absence of PEC formation was assessed by FT-IR spectroscopy. The cytotoxity of films and sponges was assessed, and cell adhesion and proliferation tests were carried out on the films. The performance of these materials was compared with that of pure CHI films and sponges.
Experimental
Materials
CHI from Paralomis granulosa (Deacetylation Degree: 85%; intrinsic viscosity: 4.57 dL/g in 0.1 M Acetic acid + 0.2 M NaCl at 25 °C; ashes: 3%; purity: 97%; heavy metals: not detected) was a gift from IDEBIO S.L. It was purified as follows: CHI was dissolved in a 2% acetic acid aqueous solution until a homogeneous 1% CHI solution was attained. The solution was filtered to remove any insoluble particles and neutralized to pH 8.0 with a 10% NaOH solution. The precipitated CHI gel obtained was washed several times with deionised distilled water and lyophilized to produce porous CHI sponges. Chondroitin sulphate (CHOS) (JP, CODE: F001101, 0/0006) and sodium hyaluronate (HA) (LMW, CODE: F019701, 3/0001) were kindly supplied by BIOIBERICA. Both were oral grade samples and were used without further purification. Thermanox (TMX) control discs were supplied by Labclinics S. L. and plasticware by Sarstedt. Tissue culture media, additives, tripsin, phosphate buffered saline (PBS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were supplied by Sigma. Alamar Blue (AB) was supplied by SEROTEC. All other chemicals; reagents and solvents were analytical grade.
Preparation of films
CHI films were prepared by depositing four drops of a 2% CHI solution in 1% acetic acid, over small glass disks of 10 mm diameter and 1 mm thickness, and leaving them to evaporate at room temperature. The films were neutralized by addition of 3–4 drops of 10% NH4OH solution in methanol. After drying the addition of NH4OH solution was repeated. Afterwards the films were thoroughly washed with a 70/30 (v/v) ethanol:water mixture and left to dry at room temperature. Sixty films were prepared for all the cell experiments.
CHI–CHOS films were made as follows: a 0.4% CHOS solution in a 70/30 (v/v) ethanol:water mixture was prepared by dissolving CHOS in water first (0.04 g of CHOS in 3 mL of water) and adding ethanol to complete 10 mL solution (no precipitation of the solution was observed). CHI–CHOS films were formed by adding six drops of the freshly made 0.4% CHOS solution to CHI films (n = 20) prepared according to the last paragraph and leaving the solution to evaporate.
CHI–HA films were obtained by adding six drops of a freshly made 0.4% HA solution in a 70/30 (v/v) ethanol:water mixture -prepared in the same way as the 0.4% CHOS solution- to CHI films and leaving the solution to evaporate. 20 films of CHI-HA films were prepared for all the experiments.
Preparation of sponges
CHI sponges were prepared by direct lyophilization of solutions. The sponges were deposited in a 24 wells plate and kept for biological studies.
Chitosan-chondroitin sulphate sponges were prepared by dropping a freshly made 0.4% CHOS solution in a 70/30 (v/v) ethanol:water mixture over CHI sponges placed in a 24 wells plate. The sponges were left to dry at room temperature.
Chitosan-hyaluronic acid sponges were prepared as follows: 50 mL of 1% CHI solution in aqueous HCl (pH 5.5) were added to 50 mL of 1% HA aqueous solution with magnetic stirring. A precipitate of the polyelectrolyte complex was formed and stirring was kept for 30 min. The precipitated complex was separated from the solution by centrifugation for 30 min at 6000 rpm. The precipitate was dissolved by adding 15 mL of 0.66 M NaCl and two drops of 2 N HCl giving rise to a viscous acid solution (pH ∼ 1). The solution was lyophilized. The lyophilizate was freed from NaCl by three successive 30 min immersions in 70:30 ethanol/water mixtures. Afterwards it was immersed in 10% NH4OH methanolic solution for 20 min, washed with a 70:30 ethanol/water mixture and lyophilized. Samples of the sponge obtained were placed in a 24 wells plate.
The morphology of films and sponges was determined by Environmental Scanning Electron Microscopy ESEM, with a XL30 Philips equipment by direct analysis of the surface at the magnification indicated in the corresponding figures.
Cytotoxicity test
Specimens and in vitro cell culture for biocompatibility experiments
The discs with CHI, CHI–CHOS and CHI–HA films were used for direct and indirect biocompatibility experiments. All specimens were sterilised with poly(ethylene oxide). The negative control was tissue culture plastic, Thermanox, an international standard, and the positive control (toxic agent) Triton-0.5%. The experiments were carried out using fibroblasts obtained from human embryonic lung and supplied by the cell culture laboratory of the Avila’s Provincial Hospital (Spain). Cells were cultured at 37 °C and 5% CO2. The culture medium was minimal essential medium eagle (MEM), modified with HEPES (Sigma) and supplemented with 10% fetal bovine serum (FBS), 200 mM l-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin. The culture medium was changed at selected time intervals with care to cause little disturbance to culture conditions.
MTT assay
TMX, CHI, CHI-CHOS and CHI-HA films and sponges were set up in 5 mL of MEM. They were placed on a roller mixer at 37 °C and the medium was removed at different time periods (1, 2 and 7 days) and replaced with other 5 mL of fresh medium. All the extracts were obtained under sterile conditions. Fibroblasts cells were seeded at a density of 11 × 104 cells/mL in complete medium in a sterile 96-well culture plate and incubated to confluence. Then, the medium was replaced with the corresponding eluted extract and incubated at 37 °C for 24 h. A solution of MTT was prepared in warm PBS (0.5 mg/mL) and the plates were incubated at 37 °C for 4 h. Excess medium and MTT were removed and dimethylsulphoxide (DMSO) was added to all wells in order to solubilize the MTT taken up by the cells. This was mixed for 10 min and the absorbance was measured with a Biotek ELX808IU detector using a test wave length of 570 nm and a reference wave length of 630 nm.
Lactate dehydrogenase release assay
The lactate dehydrogenase (LDH) released by dead cells was determined using a commercial test kit (TOX-7, Sigma). The detection principle was based on the reduction of NAD by the action of LDH. The resulting reduced NAD (NADH) is utilized in the stoichiometric conversion of a tetrazolium dye. To complete this assay, human fibroblasts were seeded at a density of 14 × 104 cell/mL and incubated at 37 °C in humidified air with 5% CO2 for 1, 2, and 7 days over the testing dry specimens placed in 24-well culture plate. Lapsed these times the culture medium was collected, and 25 μL of this medium was transferred (n = 8) to a 96-well plate. In each one of these wells, 50 μL of the kit components mixture (equal amounts of substrate solution, cofactor and dye solution) was added. After 30 min of incubation at room temperature, 7.5 μL of HCl 1N was added for each test sample, and the absorbance was measured at 490 nm with a reference wavelength of 630 nm on a Biotek ELX808IU detector.
Statistical analysis of biocompatibility test
The statistical analysis of the extracts of cured systems was made by analysis of variance (ANOVA). In all statistical analyses p < 0.05 and p < 0.001 was considered as statistically significant.
Adhesion and proliferation test
Adhesion and proliferation was followed with Alamar Blue (AB). AB is a redox indicator that changes colour with the chemical reduction of the culture medium occurring as the result of cells growth and proliferation. This reagent can be withdrawn and replaced with fresh medium, for monitoring cell proliferation. It is soluble, stable in culture media and non-toxic to cells. The test was performed using fibroblast cells at a density of 14 × 104 cells/mL. Determinations were carried out at 24 h and were repeated at 4, 7 and 14 days. Test specimens and TMX control were placed in 24-well sterile culture plate. Alamar Blue dye (1 mL of 10% alamar blue solution in phenol red free DMEM medium) was added to each specimen. After 4 h of incubation the absorbance was measured at an excitation wavelength of 570 nm. Results were normalized with respect to a negative control (TMX = 100%) and statistically tested with ANOVA (p < 0.05).
Adhesion and proliferation of cells was also followed by direct analysis of the seeded CHI, HA, and CHOS films after periods of 1 and 2 days, by ESEM microscopy without any treatment of the corresponding surface.
Results and discussion
Preparation of films and sponges
The procedures used here to prepare the films and sponges for the CHI–CHOS and CHI–HA systems were conceived to avoid complexation of the corresponding polymers. This is very difficult to attain if aqueous solutions of the polyelectrolytes are used, since it has been shown that CHI–CHOS and CHI–HA form very stable complexes even in very acid media [16].
In the case of CHOS (Scheme 1a) the strong –SO3 − groups by themselves will bring about complexation with the –NH3 + groups of CHI no matter how low the pH. On the other hand, it has been shown that the weak polyacid HA, also reacts with CHI forming a polyelectrolyte complex (Scheme 1b) at very low pH [17].
The preparation of chitosan-chondroitin sulphate films and sponges by depositing over the chitosan film a hydro-alcoholic CHOS solution should overcome this problem, due to the insolubility of CHI in this medium.
CHI–HA films were prepared in a similar manner as CHI–CHOS films. In the case of CHI–HA sponges a different strategy was followed. Firstly the CHI–HA polyelectrolyte complex was formed and isolated from solution. Then, the complex was broken down and dissolved in an acid solution with a high salt concentration (0.66 M NaCl). The solution was lyophilized. The resulting CHI–HA membrane was washed to remove the salt, neutralized and freeze dried.
Infrared spectra
In Fig. 2 the FTIR spectra of chondroitin sulphate (a), CHI–CHOS PEC (b), CHI (c) and CHI–CHOS sponge (d) are shown. Although the similarities in CHI and CHOS chemical structures makes the comparison difficult, it is interesting to stress that there is a signal (relatively well defined) at 1,520 cm−1 which can be attributed to the –COO− and –NH3 + groups of CHI in the complexed chains [18, 19]. This signal is absent in the spectra of the pure chondroitin sulphate sodium salt (a) and the CHI–CHOS sponge (d). CHI spectrum exhibits the distinctive absorption bands at 1,658 cm−1 (Amide I) and 1,595 cm−1 (amide II, CO–NH free) [20] which are absent in the spectrum of the complex (b) and 1,314 cm−1 (Amide III). The CHOS spectrum shows a very intense and broad band centered at 1,630 cm-1 with a shoulder at 1,567 cm-1, also assigned to the amide I and II vibrations respectively. It is clear from the spectra of the pure CHI in Fig. 2c and the CHI–CHOS sponge, (Fig. 2d), that the main signals assigned to CHI clearly have not changed with the addition of the CHOS and therefore, it can be considered that using the technique of preparation described in this work, the formation of the interpolymer complex is avoided practically.
Figure 3 shows the IR spectra of HA (a), CHI-HA sponge (b) and CHI (c). As expected, there is again a great similarity between the spectra of the polymers involved. However, it can be noticed the presence of an absorption peak at 1,259 cm−1 in the spectrum of the sponge which is absent in the spectrum of HA. There is also a decrease in the relative intensity of the peak at 1,378 cm−1 with respect to the peak at 1,408 cm-1 in the spectrum of the CHI-HA sponge (A1378/A1408 = 0.322) in comparison with the same peak ratio in HA spectrum (A1378/A1408 = 0.443). These two facts indicate the presence of both polymers in the sponge, but as in the case of the CHI-CHOS sponge there is no indication of complex formation in the CHI–HA system.
SEM microscopy of films and sponges
Films of CHI and CHI–CHOS prepared by casting, exhibited an homogeneous, very compact and smooth surface, which could be very interesting for cells proliferation test, since smooth highly hydrophilic surfaces in general favour the attachment of cells and therefore the prioliferative process. The very smooth surface of the cast films was confirmed by ESEM.
In contrast, the porosity of CHI sponges becomes apparent in the ESEM micrograph in Fig. 4a. Even more, when these sponges were treated with the hydro-alcoholic CHOS solution, the open porous structure remained as it is shown in Fig. 4b.
On the other hand, CHI–HA sponges were prepared in a different way (lyophilisation), and as a consequence they showed a different morphology, with pores of higher size in an almost parallel arrangement. This can be well appreciated in the electron micrographs shown in Fig. 5.
Biocompatibiliy of films and sponges
The results of the MTT test are shown in Fig. 6. For the first day all the samples have the same behaviour than the control, Thermanox (TMX), except for the CHI–CHOS sponge which presents a viability of 80% respect to the control. Results obtained after two and seven days also demonstrated a viability of around the 80% respect to the negative control TMX. Although this can be considered a good results respect to the general criteria for cell compatibility, we analysed the results by a complementary assay based on lactate deshydrogenase enzyme. Figure 7 shows the spectroscopic absorption related directly with LDH release and it is clear that at one day no significative differences are obtained, but at two and seven days the CHI–CHOS samples give a higher LDH release than the others. This means that the CHI–CHOS system affects more to the cell attachment. This could be related with the partial solubilization of the surface in the layered CHI–CHOS supports. This is also observed in the Alamar blue assay (Fig. 8), but the differences are not very important.
In fact, the adhesion and proliferation assays (Fig. 8) indicate that cells are capable of proliferating satisfactorily in these materials. In fact, the percentage of cells growth respect to the control TMX is in all the cases higher than 70%, and according to the statistical approach, it seems to demonstrate that the swollen matrices do not affect to the proliferation of fibroblasts during the period of the experiment.
In a precedent study Denuziere et al. compared the rate of proliferation of chondrocytes in contact with pure CHI and CHI complexed with CHOS and HA. They found that cells seeded in the complexed systems had a decreased proliferation as compared to CHI [15].
Figure 9 shows the morphology of fibroblasts after one day seeded, on films of CHI (Fig. 9a), chitosan/hyaluronic acid (Fig. 9b) and chitosan/chondroitin sulphate (Fig. 9c). It is clear that a very good adhesion of cells over the films is observed, but in the presence of hyaluronic acid (Fig. 9b) or chondroitin sulphate (Fig. 9c) a higher number of cells and better adhesion respect to the pure CHI films was observed. The micrograph corresponding to pure CHI shows a few rounded cells in contrast to the HA and CHOS systems, which show clear adhered fibroblasts, with their characteristic elongated morphology.
Figure 10 shows the morphology of fibroblasts after 2 days seeded on the corresponding CHI, HA and CHOS films, respectively. A good proliferation process is well detected in all the cases, cells seeded on systems with HA or CHOS maintain its characteristic morphology and form an homogeneous monolayer covering most of the surface of the corresponding films, in good agreement with the results of MTT and Alamar Blue tests.
Complex formation involves the neutralization of charges of the polyelectrolytes involved, as schematically represented below (Scheme 2).
It has been stated that the protonated amino groups of CHI are responsible for the bioadhesive character of the polymer [21]. Therefore, this property is bound to be strongly affected when materials are prepared with chitosan-glycosaminoglycans PECs as it was stated by Denuziere et al. However, when chitosan-glycosaminoglycans films and sponges are prepared without complex formation it is possible to obtain novel materials exhibiting good cell attachment and proliferation, with potential application as biocompatible scaffolds for tissue engineering.
References
R. A. A. MUZARELLI, Chitin (Pergamon Press, Oxford, 1977).
T. CHANDY and C. P. SHARMA, Artif. Cells, Artif. Org. 18 (1990) 1.
Y. SHIGEMASA and S. MINAMI, Biotechnol. Gen. Eng. Rev. 13 (1995) 383.
H. SASHIWA, K. SAITO, H. SAIMOTO, S. MINAMI, Y. OKAMOTO, A. MATSUHASHI and Y. SHIGEMASA, Chitin Enzymology (European Chitin Society, Lyon and Ancona, 1977).
W. G. MALETTE and H. J. QUIGLEY, US Patent No. 4,532,134, 1985.
U. HIROSHI, H. YAMADA, I. TANAKA, N. KABA, M. MATSUURA, M. OKUMURA, T. KADOSAWA and T. FUJINAGA, Biomaterials 20 (1999) 1407.
I. V. YANNAS, Collagen III (CRC Press, Boca Raton, Florida, 1988).
W. Y. CHEN and ABATANGELO G., Wound Repair Regen. 7 (1999) 79.
G. PARTSCH, C. SCHWARZER, J. NEUMULLER, A. DUNKY, P. PETERA, H. BROLL, G. ITTNER and S. Z. JANTSCH, Rheumatology. 48 (1989) 123.
T.C. LAURENT and J. R. E. FRASER FASEB J., 6 (1992), 2397.
C. B. KNUDSON and W. KNUDSON FASEB J. 7 (1993), 1233.
J. - P. BALI, H. COUSSE and E. NEUZIL, Semin. Arthritis Rheum. 31 (2001) 58.
S. B. LEE, Y. MOO LEE, K. W. SONG and M. H. PARK, J. Appl. Polym. Sci. 90 (2003) 925.
H. LIU, J. MAO, K. YAO, G. YANG, L. CUI and Y. CAO, J. Biomater. Sci. Polymer Edn. 15 (2004) 25.
A. DENUZIERE, D. FERRIER, O. DAMOUR and A. DOMARD, Biomaterials 19 (1998) 1275.
A. DENUZIERE, D. FERRIER and A. DOMARD, Carbohydr. Polym. 29 (1996) 317.
L. RUSU-BALAITA, J. DESBRIÈRES, M. RINAUDO, Polymer Bull. 50 (2003) 91.
S. J. KIM, S. R. SHIN, K. B. LEE, Y. D. PARK and S. I. KIM, J. Appl. Polym. Sci. 91 (2004) 2908.
S. B. LEE, Y. M. LEE, K. W. SONG and M. H. PARK, J. Appl. Polym. Sci. 90 (2003) 925.
B. STUART, Modern Infrarred Spectroscopy, (New York, John Wiley and Sons, 1996).
O. GÅSERØD, A. G. JOLLIFFE, F. C. HAMPSON, P. W. DETTMAR and G. SKJÅK-BRÆK, Int. J. Pharm. 175 (1998) 237.
Acknowledgements
The authors acknowledge to IDEBIO and BIOIBERICA companies for providing the samples of CHI, hyaluronic acid and chondroitin sulphate. Support from CYTED and CICYT grants is also acknowledged. This work has been carried out in the framework of the NoE EXPERTISSUES. Mar Fernández is supported by the Ramón y Cajal Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peniche, C., Fernández, M., Rodríguez, G. et al. Cell supports of chitosan/hyaluronic acid and chondroitin sulphate systems. Morphology and biological behaviour. J Mater Sci: Mater Med 18, 1719–1726 (2007). https://doi.org/10.1007/s10856-007-3032-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3032-7