Abstract
The thermal properties, crystalline structure and shape memory effects of poly(l-lactide) (PLLA) and poly(l-lactide-co-ε-caprolactone) (PCLA) copolymers are systematically investigated by differential scanning calorimetry (DSC), X-ray diffraction (XRD) and tensile tests. The effects of the deformation strain on the shape recovery rate and recovery stress are also revealed. The polymers have the PLLA crystal and the amorphous phase, which are served as the fixed phase and reversible phase, respectively. The shape recovery rate and the recovery stress are significantly affected by the compositions and the deformation strain. With the increase of the deformation strain, the shape recovery rate decrease and higher shape recovery rate can be obtained in the polymers which have higher ε-CL content. However, the variation of recovery stress with the deformation strain is quite different and the maximum recovery stress of all polymers exceeds 3 MPa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shape memory alloys, such as TiNi alloys have been applied widely in medical fields due to their unique shape memory effect, high strength and excellent biocompatibility [1]. But at the same time, these alloys show some obvious disadvantages, such as high manufacturing cost, limited recoverable deformation and nonbiodegradability. However, for medical implantations, the materials are expected to possess both biodegradability and shape memory effect. With good biodegradability, they would neither stay in the human body for a long time nor require a second surgery to be removed from the human body. With shape memory effect, they could be implanted with the minimal shape and recovery to its original shape when in the body. Recently, biodegradable shape memory polymers attracted much attention and several kinds of biodegradable polymers have been reported [2–7]. For example, Lendlein studied the shape memory properties of multiblock copolymers based on oligo(ε-caprolactone) and oligo(p-dioxanone) segments [6]. Nagata coworkers have synthesized photocurable biodegradable multiblock copolymers with shape memory effects [7]. But as indicated in their papers, the synthesis and post treatment methods of the polymers were complicated and the mechanical properties are low comparatively which results in low recovery stress. Therefore, it is necessary for researchers to find biodegradable polymer systems with good shape recovery rate and high recovery stress.
Poly(l-lactide) (PLLA), poly(ε-caprolactone) (PCL) and their copolymers occupy an important position in the family of biodegradable polymers and have been widely used in the medical field because of their excellent biodegradabilities and biocompatibilities [8, 9]. During the past decades, much research work focuses on the synthesis, mechanical and degradation properties of the PCLA copolymers [10–12]. However, to our knowledge, there have been very few reports on shape memory effects of PCLA copolymers and especially no report on the recovery stress has been found to date.
In this study, the shape memory effects of PLLA and PCLA copolymers are first investigated, which open a new application fields in minimal invasive surgery, such as stents, suture, internal fixation devices and so on. Meanwhile, the effects of the deformation strain on the shape recovery rate and the recovery stress are also discussed.
Experimentals
Materials
l-Lactide (l-LA) was synthesized according to the literature [13], and purified by re-crystallization using ethyl acetate as solvent. ε-CL (Aldrich) was dried over molecular sieve 4 Å and purified by distillation under reduced pressure. Stannous octoate (Sigma, A. R.), chloroform and methanol were used as received.
PLLA and PCLA copolymers were prepared by ring opening polymerization of l-LA and the calculated amounts of two monomers (l-LA and ε-CL), respectively. The polymerization was conducted at 130 °C for 48 h with stannous octoate as initiator (initiator/monomer ratio = 1/8000(mol/mol)). The products were dissolved in chloroform and precipitated by pouring the polymer solution into excess methanol and then dried under vacuum. The polymer films were prepared by casting chloroform solutions of polymers with a concentration of 8 wt% onto a mold. After solvent evaporation at room temperature, the films were removed from the mold and dried under vacuum to optimize solvent removal.
Characterization
Intrinsic viscosities of polymers were measured with a Ubbelohde viscometer in chloroform at 25 °C and the results were listed in Table 1. 1HNMR spectroscopy was carried out with a Bruker DMX 300 spectrometer and chloroform was used as the solvent. Differential scanning calorimeter (DSC) measurements were performed on a Perkin-Elmer Diamond DSC at heating and cooling rate of 10 °C/min. X-ray diffraction spectra was carried out with a Rigaku D/max-rb rotating anode X-ray diffractometer at the conditions of Cu Kα, 50 kV and 40 mA.
Shape memory effects
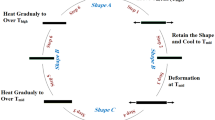
Shape memory effects of the polymers were investigated by the tensile test, which were performed with a WDS-5 tensile tester (Changchun Chaoyang), equipped with a constant-temperature heating chamber; the crosshead speed was 50 mm/min. The specimens had dimensions of 40 mm in length, 5 mm in width and 0.4 mm in thickness. The procedure for investigating the shape memory effect was as follows: (1) applying a deformation (εm) to the sample at a constant crosshead speed of 50 mm/min at Tk, (2) cooling the sample to T1 with the same εm, (3) maintaining the sample at T1 for 5 min after removal the load, and (4) raising the temperature from T1 to Tk and then for 5 min. Under these conditions, shape recovery rate (Rr) was defined as follows [14]:
where Tk = Tg + 15 °C, T1 = Tg−15 °C, εm was the elongation strain and εp was the recovery strain at Tk.
For measurement of the recovery stress, the test procedure was similar to the mentioned above (1)∼(3), the difference was the last step, (4) the specimen was clamped with its length fixed and then was heated until Tk, and the tensile stress was recorded as the recovery stress.
Results and discussion
Thermal properties and crystalline structure
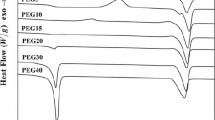
Figure 1 shows the DSC curves of the first heating and cooling for PLLA and PCLA copolymers. An endothermic peak can be observed on the DSC heating curves of each sample, which corresponds to the melting of PLLA crystal. Only the PLLA shows a clear exothermal peak at about 110 °C due to the cold crystallization. The glass transition temperature (Tg), the melting temperature (Tm) and the heat of fusion (ΔHm) are summarized in Table 1. It can be seen that copolymerization with ε-CL has a dramatic impact on thermal properties and both the Tg and the Tm decrease with the increase of the amount of ε-CL. The introduction of ε-CL units which have five methylene groups increases the chain flexibility and mobility and results in a considerable decrease in the Tg and Tm. It also can be seen that the crystallinity of the polymers decreases obviously with the ε-CL content increasing, which is attributed to the increase of chain flexibility and chain disorder with the addition of ε-CL.
The X-ray diffraction pattern is shown in Fig. 2. It is seen that there are two main peaks at 2θ = 16.7° and 19.1° which characterize the crystalline of PLLA; the peak positions coincide with those reported by other authors [15]. Moreover, the peak intensities decrease with increasing the ε-CL content. However, the typical diffraction peaks of crystalline PCL reported [15] at 2θ = 21.4° cannot be observed in the patterns of the copolymers, which indicates that PCL is amorphous phase in the copolymers. The width of the diffraction peak seems to be independent of the amount of ε-CL. This observation suggests that the crystal size of PLLA is not largely influenced by the presence of ε-CL.
Shape memory effects
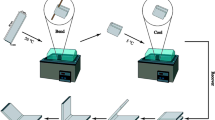
The shape memory effect of PLLA is photographically shown in Fig. 3. The original shape of the sample is a straight slice and the temporary shape is a spiral after being deformed at 65 °C, followed by quenching to ice water. Then, the deformed sample is reheated to 70 °C, the transition from the temporary to the original shape occurs within 20 s at 70 °C.
Figure 4 shows the loading and unloading curves of the polymers stretching at Tg + 15 °C. All the curves demonstrate the elastic properties of the materials and none of these polymers shows a yield point. It can also be seen that the tensile strength decrease with the increase of the ε-CL content. The cyclic tensile tests are used to characterize the shape memory effects of these samples. The sample is first elongated at Tg + 15 °C to εm of 100%, at a constant elongation rate of 50 mm/min. Then the sample is cooled to Tg−15 °C with the strain keeping at εm, after that the sample is unloaded. The sample is subsequently heated to Tg + 15 °C over a period of 10 min, and then keep at this temperature for a further 5 min, so allowing the strain to recover. This complete one thermomechanical cycle (N = 1), leaving a residual strain, εp, at which the next cycle (N = 2) starts. A typical stress–strain diagram for a thermocyclic test is shown in Fig. 5. According to the stress–strain diagram, the shape recovery rate can be determined, and the effect of the deformation strain on it is shown in Fig. 6.
Generally, shape memory polymers consist of two phases, a thermally reversible phase for maintaining transient shape and a fixed phase for memorizing original shape. Crystal, glassy state, entanglement or crosslinking can be used as a fixed phase [16]. When they are deformed at the rubbery plateau above the Tg and subsequently cooled down below Tg under constant strain, the deformed shape is fixed because molecular mobilities are frozen. As they are reheated above Tg, the original shape is recovered by the elastic force generated during the deformation. As discussed in 3.1, the structure of PLLA and PCLA copolymers consists of crystalline hard domains of PLLA and amorphous soft phase. The crystalline PLLA domains are expected to serve as physical cross-linked points, while the flexibility of the amorphous phase provides the large elongation and the shape recovery.
Figure 6 demonstrates the effects of deformation strain on the shape recovery rate for the polymers. It can be seen that the shape recovery rate of all samples decreases with an increase of deformation strain and at a certain deformation strain, the shape recovery rate increase with the increase of ε-CL content.
In general, when the deformation is applied to the samples, the amorphous phase having low modulus will be extended in the first stage, and the number of extended amorphous chain would increase with increasing the deformation strain. However, at a high deformation strain value, two factors unfavorable for recovery should be taken into consideration. One is the relaxation of the extended amorphous chain due to their dislocation from the PLLA crosslinks; another should be the plastic deformation of crystalline domain because stress transfer to the crystalline domain as the deformation strain increases further and with the increase of the deformation strain, the irreversible deformation formed by the deformation of PLLA crystal increases. The combination of the two aspects can explain the decrease of the shape recovery rate with the increase of the deformation strain. Moreover, the crystallinity of polymers decrease with the ε-CL content increasing, that is to say, the number of amorphous phase increases with the increase of ε-CL content, which is responsible for the shape recovery, thus, the shape recovery rate increases with the increase of the ε-CL content.
To evaluate the driving force for shape recovery, the recovery stress is measured by tensile test while a pre-stretched specimen is heated to a certain temperature with the specimen length fixed. As shown in Fig. 7, the variation of recovery stress exhibits different trends for the polymers with different composition. PLLA reaches a maximum recovery stress around 4.0 MPa at 50% deformation strain, but the recovery stress of PCLA90 and PCLA80 increase gradually with the increase of deformation strain. It also can be seen from Fig. 7 that at low deformation strain conditions, higher recovery stress can be obtain in the polymers which have lower ε-CL content. Moreover, all the samples display maximum recovery stress exceeding 3 MPa, which is superior to those exhibited by other biodegradable shape memory polymers recently developed [6].
As we know, the recovery process is provided by comparatively highly oriented amorphous chains and comes from the relaxation of internal stress that is frozen in the sample after deformation (orientation). Thus, the experimental results mentioned in Fig. 7 can be explained as follows: on the one hand, with increasing the deformation strain, the number of orientation amorphous chain would increase, which leads to higher recovery stress. On the other hand, recovery stress relates to the shape recovery rate. As we know, the higher shape recovery rate means the lower plastic deformation, which suggests that the higher proportion of applied stress can be stored in the specimen. Thus, the recovery stress increase as a result of the shape recovery rate increasing. The combination of the two aspects can explain the variation of recovery stress with the content of ε-CL and it can be concluded that there exists an optimal deformation strain value corresponding to the maximum recovery stress. Meanwhile, recovery stress is proportional to the applied stress because the inner stress stored in the specimen during pre-deformation and fixed processes is the driving force for shape recovery. According to the results of Fig. 4, the applied stress of PLLA is higher than those of PCLA90 and PCLA80, therefore, the higher recovery stress of PLLA can be obtained at a certain conditions.
Conclusion
-
1.
PLLA and PCLA copolymers are semicrystalline, and the PLLA crystal serves as physical cross-linked, while the amorphous phase acts as reversible phase, which are responsible for the shape memory effect.
-
2.
The shape recovery rate decreases with the increase of deformation strain, which is due to the amorphous chain dislocation of the physical crosslinks and the plastic deformation of PLLA crystal at large deformation strain. The shape recovery rate increases with the increase of the ε-CL content which is attributed to the increase of the number of amorphous phase.
-
3.
There exists a maximum recovery stress when the deformation strain increases and all the samples display maximum recovery stress exceeding 3 MPa, which is very significant for medical applications.
References
T. DUERIG, A. PELTON and D. STOCKEL, Mater. Sci. Eng. A. 273–275 (1999) 149
Y. SHIKINAMI, US Patent 6281262 (2001)
R. LANGER, A. LENDLEIN, A. SCHMIDT, and H. GRABLOWITZ, US Patent 6160084 (2000)
G. ZHU, Q. XU, G. LIANG and H. ZHOU, J. Appl. Polym. Sci. 95 (2005) 634
C. MIN, W. CUI, J. BEI and S. WANG, Polym. Adv. Tech. 16 (2005) 608
A. LENDLEIN and R. LANGER, Science 25 (2002) 1
M. NAGATA and Y. SATO, J. Polym. Sci. Part A: Polym. Chem. 43 (2005) 2426
J. C. MIDDLETON and A. J. TIPTON, Biomaterials 21 (2000) 2335
D. COHN and G. LANDO, Biomaterials 25 (2004) 5875
Q. Q. QIU, P. DUCHEYNE and P. S. AYYASWAMY, J. Biomed. Mater. Res. 52 (2000) 66
F. DENG, K. S. BISHT, R. A. GROSS and D. L. KAPLAN, Macromolecules 32 (1999) 5159
T. MIYATA and T. MASUKO, Polymer 38 (1997) 4003
S. H. HYON, K. JAMSHIDI and Y. IKADA, Biomaterials 18 (1997) 1503
B. K. KIM, Y. S. LEE and M. XU, Polymer 37 (1996) 5781
O. JEON, S. H. LEE, S. H. KIM, Y. M. LEE and Y. H. KIM, Macromolecules 36 (2003) 5585
J. R. LIN and L. W. CHEN, J. Appl. Polym. Sci. 69 (1998) 1563
Acknowledgement
This work was supported by the 973 National Technological Project of China (No. 2006CB708609).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, X.L., Sun, Z.J., Cai, W. et al. Study on the shape memory effects of poly(l-lactide-co-ε-caprolactone) biodegradable polymers. J Mater Sci: Mater Med 19, 395–399 (2008). https://doi.org/10.1007/s10856-006-0100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-006-0100-3