Abstract
Calcium phosphate ceramic has been widely used as bone substitute materials. Neumerours approaches have been investigated to develop tissue-engineered scaffold from hydroxyapatite because of its advantages like osteoconduction. We have developed porous ceramic matrices from nanoparticles of calcium phosphate containing zinc and magnesium. Mimicking the grain size of natural bone enhances the bone forming function of cells. Osteoblast-like MG63 cells were cultured on to these porous ceramic matrices. Cell adhesion and spreading onto these matrices were studied for 24 h and 5 days in vitro. It was observed that on calcium phosphate matrix, containing a combination of zinc and magnesium, the osteoblast adhesion and spreading was significant on 5th day. This appeared to be comparable to the hydroxyapatite control. This makes it a promising candidate as a bone tissue-engineering scaffold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ceramics are widely used as bone substitutes for the past several years. Hydroxyapatite and tricalcium phosphate (HA & TCP) are approved by FDA as bone substitutes in humans. Ceramic-based bone graft substitutes include calcium phosphate, calcium sulfate, and bioglass used alone or in combination. The design of biomaterials with surface properties similar to physiological bone, which demonstrates the basic criteria of osteoinduction and osteoconduction, promotes formation of new bone and improved orthopedics. Bone tissue engineering is comparatively a new approach in the repair of bony defects. The recent developments of tissue engineering in the field of orthopedic research make it possible to envisage the association of autologous cells and proteins that promote cell adhesion with osteoconductive material to create osteoinductive materials [1]. Nature of interaction between osteoblast cells and their substrate can influence the ability of these cells to produce an osteoid matrix around an implant, which in turn will determine the fate of the implant [2]. Cell spreading is an essential function of a cell, which has adhered to any surface and precedes the function of cell proliferation. Out of the bone and the ceramic material interactions that take place at the material surface, the interaction of osteoblasts is crucial in determining the tissue response at the biomaterial surface [3]. Attachment and spreading of specific bone forming cells in cell culture has been utilized for predicting the behavior of the calcium phosphate materials in vivo [4]. The process of cell interaction on materials is highly dynamic and depends on various parameters influencing the cell responses. It is well known that the size and shape of cell spreading area, as well as the number, size, shape and distribution of focal adhesion plaques are decisive for further migratory, proliferative and differentiation behavior of anchorage-dependent cells [5]. Cells usually do not survive if the attached cells are round and are not spreading. If the cell material contact area is significantly high the cells tend to skip the proliferation phase and enter sooner the differentiation program. If the adhesion is intermediate the cells are most active in migration and proliferation.
The minerals like zinc and magnesium are know to aid bone growth, calcification and bone density [6, 7]. Biphasic calcium phosphate ceramic containing zinc also promotes osteoblastic cell activity in vitro [8]. Thus the objective of this investigation is to evaluate the attachment and spreading of osteoblast like cells on to porous ceramic materials made from nanoparticles of zinc phosphate (ZnP) and calcium phosphate containing zinc and magnesium. Further, since the ceramic matrices are made from nanoparticles, it mimics the way nature itself lays down minerals.
Materials and methods
Materials
Zinc phosphate (300–800 nm), Zinc calcium phosphate (>1 micron) and Zinc calcium magnesium phosphate (300–800 nm) nanoparticles were prepared by wet chemical precipitation method similar to which has been reported earlier with a slight modification [9]. These particles were characterized using XRD.
Preparation of matrices
Zinc phosphate (ZnP) nanoparticles, calcium zinc phosphate (CaZnP) nanoparticles, calcium zinc magnesium phosphate (CaZnMgP) nanoparticles and hydroxyapatite, Ca10(PO4)62H2O (HA) powder were mixed with the porogen, pressed [3 ton) in the form of a 12 mm diameter and 1.5 mm thick discs and sintered at 1,100°C for 1 h to obtain porous ceramic matrices. All materials were cleaned with distilled water and acetone, air-dried and steam sterilized.
Cell culture
MG-63 osteoblast-like cells (National Centre for Cell Sciences, Pune, India isolated from osteosarcoma) were used for these experiments. The cells were seeded onto HA, ZnP, CaZnP and CaZnMgP discs, which were placed in tissue culture plates as reported earlier [10]. Cells on these surfaces were evaluated by Scanning Electron Microscope (SEM) after fixing and dehydrating. It was dried in a Critical Point Dryer (Hitachi-HCP-2). A thin layer of gold was sputter coated onto samples prior to examination in SEM (Hitachi-S2400).
Results and discussion
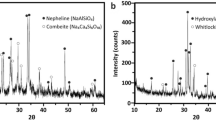
Ceramic nanoparticles of ZnP, ZnCaP and ZnCaMgP are prepared by wet chemical method. The particle size has been evaluated using TEM and the photograph is given in Fig. 1. The particles prepared are in the range of 300–800 nm. Efforts are ongoing to reduce the particle sizes to less than 100 nm. Porous ceramic discs with interconnected pores and an average pore size of 200 microns are obtained from these nanoparticles and also with standard HA powder. The XRD spectra of these nanoparticles and of HA are given in Fig. 2. From the X-ray diffraction studies the chemical formula of these particles has been evaluated as Zn3(PO4)24H2O (ZnP), CaZn2(PO4)22H2O (CaZnP) and CaZn2(PO4)24H2O + MgH2 (CaZnMgP). All particles seems to be crystalline from the XRD analysis.
Figures 3–6 demonstrates the adhesion and spreading of osteoblast cells onto the different ceramic matrices. On HA (control) and CaZnP surfaces osteoblast cells are seen attached to the surface and starts spreading in 24 h of incubation. On ZnP surface, significantly less number of cells are visible and these cells are also not spreading (24 h). However, it is interesting to observe that on CaZnMgP surface osteoblast cells seems to be completely spread which exhibits a smooth surface texture, with some cells sitting above it (24 h). On day 5 cells seem to be completely spread on HA (control) as well as on CaZnMgP surfaces with a significantly high spreading on CaZnMgP surface. Comparing the surface morphology of CaZnMgP before (Fig. 7) and after cell adhesion studies it is evident that the surface smoothness seen on CaZnMgP surface on day 5 is due to the complete spreading of cells onto the surface of the matrix. This is obvious while comparing the 1k × and 30 × magnification micrographs of Fig. 4C, D and 7. On CaZnP surface cell attachment at day 5 is almost similar to that at 24 h. However, on ZnP surface no cells are found after 5 days of incubation. Earlier reports also indicate that osteoblast spreading/proliferation is slower for more amorphous surfaces mainly due to less organised cytoskeleton [11, 12].
Conclusion
An important objective of bone tissue engineering is to develop improved scaffold materials or arrangement to control osteoblast behavior significantly affecting its response. Osteoblastic cells on hydroxyapatite exhibited unique attachment and subsequent behavior in vitro, which may explain why mineralized tissue formation is better on HA. Divalent cations including Mg2+ are known to be active in cell adhesion mechanisms [13, 14]. The present investigation demonstrates that the cells are spreading well on matrix containing an optimum amount of calcium, zinc and magnesium. It seems that the presence of calcium and magnesium encourages the spreading and adhesivity of osetoblasts cells onto bioceramic matrices. Cells are attached and spread completely on the ZnCaMgP matrix and this matrix appears to be comparable to the control group (hydroxyapatite matrix). This makes ZnCaMgP matrx a promising candidate as a bone tissue-engineering scaffold similar to HA.
References
K. ANSELME, Biomaterials 21 (2000) 667
D. A. PULEO and R. BIZIOS, J. Biomed. Mater. Res. 26 (1992) 291
A. HUNTER, C. W. ARCHER, D. S. WALKER and G. W. BLUNN, Biomaterials 16 (1995) 287
U. MEYER, A. BÜCHTER, H. P. WIESMANN, U. JOOS and D. B. JONES, Euro. Cells Mater. 9 (2005) 39
L. BAČÁKOVÁ, E. FILOVÁ, F. RYPÁČEK, V. ŠVORČÍK and V. STARÝ, Physiol. Res. 53 (2004) s35
A. HIGASHI, T. NAKAMURA, S. NISHIYAMA, M. MATSUKURA, S. TOMOEDA, Y. FUTAGOSHI, M. SHINOHARA and I. MATSUDA, J. Am. Coll. Nutr. 12 (1993) 61
K. L. TUCKER, M. T. HANNAN, H. CHEN, L. A. CUPPLES, P. W. F. WILSON and D. P. KIEL, Am. J. Clin. Nutr. 69 (1999) 727
Y. SOGO, A. ITO, K. FUKASAWA, T. SAKURAI and N. ICHINOSE, Mater. Sci. Technol. 20 (2004) 1079
T. MORCOL, P. NAGAPPAN and L. NERENBAUM, Int. J. Pharm. 277 (2004) 91
A. JOHN, T. V. KUMARY and H. K. VARMA, Biomater. Appl. 18 (2003) 63
M. D. BALL, S. DOWNES and C. A. SCOTCHFORD, Biomaterials 22 (2001) 337
L. CHOU, B. MAREK and W. R. WAGNER, Biomaterials 20 (1999) 977
J. H. Fitton, Divalent Cation Modulation of Cell Adhesion to Surface, In: Proceedings 5th Annual Conference of Australian Soc. Biomater. p,.A 22 (1995)
R.U. HYNES, Cell 69 (1992) 11
Acknowledgments
We are thankful to the Director, SCTIMST and the Head, BMT Wing for providing facilities for this work. The XRD analysis by Dr. HK Varma and Mr. S Vijayan, TEM by Dr. Annie John, SEM by Mr. Sreekumar and cell culture by Dr. T.V. Kumary is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, W., Sharma, C.P. Effect of calcium, zinc and magnesium on the attachment and spreading of osteoblast like cells onto ceramic matrices. J Mater Sci: Mater Med 18, 699–703 (2007). https://doi.org/10.1007/s10856-006-0005-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-006-0005-1