Abstract
In this study, a series of Sr3LaNb3O12 phosphors doped with Dy3+ ions was prepared by a high-temperature solid-state reaction. The crystal structure, phase purity, particle morphology, photoluminescence (PL) properties, and thermal stability of the phosphors were described. And the Sr3LaNb3O12 crystal symmetry is hexagonal. Under the optimal excitation wavelength of 352 nm, the three prominent emission peaks of the Sr3LaNb3O12:Dy3+ focused on 483 nm (4F9/2-6H15/2), 575 nm (4F9/2-6H13/2), and 668 nm (4F9/2-6H11/2). The optimal Dy3+-doped concentration in the Sr3LaNb3O12 phosphor was 0.05 mol. The phosphor had an abnormal thermal quenching phenomenon and activation energy (Ea = 0.29 eV). The prepared white light-emitting diode (w-LED) had a low correlation color temperature of 4162 K, chromaticity coordinates (0.365, 0.368), and the color rendering index 91. The feasibility of the Sr3LaNb3O12:Dy3+ luminescent material for w-LED was verified by its performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
White light-emitting diodes (W-LEDs) have the advantages of low energy consumption, environmental protection, and higher efficiency, which remedies some disadvantages of incandescent and traditional fluorescent lamps [1,2,3,4,5,6,7,8]. Nowadays, commercial w-LEDs are based on blue light-emitting diode (LED) chips using the YAG:Ce3+. The blue chips may take a toll on health [9, 10]. For example, it leads to the inhibition of melatonin secretion, myopia, and yellow spots [11, 12]. The phosphor conversion to n-UV chips stimulating w-LEDs can solve these problems. Therefore, it is of great significance to develop a new yellow phosphor for w-LEDs based on n-UV chips.
In recent years, Dy3+ ions doped phosphors have been studied for w-LED in many rare-earth ions. And Dy3+ ions doped phosphors have good optical properties in many reports [13,14,15]. The rare-earth Dy3+ ions are highly efficient activators and usually have a blue peak, prominent yellow emission peak, and low-intensity red emission peak, corresponding to 4F9/2-6HJ/2 (J = 15, 13, 11) transitions [16,17,18]. White light is obtained by taking the host materials and doped Dy3+ ions in combination. Emission peaks of rare-earth Dy3+ ions doped materials can show white light and the CIE coordinates can be reflected in the white light area. Therefore, many Dy3+-doped materials are ideally combined with an n-UV chip and successfully applied in the w-LED [19,20,21].
An excellent host material favors the luminescence properties of doped ions. The hexagonal perovskite Sr3LaNb3O12 is a good microwave dielectric material with low loss, whose space group is R_3m. At present, many hexagonal perovskite phosphors are applied in w-LEDs. Peng et al. compounded Sr3La(VO4)3:Sm3+ phosphors which have good thermal stability [22]. Priyanka et al. researched the luminescence properties of Dy3+-activated BaYAlZn3O7 phosphors, a fine cold-white-emitting material [23]. Zhang et al. studied luminescence properties and the thermal stability of Sr2CaLa(VO4)3:Sm3+ phosphors with higher activation energy [24]. These show that the phosphors with hexagonal perovskite structures have good optical properties. However, the luminescence properties of Sr3LaNb3O12:Dy3+ phosphors haven’t been studied.
Here, novel Dy3+-doped Sr3LaNb3O12 phosphors were compounded by a solid-state reaction. The structural and optical properties of phosphors were researched, including phase purity, crystal structure, particle morphology, element composition, CIE chromaticity coordinates, photoluminescence properties, and the abnormal thermal stability [25,26,27]. Finally, w-LEDs were prepared using multicolor phosphors based on an n-UV chip.
2 Experimental section
Dy3+-doped Sr3La1-xDyxNb3O12 (x = 0.01, 0.02, 0.05, 0.10, 0.15, 0.20, 0.25, and 0.30 mol) phosphors were prepared by the traditional high-temperature solid-state technique. High purity raw materials were SrCO3 (99%), La2O3 (99.99%), Dy2O3 (99.99%), and Nb2O5 (99.9%). These raw materials were weighed by following the equilibrium equation and ground for around 16 min in the agate mortar. Then the samples were put in the small crucibles. The samples firstly were heated at 1200 °C for four hours in the muffle furnace. When the preparation firing reaction was completed, they were cooled to room temperature. Afterward, the prepared samples were continued to be heated at 1320 °C for twelve hours. After a series of reactions were completed, the samples were fetched and completely smashed. Then color and hardness of products were recorded and used for the next luminescence characterization. The related experimental equation is given as follows:
The phase purity analysis of Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) phosphors were revealed on the X-ray diffractor (XRD, Bruker D2 PHASER) with Cu Ka source (λ = 0.154184 nm) by scanning from 10° to 70° range. The crystal structure was refined the Maud software. The sample particle size was obtained passing by a laser particle size analyzer (Malvern MS2000). The appearance and composition of the phosphor element were determined with scanning electron microscopy (Nova Nano SEM-450) equipped with energy-dispersive X-ray spectrum (EDS). The photoluminescence (PL) spectra and temperature-dependent spectra were measured via Edinburgh FLS 980 spectrometer, which used a 450 W Xenon lamp as the excitation source.
The prepared yellowing Sr3LaNb3O12:0.05Dy3+ phosphor was mixed with a bit of commercial red phosphor ((Sr, Ca)AlSiN3:Eu2+) and blue phosphor (BaMgAl10O17:Eu2+ (BAM)) to obtain the required mixture, whose ratio is 98%, 0.5%, and 1.5%, respectively. The mixture was added with a moderate amount of shadowless adhesive and combined with a 388 nm n-UV InGaN chip. The obtained product was cured under a UV lamp for 5 min, and finally, the w-LED can be fabricated. Then CIE chromaticity coordinates, electroluminescence (EL) spectrum, Ra, and correlation color temperature (CCT) can be achieved using the USB 4000 fiber optic spectrometer (Ocean Optics).
3 Results and discussion
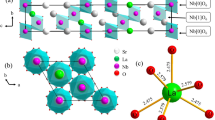
The crystal structure of the Sr3LaNb3O12 phosphor is shown in Fig. 1. It indicates that M (M = Nb/Sr/La) ions occupy a 3a lattice site in the proportion of 14% La, 28% Sr, and 58% Nb. M coordinating with six O ions at the position 3a lattice site forms the group, which is a regular octahedral biconical whose M–O distance is 1.91 Å. In the standard data, the cell parameters of the Sr3LaNb3O12 are a = b = 5.6590 Å, c = 27.1780 Å, a/c = 0.2082 Å, V = 753.8 Å3, and Z = 3.
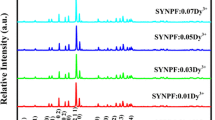
The standard (PDF#00–041-0148) card of the Sr3LaNb3O12 and eight XRD patterns of the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) samples are represented in Fig. 2a. The peaks of XRD have very narrow diffraction and show no evident mismatched peaks, which proves that the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) samples were successfully synthesized, and these phosphors are high phase purity. Using the substitution formula of the Dy3+ can calculate the substitution of the host lattices [28, 29]:
where RS is represented for the radius of the substituted cation, CN is on behalf of the coordination number, and Dr stands for the difference between the radius percentage, Rd represents the radius of the doped ions. The radius of Sr2+ ions is 1.180 Å and the radius of Nb3+ is 0.720 Å, whose Dr is 22.71% and -26.67%. But the radius of Dy3+ ions is 0.912 Å and its Dr is 11.62% which is the smallest and far less than 30%. Dy3+ ions and La3+ ions stay in the uniform valence state. The results indicate that the Dy3+ ion prefers to replace the La3+ ion. So The Dy3+ can quickly get into the host lattice, and it has no evident effect on the Sr3LaNb3O12 crystal system.
In the Fig. 2b, the Rietveld refinement of the Sr3LaNb3O12:0.01Dy3+ phosphor was researched by using Maud software [30]. In Tables 1 and 2, the crystal structure parameters and Rietveld refinement data are shown. The lattice parameters of the Sr3LaNb3O12:0.01Dy3+ sample is a = b = 5.65 Å, c = 27.19 Å, a/c = 0.2078, V = 753.28 Å3, and Z = 3. The obtained fitting parameters of Sr3LaNb3O12:0.01Dy3+ sample are Rwp = 14.95% and χ2 = 7.57, which indicates that the Dy3+ ions well replace La3+ ions in the Sr3LaNb3O12 phosphors.
The surface morphology and size of phosphor is also necessary to study the use of w-LEDs. Figure 3a indicates SEM image of the Sr3LaNb3O12:0.01Dy3+ phosphor at 2400 magnification. The products are large conglomerations due to a high-temperature process. Figure 3b shows the particle size distribution of phosphor. D(50) is 24.418 μm, which represents that 50% of the particles were less than 24.418 μm. D(90) is 68.662 μm, which represents that 90% of the particles were less than 68.662 μm. The particle size distribution of 22.556 μm is the largest cell volume percentage, which describes that they are suitable for use in solid-state lighting.
To confirm the elemental composition of samples, the type of the element can be analyzed using the EDS spectrum. By the EDS spectrum of the Sr3LaNb3O12:Dy3+ phosphor, all the elemental peaks can be observed containing Sr, La, Nb, O, and Dy. Figure 4b–g shows all the elements were even distribution. The weight percentages of the elements Sr, La, Nb, O and Dy are 29.27%, 16.74%, 31.34%, 22.29%, and 0.36%. The atomic percentages are 15.27%, 5.51%, 15.42%, 63.70%, and 0.10%. The results were consistent with the outcome of Sr3LaNb3O12:0.01Dy3+ phosphors. These proved that we successfully made the Sr3LaNb3O12:Dy3+ phosphors.
The excitation spectrum of the Sr3LaNb3O12:0.05Dy3+ is shown in Fig. 5a (λem = 575 nm). The O2−—Dy3+ charge transfer (CT) causes a weak broad band ranging from 240 to 310 nm [31]. In the 300–500 nm excitation spectrum, the seven remarkable peaks lie in 326, 352, 366, 388, 426, 454, and 475 nm, corresponding to the transition of the 6H15/2 level to the 6P3/2, 6P7/2, 6P5/2, 4I13/2, 4G11/2, 4I15/2, and 4F9/2 level [32, 33]. Among these excitation peaks, the 352 nm excitation is the most favorable wavelength because of the Dy3+ transition (6H15/2-6P7/2), which will be suitable for the development of the n-UV-based w-LEDs.
Figure 5b describes the emission spectrum of Sr3LaNb3O12:0.05Dy3+ under the 352 nm excitation, delegated the three prominent emission peaks at 483 nm (4F9/2-6H15/2), 575 nm (4F9/2-6H13/2), and 668 nm (4F9/2-6H11/2). The most substantial peak was the yellow emission (4F9/2-6H13/2), but it was easily impacted by the external coordination environment, which is ascribed to the pure electric dipole transition. In contrast, the lower peak was the blue emission (4F9/2-6H13/2), which was not affected by the crystal field. The blue area (483 nm) of Dy3+ ions was lower than the yellow emission (575 nm) [34]. This phenomenon was identified with the fact that the Dy3+ ions occupied a lower symmetrical position.
To study the relationship between the doped Dy3+ concentration and the emission intensities, the emission spectra (λex = 352 nm) of the Sr3LaNb3O12:xDy3+ phosphors (x = 0.01–0.30 mol) were shown in Fig. 6a. Figure 6b clearly describes the relationship between the doping concentration of Dy3+ and the peak intensity of Sr3LaNb3O12:xDy3+ by normalizing the maximum emission intensity. At the low doping concentration of Dy3+ ions, the luminous intensities gradually rises till the distance of centers reaches the critical distance where the optimal doping concentration was 0.05 mol in the Sr3LaNb3O12:Dy3+. After this point, the luminescence intensity presents the trend of falling, and the energy transfer ability has weakened. Figure 6c indicates the corresponding energy level schemes of the Dy3+. Under the excitation of 352 nm, electrons are firstly transmitted from the ground state 6H15/2 level to the higher excited state 4I15/2 level. Then the excited electrons go through the non-radiative (NR) transition to 4F9/2 level. In the end, the typical radiative transitions 4F9/2 to 6HM/2 (M = 15, 13, and 11) of Dy3+ ions by can be observed, corresponding to the blue peak at 483 nm, the yellow main peak at 575 nm, and the red peak at 668 nm. Meanwhile, this process quickly releases the energy of phosphors through the non-radioactive transition.
a The PL emission of the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) at 352 nm, b The relationship between the doping concentrations of Dy3+ and the peak intensities of Sr3LaNb3O12:xDy3+, c The energy electron transition processes of Dy3+ levels diagram, d The relationship between lg (I/x) and lg(x) in the Sr3LaNb3O12:Dy3+ samples
To study the interaction mechanism between Dy3+ ions, the critical distance (Rc) is calculated. The value of the Rc is calculated by the subsequent formula [35, 36]:
where N is the number of cations, Xc is the optimal Dy3+-doped concentration of the Sr3LaNb3O12, and V is the volume of the cell. By substituting the value of Xc (0.05 mol), V (752.11 Å3), and N (3), the energy transfer distance can be calculated (21.25 Å), which is far larger than the critical distance (5 Å) of exchange. Therefore, the concentration quenching (CQ) is caused by the multipole-multipole interaction.
To further explore the connection between the Dy3+-doping concentration and the luminous intensity, Dexter’s model can be used. Dexter’s model established the energy transfer between the same kinds of activators. On the basis of Dexter’s theory, the specific role of the multipole energy transfer mechanism can be explored [37, 38]:
where I stands for emission intensity of different Dy3+ concentrations; x is the Dy3+ concentration; β and K are constants at a certain excitation wavelength in the same system. α represents a multipole interaction constant including three types of mechanism interaction: dipole–dipole, dipole-quadrupole, and quadrupole–quadrupole, corresponding to the α = 6, 8, and 10. Figure 6d shows that the connection with x and I/x has a negative correlation relation, and the slope is determinately -1.81. So the value of α is 5.43, which is close to 6. Therefore, the combined action of dipole–dipole causes the CQ of the Dy3+-doped Sr3LaNb3O12 phosphors.
The intensity of 4F9/2-6H15/2 and 4F9/2-6H13/2 transition at different concentrations is shown in Fig. 7. 4F9/2-6H15/2 represents the magnetic dipole (MD) transition, and 4F9/2-6H13/2 transition denotes the electric dipole (ED) transition. ED intensity is affected by the ligand ions in the crystal field [39]. The intensity of MD transition is weaker than the ED, which states that Dy3+ occupies the low symmetry site in the Sr3LaNb3O12 matrix. Here, the asymmetric ratio ED/MD (R) play an important role in analyzing the symmetry of the lattice environment around Dy3+. The R by calculating is 3.27, 3.00, 2.93, 2.77, 2.60, 2.42, 2.11, and 3.19, corresponding to x = 0.01, 0.02, 0.05, 0.10, 0.15, 0.20, 0.25, and 0.30, respectively. The R of Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) phosphors is greater than 1, which explains that Dy3+ ion occupied inversion symmetric lattice sites in the Sr3LaNb3O12 crystal structure [40].
Thermal stability plays an important role that is analyzed to decide whether the phosphors are suitable for the w-LED application. When the LED chip temperature can reach about 420 K, excellent phosphors can sustain high temperatures and against a color shift. As follows, the effect of changing temperature in the range of 300–480 K on the luminescent intensity of the Sr3LaNb3O12:0.02Dy3+ and Sr3LaNb3O12:0.05Dy3+ were also studied in Fig. 8a, b. The emission intensity rise and then fall when the temperature increase but the emission peaks of both phosphors hardly shift. It can also be observed through the normalized integral emission intensity. Figure 8c, d clearly plots the normalized integrated PL intensities versus temperature for representative samples. It engenders thermal quenching, and compared with the luminous intensity of the initial temperature, the intensity of 480 K has not obviously fallen. For the Sr3LaNb3O12:0.02Dy3+ phosphor, the PL intensity increased abnormally by approximately 6% from 300 to 340 K. For the Sr3LaNb3O12:0.05Dy3+ phosphor, the PL intensity increased abnormally by approximately 13% from 300 to 360 K. Then the intensities of the both phosphors decreased with the further increase in temperature.
The phosphor exhibited abnormal thermal quenching, first discovered in Na3Sc2(PO4)3:Eu2+ blue phosphors in 2017 [41]. It was later found in several phosphors [42,43,44,45,46]. In the Sr3LaNb3O12 crystal, Sr, La, and Nb ions occupy the same crystal sites. Moreover, the radius of Sr2+ (r = 1.18 Å, CN = 6) is very close to that of Dy3+ (r = 0.912 Å, CN = 6). Dy3+ may enter into the Sr2+ lattice of Sr3LaNb3O12, an unbalanced charge substitution causing lattice defects [47]. Therefore, this abnormal thermal quenching phenomenon can ascribe to the lattice defects, which function like electron capture centers. In the end, the remaining energy of the excited electron was transferred and stored in Dy3+, which was higher than the loss caused by NR transition. Therefore, the emission intensity became stronger as temperature increased. It showed good thermal stability and potential in high-power photoelectric devices, especially w-LEDs.
Besides the variation of emission spectral intensity, the change of CIE chromaticity coordinates can also be used to evaluate the stability of phosphors at different temperatures. The CIE chromaticity coordinates of the Sr3LaNb3O12:0.02Dy3+ and Sr3LaNb3O12:0.05Dy3+ vary only slightly from 300 to 480 K in Fig. 9a, b. Therefore, the phosphors are almost the same color. The changes in CIE chromaticity coordinates of the Sr3LaNb3O12:0.02Dy3+ and Sr3LaNb3O12:0.05Dy3+, respectively are (∆x = 3.89 × 10–5, ∆y = 6.94 × 10–5) and (∆x = 3.33 × 10–6, ∆y = 0.89 × 10–5) by calculating. So the phosphors manifest good coloring stability at high temperatures.
For the sake of learning the mechanism of thermal quenching, the research knows more about the activation energy (Ea) of thermal quenching. By the Arrhenius model combined with experimental data, the relational graph of ln[(I0/I) − 1] ~ 1/T was used to achieve the activation energy in Fig. 10. The following is the Arrhenius equation [48,49,50]:
where I0is the initial luminous intensity; k is a Boltzmann constant, which is 8.62 × 10–5 eV/K; I(T) is the luminous intensity in different temperatures; c is a constant; Ea is the constant activation energy. The calculated Ea of the Sr3LaNb3O12:Dy3+ was 0.29 eV, which further indicated that the Sr3LaNb3O12:xDy3+ have good thermal stability.
CIE chromaticity coordinates play an important role in evaluating the luminescence properties of the phosphors. The CIE chromaticity coordinates of the Sr3LaNb3O12:xDy3+ (x = 0.01, 0.02, 0.05, 0.10, 0.15, 0.20, 0.25, and 0.30 mol) phosphor samples namely are (0.426, 0.452), (0.420, 0.448), (0.425, 0.450), (0.421, 0.449), (0.424, 0.446), (0.422, 0.442), (0.416, 0.434), and (0.435, 0.455), respectively, as listed in Table 3. The CIE chromaticity coordinates of the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) samples are shown in Fig. 11. They all drop into the yellow-light region of the CIE 1931 chromaticity coordinates, and all points are distributed in one piece. In addition, with increasing doping concentration, the CIE chromaticity coordinates (x, y) show a little change, and the chromaticity coordinate displacement (∆x, ∆y) is (0.019, 0.022). The small change indicate the different Dy3+-doped concentrations do not make a difference in color. The data indicated that the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) phosphors have good color stability to apply in w-LEDs.
CCT is also important for the luminescence properties of phosphors. The CCT value affects whether the lamp emits warm or cold light. The light shows a cold lamp when CCT is more significant than 4000 K, the light emits a warm lamp while CCT is less than 3200 K. By the McCamy formula, CCT can be calculated [51, 52]:
where inverse slope line, n = (x-xe)/(y-ye), (x, y) are the chromaticity coordinates of the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) samples, and (xe, ye) are the coordinates of the epicenter, which are (0.332, 0.186). The CCT value corresponding to the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) phosphors can be calculated in Table 2. These CCTs correspond to 3245, 3375, 3252, 3346, 3258, 3271, 3275, and 3242 K, respectively, which prove that these phosphors could be applied in w-LEDs.
The QY play an important role in w-LED application, which is calculated by the following equation [53]:
where LS is the emission spectrum of the Sr3LaNb3O12:0.05Dy3+, ES and ER represent the excitation light with and without the sample in the integrating sphere, respectively. The calculating QY value of the Sr3LaNb3O12:0.05Dy3+ phosphor is 18.24% under 352 nm in Fig. 12, which shows that the Sr3LaNb3O12:0.05Dy3+ phosphor is suitable for application for w-LEDs.
The synthesized Sr3LaNb3O12:0.05Dy3+ yellow phosphor, BAM blue phosphor, and (Sr, Ca)AlSiN3:Eu2+ red phosphor were combined with the n-UV InGaN chip (388 nm) to make up a w-LED. The prepared w-LED has high Ra (91), low CCT (4162 K), and CIE coordinates (0.365, 0.368). Figure 13a reveals that the manufactured w-LED compares with the commercial w-LED in terms of CRI values. Ra plays a vital role in describing a parameter of the w-LED luminescence properties. The histogram comparison of the particular rendering index (R1–R14) shows the rendering performance of different colors. It is significantly higher than the commercial w-LED values (Ra = 80, R9 = 15) [54,55,56]. Thus, a new component phosphor is used to produce w-LED to make up for the traditional limitations and has excellent potential in the future. Figure 13b shows the CIE coordinates for preparing the w-LED, which is lying white area and approaching the equal-energy point coordinates (0.333, 0.333). Therefore, this w-LED can become an excellent alternative to commercial w-LEDs.
4 Conclusions
In summary, the Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) of space group R_3m perovskite was successfully synthesized by high-temperature solid-phase method. The XRD patterns proved that the prepared Sr3LaNb3O12:xDy3+ (x = 0.01–0.30 mol) phosphors were a pure phase, and the Dy3+ ions can quickly replace the La3+ ions which has no effect on the crystal structure. The element distribution maps of the prepared samples are distributed. Sr3LaNb3O12:0.05Dy3+ had the most optimal luminescence intensity among a series of Sr3LaNb3O12 doped with different concentrations of Dy3+. Under the excitation of 352 nm, Sr3LaNb3O12:0.05Dy3+ had a prominent yellow peak of 575 nm, which was 4F9/2-6H13/2. The observed CQ phenomenon was caused by the interaction of dipole–dipole. The abnormal thermal phenomenon was due to lattice defects where Dy3+ replaces Sr2+. The activation energy of phosphor was calculated to be 0.29 eV. The CIE coordinates were located in the yellow region. The changes in temperature and doping concentration had only a slight effect on the color stability. White light with low CCT (4162 K) and Ra (91) was successfully prepared by an n-UV InGaN chip (388 nm), whose CIE chromaticity coordinates were (0.365, 0.368). Sr3LaNb3O12:Dy3+ was expected to replace commercial yellow phosphor in w-LED applications due to its high thermal stability and excellent optical performance.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy orethical restrictions.
References
G. Li, X. Lu, Q. Mao, G. Du, M. Liu, L. Chu, J. Zhong, Suppression of charge imbalance via Li+-Mn4+ co-incorporated Sr2YSbO6 red phosphors for warm w-LEDs. Mater. Today Chem. 23, 100744 (2022)
Y. Chen, Y.M. Lan, D. Wang, G.X. Zhang, W.L. Peng, Y.Q. Chen, X. He, Q.G. Zeng, J. Wang, Luminescence properties of Gd2MoO6:Eu3+ nanophosphors for WLEDs. Dalton T. 50, 6281–6289 (2021)
Y.X. Cao, X.C. Wang, J.Y. Ding, X.P. Zhou, T. Seto, Y.H. Wang, Constructing a single-white-light emission by finely modulating the occupancy of luminescence centers in europium-doped (Ca1-xSrx)9Bi(PO4)7for WLEDs. J. Mater. Chem. C 8, 9576–9584 (2020)
X.W. Li, H.L. Xu, X.M. Xia, F.Y. Xie, S.L. Zhong, D.K. Xu, One-step synthesis of Sc2W3O12:Eu3+ phosphors with tunable luminescence for WLED. Ceram Int. 45, 10461–10467 (2019)
M. Chen, H. Hu, N. Yao, X. Yuan, Q. Zhong, M. Cao, Y. Xu, Q. Zhang, Solvothermal synthesis of cesium lead halide nanocrystals with controllable dimensions: a stoichiometry defined growth mechanism. J. Mate.r Chem. C. 7, 14493–14498 (2019)
L. Ding, Y. Wang, P. Zhu, Y. Bai, One-step plant-inspired reaction that transform membrane hydrophobicity into high hydrophilicity and underwater super oleophobicity for oil-in-water emulsion separation. Appl. Surf. Sci. 479, 423–429 (2019)
H.M. Li, R. Pang, G.Y. Liu, W.Z. Sun, D. Li, L.H. Jiang, S. Zhang, C.Y. Li, J. Feng, H.J. Zhang, Synthesis and luminescence properties of Bi3+-activated K2MgGeO4: a promising high-brightness orange-emitting phosphor for WLEDs conversion. Inorg Chem. 57, 12303–12311 (2018)
J.H. Li, Z.H. Zhang, X.H. Li, Y.Q. Xu, Y.Y. Ai, J. Yan, J.X. Shi, M.M. Wu, Luminescence properties and energy transfer of YGa1.5Al1.5(BO3)4: Tb3+, Eu3+ as a multi-colour emitting phosphor for WLEDs. J. Mater. Chem. C 5, 6294–6299 (2017)
G. Hu, Y. Sun, J. Zhuang, X. Zhang, H. Zhang, M. Zheng, Y. Xiao, Y. Liang, H. Dong, H. Hu, B. Lei, C. Hu, Y. Liu, Enhancement of fluorescence emission for tricolor quantum dots assembled in polysiloxane toward solar spectrum-simulated white light-emitting devices. Small 16, e1905266 (2020)
N. Schulte-Römer, J. Meier, M. Söding, E. Dannemann, The LED paradox: how light pollution challenges experts to reconsider sustainable lighting. Sustainability. 11, 6060 (2019)
L. Franke, D. Sülflow, K. Stark, H. Piazena, R. Uebelhack, Acute effect of blue light exposition on well-being and melatonin secretion in humans. Eur. Psychiat. 24, 1–1 (2020)
S. Thakur, R. Dhakal, P.K. Verkicharla, Short-term exposure to blue light shows an inhibitory effect on axial elongation in human eyes independent of defocus. Invest. Ophth. Vis. Sci. 62, 22 (2021)
Q. Wang, Z. Mu, L. Yang, S. Zhang, D. Zhu, Y. Yang, D. Luo, F. Wu, The synthesis and the luminescence properties of Sr2Ga3La1-xDyxGe3O14. Physica. B 530, 258–263 (2018)
Q. Wang, Z. Mu, S. Zhang, Q. Zhang, D. Zhu, J. Feng, Q. Du, F. Wu, A novel near infrared long-persistent phosphor La2MgGeO6:Cr3+, RE3+ (RE = Dy, Sm). J. Lumin. 206, 618–623 (2019)
L. Yang, Z. Mu, S. Zhang, Q. Wang, D. Zhu, Y. Zhao, D. Luo, Q. Zhang, F. Wu, Dy3+ Doped Ca9Gd(PO4)7: a novel single-phase full-color emitting phosphor. J. Mater. Sci-Mater. El. 29, 6548–6555 (2018)
L. Camargo, P.R. González, M. Santiago, H.O. Dávila, J. Marcazzó, A preliminary study of the radioluminescence and optically stimulated luminescence of CaF2:Ce,Dy phosphor. J. Lumin. 246, 118845 (2022)
H.Y. Wu, H.M. Li, L.H. Jiang, R. Pang, S. Zhang, D. Li, G.Y. Liu, C.Y. Li, J. Feng, H.J. Zhang, Synthesis, structure and optical properties of novel thermally robust Dy3+-doped Ca9Sc(PO4)7 phosphors for NUV-excited white LEDs. J. Rare. Earth. 39, 277–283 (2021)
L. Li, F. Qin, Y. Zhou, Y. Zheng, J. Miao, Z. Zhang, Photoluminescence and time-resolved-luminescence of CaWO4:Dy3+ phosphors. J. Lumin. 224, 117308 (2020)
X. Wu, X. Zhao, Q. Ren, L. Du, M. Pei, O. Hai, Tuning of emission by effect of local symmetry in BaLaLiWO6:Dy3+/Eu3+ for WLEDs. Ceram. Int. 48, 18793–18802 (2022)
P. Vinodkumar, S. Panda, G. Jaiganesh, R.K. Padhi, U. Madhusoodanan, B.S. Panigrahi, SrBPO5: Ce3+, Dy3+ - a cold white-light emitting phosphor. Spectrochim. Acta. A 253, 119560 (2021)
K. Dev, A. Selot, G.B. Nair, V.L. Barai, F.Z. Haque, M. Aynyas, S.J. Dhoble, Energy transfer from Ce3+ to Dy3+ ions for white light emission in Sr2MgAl22O36:Ce3+, Dy3+ phosphor. J. Lumin. 206, 380–385 (2019)
P. Du, J.S. Yu, Photoluminescence, cathodoluminescence and thermal stability of Sm3+-activated Sr3La(VO4)3 red-emitting phosphors. Luminescence 32, 1504–1510 (2017)
P. Sehrawat, R.K. Malik, R. Punia, S.P. Khatkar, V.B. Taxak, Augmenting the photoluminescence efficiency via enhanced energy-relocation of new white-emanating BaYAlZn3O7:Dy3+ nano-crystalline phosphors for WLEDs. J. Alloy. Compd. 879, 160371 (2021)
L. Zhang, X. Zhang, C. Zhao, Crystal structure, luminescence properties and thermal stability of novel Sr2CaLa(VO4)3: Sm3+ phosphor synthesized by the combustion method. J. Alloy. Compd. 899, 163378 (2022)
M. Wang, X. Hu, Z. Zhan, T. Sun, Y. Tang, Facile fabrication of CeVO4 hierarchical hollow microspheres with enhanced photocatalytic activity. Mater. Lett. 253, 259–262 (2019)
T. Sun, M. Wang, Y. Tang, J. Ding, Y. Zhang, Copolymer-assisted synthesis and photoluminescent properties of 3D hierarchical EuF3 spherical architectures. Mater. Lett. 177, 120–123 (2016)
M. Wang, X. Yang, S. Tian, Y. Guo, T. Sun, M. Wang, Y. Tang, Constructing novel hierarchical porous hydrangea-like ZnWO4 microspheres with enhanced photocatalytic performance. Mater. Lett. 264, 127417 (2020)
F. Fan, L. Zhao, Y. Shang, J. Liu, W. Chen, Y. Li, Thermally stable double-perovskite Ca3TeO6:Eu3+ red-emitting phosphors with high color purity. J. Lumin. 211, 14–19 (2019)
P. Cai, L. Qin, C. Chen, J. Wang, S. Bi, S.I. Kim, Y. Huang, H.J. Seo, Optical thermometry based on vibration sidebands in Y2MgTiO6:Mn4+ double perovskite. Inorg. Chem. 57, 3073–3081 (2018)
C.X. Luo, Y.X. Zhang, T.F. Deng, Pressureless sintering of high performance silicon nitride ceramics at 1620 degrees C. Ceram. Int. 47, 29371–29378 (2021)
K. Mishra, S.K. Singh, A.K. Singh, S.B. Rai, Optical characteristics and charge transfer band excitation of Dy3+ doped Y2O3 phosphor. Mater Res Bull. 47, 1339–1344 (2012)
P. Dewangan, D.P. Bisen, N. Brahme, S. Sharma, R.K. Tamrakar, I.P. Sahu, K. Upadhyay, Influence of Dy3+ concentration on spectroscopic behaviour of Sr3MgSi2O8:Dy3+ phosphors. J. Alloy. Compd. 816, 152590 (2020)
B.C. Jamalaiah, Y.R. Babu, Near UV excited SrAl2O4:Dy3+ phosphors for white LED applications. Mater. Chem. Phys. 211, 181–191 (2018)
J.X. An, S. Zhang, R.W. Liu, G.X. Hu, Z.W. Zhang, Y.Y. Qiu, Y.Y. Zhou, F.M. Zeng, Z.M. Su, Luminescent properties of Dy3+/Eu3+ doped fluorescent glass for white LED based on oxyfluoride matrix. J. Rare. Earth. 39, 26–32 (2021)
G.S.R. Raju, E. Pavitra, H. Patnam, G.L. Varaprasad, N.R. Chodankar, S.J. Patil, K.S. Ranjith, M.V.B. Rao, J.S. Yu, J.Y. Park, Y.S. Huh, Y.K. Han, Rationalized crystal structure augmented highly efficient far-red-emitting double perovskite niobate phosphor for indoor plant growth LED applications. J. Alloy Compd. 903, 163881 (2022)
L. Tang, L. Zhou, X. Yan, K. Zhong, X. Gao, J. Li, A new NIR-emissive fluorescence turn-on probe for Hg2+ detection with a large Stokes shift and its multiple applications. J. Photoch. Photobio. A 387, 112160 (2020)
Y. Tang, Y. Liu, W.M. Ning, L.S. Zhan, J.Q. Ding, M.L. Yu, H.J. Liu, Y.H. Gao, G.H. Xie, C.L. Yang, Manipulating Forster and Dexter interactions between a thermally activated delayed fluorescence host and a phosphorescent dopant for highly efficient solution-processed red and white OLEDs. J. Mater. Chem. C 10, 4637 (2022)
G. Sowjanya, R. Doddoji, V.R.M. Reddy, Structural, luminescence, energy transfer mechanism, and photometric properties of Sm3+-doped KYBO phosphors. Radiat. Phys. Chem. 190, 109791 (2022)
M. Rajendran, S. Vaidyanathan, Zero-concentration quenching: a novel Eu3+ based red phosphor with non-layered crystal structure for white LEDs and NaSrY(MoO4)3:Sm3+ based deep-red LEDs for plant growth. Dalton T. 49, 9239–9253 (2020)
A. Hooda, S.P. Khatkar, A. Khatkar, S. Chahar, S. Devi, J. Dalal, V.B. Taxak, Characteristic white light emission via down-conversion SrGdAlO4:Dy3+ nanophosphor. Curr. Appl. Phys. 19, 621–628 (2019)
Y.H. Kim, P. Arunkumar, B.Y. Kim, S. Unithrattil, E. Kim, S.H. Moon, J.Y. Hyun, K.H. Kim, D. Lee, J.S. Lee, W.B. Im, A zero-thermal-quenching phosphor. Nat. Mater. 16, 543–550 (2017)
Y.Z. Yin, W.H. Yang, Z. Wang, Y. Zhang, M.C. Zhu, C. Dou, Y. Che, S.J. Sun, C. Hu, B. Teng, J.Q. Zhao, J.Y. Lu, R.Y. Sun, D.G. Zhong, Achieving zero-thermal quenching luminescence in ZnGa2O4: 0.02Eu3+ red phosphor. J. Alloy Compd. 898, 162786 (2022)
X.L. Wu, R. Shi, J.L. Zhang, D.W. Wen, Z.X. Qiu, X.G. Zhang, W.L. Zhou, L.P. Yu, S.X. Lian, HighlyS efficient and zero-thermal-quenching blue-emitting Eu2+-activated K-beta-alumina phosphors. Chem. Eng. J. 429, 132225 (2022)
S.H. Wang, Y.Q. Xu, T. Chen, W.H. Jiang, J.M. Liu, X. Zhang, W. Jiang, L.J. Wang, A red phosphor LaSc3(BO3)4:Eu3+ with zero-thermal-quenching and high quantum efficiency for LEDs. Chem. Eng. J. 404, 125912 (2021)
S.Q. Piao, Y.C. Wang, X.F. Zhou, W.Y. Geng, J.S. Zhang, X.Z. Zhang, D.Y. Wu, Y.Z. Cao, X.P. Li, B.J. Chen, Defect engineering in a Eu2+-doped beta-Al2O3 structure blue phosphor and its controllable zero-thermal quenching luminescence. ACS Sustain. Chem. Eng. 9, 7882–7890 (2021)
X.G. Zhang, Z.P. Zhu, Z.Y. Guo, F.W. Mo, Z.C. Wu, A zero-thermal-quenching and color-tunable phosphor LuVO4: Bi3+, Eu3+ for NUV LEDs. Dyes Pigments 156, 67–73 (2018)
R. Shi, L.X. Ning, Z.Q. Wang, J.T. Chen, T.K. Sham, Y. Huang, Z.M. Qi, C.J. Li, Q. Tang, H.B. Liang, Zero-thermal quenching of Mn2+ red luminescence via efficient energy transfer from Eu2+ in BaMgP2O7. Adv. Opt. Mater. 7, 1901187 (2019)
J. Crapse, N. Pappireddi, M. Gupta, S.Y. Shvartsman, E. Wieschaus, M. Wuhr, Evaluating the Arrhenius equation for developmental processes. Mol. Syst. Biol. 17, e9895 (2021)
L.Z. Liu, R.Y. Liu, T. Xu, Q. Zhang, Y.B. Tan, Q.L. Zhang, J.D. Ding, Y.F. Tang, Enhanced catalytic oxidation of chlorobenzene over MnO2 grafted in situ by rare earth oxide: surface doping induces lattice oxygen activation. Inorg. Chem. 59, 14407–14414 (2020)
N. Zhou, P. Gao, Y. Yang, Y. Zhong, M. Xia, Y. Zhang, Y. Tian, X. Lu, Z. Zhou, Novel orange–red emitting phosphor Sr8ZnY(PO4)7:Sm3+ with enhanced emission based on Mg2+ and Al3+ incorporation for plant growth LED lighting. J. Taiwan Inst. Chem. E 104, 360–368 (2019)
G. Lakshminarayana, A.N. Meza-Rocha, O. Soriano-Romero, E.F. Huerta, U. Caldino, A. Lira, D.E. Lee, J. Yoon, T. Park, Analysis of fluorescence characteristics of Sm3+-doped B2O3-rich glasses for Orange-light-emitting diodes. J. Alloy Compd. 884, 161076 (2021)
S. Verma, D. Kumar, S. Dutta, V. Sharma, H.C. Swart, V. Kumar, A novel near white light emitting phosphor KSrYSi2O7:Dy3+: synthesis, characterization and luminescence properties. Vacuum 174, 109179 (2020)
C. Liu, Z. Xia, Z. Lian, J. Zhou, Q. Yan, Structure and luminescence properties of green-emitting NaBaScSi2O7:Eu2+phosphors for near-UV-pumped light emitting diodes. J. Mater. Chem. C 1, 7139–7147 (2013)
M. Gao, C. Peng, X. Dong, G. Xue, A. Wang, Y. Pan, A red phosphor of Ba3In2F12:Mn4+ with enhanced moisture stability for warm WLED application. J Lumin. 242, 118564 (2022)
Y. Xu, L. Zhang, S. Yin, X. Wu, H. You, Highly efficient green-emitting phosphors with high color rendering for WLEDs. J. Alloy Compd. 911, 165149 (2022)
Q. Liu, Z. Chen, X. Chen, Y. Zhu, W. Chen, S. Liu, P. Chen, T. Yuan, B. Wei, Eu3+ and Mn4+ co-doped BaLaMgNbO6 double perovskite phosphors for WLED application. J. Lumin. 246, 118808 (2022)
Funding
This work was supported by the Undergraduate Innovation Fund of Northwest A&F University, China (202210712058, 202210712050, S202210712394, 202110712190, 202110712188, S202110712707, S202010712314, S202010712006), the Scientific and Technological Plan of Guangdong Province, China (No. 2019B090905005), National Natural Science Foundation of China (No. 11804265), and Home for researchers (www.home-for-researchers.com), China.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yuchen Zhang, Zhikai Liang, Weixian Wang, Yifang Lin, Rong Tang, Jingfei Wang, Daomiao He, Jinhui Liu, Zhong Zhang, Lingzhi Zhao, Bin Deng, Dan Zhang, Ruijin Yu. The first draft of the manuscript was written by Yuchen Zhang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
I certify that the Yuchen Zhang and other co-authors of the article “Optical properties of yellow-emitting Sr3LaNb3O12:Dy3+ phosphors with an abnormal thermal quenching for w-LED applications” have no conflict of interest, financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Liang, Z., Wang, W. et al. Optical properties of yellow-emitting Sr3LaNb3O12:Dy3+ phosphors with an abnormal thermal quenching for white light-emitting diode applications. J Mater Sci: Mater Electron 33, 26619–26632 (2022). https://doi.org/10.1007/s10854-022-09374-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09374-4