Abstract
Lead-free Ba0.85Ca0.15Zr0.1Ti0.9O3 (BCZT) ceramic powders were synthesized using the sol–gel method. The ceramics thickness was reduced to achieve high-energy storage and large electrocaloric effect in bulk ceramics. Dielectric, ferroelectric, energy storage, and electrocaloric properties were investigated for BCZT ceramic with 400 µm. Here, pure crystalline structure and homogenous microstructure were identified by XRD analysis and SEM measurements, respectively. The dielectric measurements revealed a maximum dielectric constant associated with ferroelectric–paraelectric phase transition. The maximum of \(\varepsilon^{\prime}_{{\text{r}}}\) was 17841, around 352 K. Furthermore, the BCZT ceramic exhibited improved energy storage and electrocaloric properties. A high recoverable energy density Wrec of 0.24 J/cm3 and a total energy density Wtotal of 0.27 J/cm3 with an efficiency coefficient of ~ 88% at 423 K under an electric field of 55 kV/cm were obtained. Besides, The maximum value of ΔT = 2.32 K, the electrocaloric responsivity ζ = 0.42 K mm/kV, the refrigeration capacity RC = 4.59 J/kg and the coefficient of performance COP = 12.38 were achieved around 384 K under 55 kV/cm. The total energy density Wtotal and the temperature change ΔT were also calculated by exploiting the Landau–Ginzburg–Devonshire (LGD) theory. The theoretical results matched the experimental findings. These results suggest that the synthesized BCZT ceramic with reduced thickness could be a promising candidate for energy storage and electrocaloric applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ferroelectric materials can be defined as dielectric materials in which permanent polarization exists even after removing the applied electric field. For most applications of ferroelectric materials, the dielectric constant and loss are essential practical parameters. These applications include non-volatile memories, pyroelectric detectors, sensors, actuators, transducers, capacitors, generators, optical memories, and energy harvesting devices [1].
The most used ferroelectric materials are oxides with a perovskite structure of ABO3. A cubic lattice of space group (Pm-3m) describes this high symmetry structure [2]. Where A is an alkali cation, alkaline earth, or rare-earth, B is a transition cation of atomic radius smaller than that of site A. The structure is a network of corner-linked oxygen octahedral, with the smaller cation filling the octahedral holes and the large cation filling the dodecahedral holes.
Among perovskite ferroelectric materials, the most studied are lead titanate (PbTiO3) [3], lead zirconate titanate (PZT) [4], lanthanum zirconate titanate (PLZT) [5]. However, lead is recognized as toxic, dangerous to humans and the environment. Therefore, it is necessary to develop environmentally friendly materials with no lead [6]. Thus, several scientific works have focused mainly on BaTiO3 (BT) perovskite because it has interesting advantages such as low dielectric loss and stable electrical properties for long-term operations [7]. However, their use is limited due to their relatively high curie temperature Tc (~ 390 K) [8]. Therefore, adequate atomic substitutions are expected to modify the chemical bonds of the structure and, thus, the mobility of the ions and the resulting polarizability. A series of studies have been initiated to modify the dielectric and ferroelectric properties of BaTiO3 systems, including (Ba,Ca)TiO3 [9], Ba(Ti,Zr)O3 [10], (Ba,Ca)(Ti,Sn)O3 [11],etc. In these doped (BT) matrix systems, the dielectric loss of Ba(Ti,Zr)O3(BZT) ceramics is very low, which can be attributed to the increased substitution rate of zirconium [12]. In addition, the good temperature stability of (Ba, Ca) TiO3 (BCT) ceramics is due to the introduction of Ca at site A of the BT system, which leads to the diffuse phase transition [13].
More recently, (Ba,Ca)(Zr,Ti)O3 (BCZT) materials with Ca2+ and Zr4+ at site A and site B of the BaTiO3 perovskite structure have been widely studied for their excellent dielectric, ferroelectric and piezoelectric properties, and their composition closed to the morphotropic phase boundary MPB [14]. Among these ceramics, xBa (Zr0.2Ti0.8) O3− (1 − x) (Ba0.7Ca0.3) TiO3 ceramics with (0.48 < x < 0.52) were largely studied, and the results showed that the composition Ba0.85Ca0.15Zr0.1Ti0.9O3 has considerable dielectric, ferroelectric and piezoelectric properties compared to other components [15]. In this regard, Wang et al. reported that Ba0.85Ca0.15Zr0. 1Ti0.9O3 ceramics exhibit a high spontaneous polarization (Ps = 9.69 µC/cm2), a low coercive field (Ec = 1.89 kV/cm) and a high-dielectric permittivity (\(\varepsilon^{\prime}_{{\text{r}}}\) = 16,310) [16]. In addition, due to low-remnant polarization, high-maximal polarization, and adjustable breakdown strength (BDS), relaxor ferroelectrics (RFEs), including BCZT, have a promising future in the field of dielectric energy, and their electric polarization response can be converted into recoverable electrostatic energy.
One of the crucial aspects of functional microstructure is the synthesis method. Many papers have reported that the sol–gel process should be a rather promising approach as it offers the possibility of mixing metal ions at the atomic level [17, 18]. Besides, this method also has several potential advantages of better homogeneity, chemical purity, and stoichiometric composition, which can be difficult to achieve by the solid-state method [19]. With a variation of the sintering temperature, various BCZT properties can be extracted. Ji et al. reported that BCZT sintered at 1400 °C had a uniform microstructure with a density of 5.56 g/cm3 and had excellent electrical properties (\(\varepsilon^{\prime}_{{\text{r}}}\) = 8808, Pr = 12.24 µC/cm2, d33 = 485 pC/N) [20]. Moreover, BCZT ceramics obtained by sol–gel method and sintered at 1420 °C have outstanding electrical and energy storage properties (d33 = 558 pC/N, \(\varepsilon^{\prime}_{{\text{r}}}\) = 3375, tgδ = 0.02, and W = 0.52 J/cm3) as evaluated by Zhong Ming Wang et al. [17]. The energy density can also be demonstrated by exploiting the Landau-Ginzburg-Devonshire (LGD) phenomenological theory. The modelling result supports the experimental findings as reported for BaTiO3-BaSnO3 by Yonggang et al. [21].
Moreover, the electrocaloric effect could be investigated by indirect EC effect calculation through Maxwell relation and theoretical investigations based on the phenomenological approach via Landau–Ginzburg–Devonshire (LGD) theory. Meanwhile, a large electrocaloric responsivity (ξ = ∆T/∆E) was found (ξ = 0.19 K mm/kV) for BCZT ceramic sintered at 1420° C, as reported by Kaddoussi et al. [22]. Additional estimations of the EC effect in ferroelectric materials can be obtained by theoretical investigations exploiting the LGD phenomenological approach.
A different approach has been used to improve the electrocaloric efficiency (1) by monitoring the perovskite composition, (2) by combining ferroelectric, relaxor ferroelectric, and antiferroelectric materials, (3) using composites (ceramics/polymers), and (4) by reducing the ceramic thickness. On the other hand, according to previous studies on the energy storage density of material dielectrics, a higher breakdown strength is responsible for a larger energy storage density [23]. In this regard, the breakdown strength of dielectrics increases exponentially with decreasing ceramic thickness [24]. Thus, reducing thickness is a practical method to enhance dielectrics' breakdown strength and energy density. As a result, the dielectric thin films exhibit high breakdown strength, large energy storage density and giant electrocaloric effect compared to bulk ceramics. However, their energy capacities are low because of the low thickness or volume of thin films, making them unsuitable for cooling applications where a high level of energy is needed. This indicates that thin bulk ceramics should be more appropriate for practical energy storage density and electrocaloric effect. Thus, to achieve higher energy storage properties and a giant electrocaloric effect in bulk ceramics, enhancing the breakdown strength is necessary to tolerate strong electric fields while keeping a large dielectric volume. This can be accomplished by decreasing the ceramic’s thickness.
This work aims to improve the energy storage and electrocaloric properties of BCZT ceramic prepared by the sol–gel method while reducing its thickness. The energy storage properties were determined from the P–E hysteresis and the experimental findings were compared with the theoretical results derived from the phenomenological approach through the LGD theory. Furthermore, the electrocaloric effect was investigated by two methods: the indirect approach following Maxwell's relation and the phenomenological approach through the LGD theory.
2 Experimental
The Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramic was synthesized by the sol–gel route. Stoichiometric amounts of barium acetate Ba(CH3COO)2 (99%), calcium nitrate tetrahydrate Ca(NO3)2·4H2O (99%), zirconyl chloride octahydrate ZrOCl2.8H2O (98%), and titanium isopropylate C12H28O4Ti (97%) were used as precursors. As solvents, acetic acid (100%) and 2-methoxyethanol (99%) were chosen. Ba(CH3COO)2 and Ca(NO3)2·4H2O were initially dissolved in acetic acid, while [ZrOCl2·8H2O] was dissolved in 2-Methoxyethanol, then an appropriate amount of C12H28O4Ti was added. The precursor solution of (Zr,Ti) was added to that of (Ba,Ca) and stirred for 1 h at room temperature to give a BCZT solution, and then the pH was adjusted with ammonia to 7. The sol obtained was dried in air for 48 h to remove the solvents and then ground into a fine powder in a mortar. The powder was calcined for 4 h at 1000° C in the air to form a pure crystalline phase. The calcined powder was then pressed into cylindrical discs by uniaxial pressing to prepare pellets sintered at a high temperature of 1420° C for 4 h to improve the ceramic’s density. The pellets were polished to adjust the ceramic thickness at 400 µm.
The sintered ceramic’s phase structure was determined by X-ray diffraction (XRD, Panalytical X-Pert Pro) analysis using the Cu-Kα radiation with λ ~ 1.540598 Å. The measurements were performed at room temperature in a range of 2θ from 20° to 80° with a step size of 0.02°. Rietveld refinement analysis was realized using FullProf software. A Scanning Electron Microscope (SEM, VEGA 3-Tscan) revealed the surface’s morphologies. The density of the pellet was measured using Archimedes’ method. The dielectric measurements were examined using an HP 4284A precision impedance meter, controlled by a computer in the 20 Hz to 1 MHz frequency range. Differential scanning calorimetry (Perkin–Elmer Jade DSC) with a heating rate of 5 °C/min was utilized to highlight the various phase transitions. The surfaces of the sintered sample were covered with a conductive silver paste serving as electrodes for electrical measurements. The polarization–electric field (P–E) hysteresis loops were collected using CPE1701, PolyK, USA, with a high-voltage power supply (Trek 609–6, USA). The electrocaloric study was achieved by the indirect method from recorded (P–E) hysteresis loops at 10 Hz as a function of temperature.
3 Results and discussion
3.1 Structural and microstructural properties
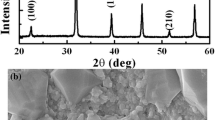
Figure 1 presents the X-ray diffraction pattern of BCZT ceramic sintered at 1420 °C for 4 h. The formation of a complete single-phase perovskite structure, with any impurity phase, is evident in the room temperature XRD data, suggesting that Ca2+ and Zr4+ have been diffused into the BaTiO3 lattice to form a solid solution [25]. The peak between 2θ ≈ 45° was split into two peaks corresponding to (002) and (200) planes, as represented in Fig. 1b. This could be the reflection of a tetragonal structure [26]. The splitting peaks of (022) and (200) around 2θ ≈ 45° imply the existence of an orthorhombic phase which can also be confirmed by the formation of the triplet around 2θ ≈ 66° [27], as displayed in Fig. 1c. The coexistence of orthorhombic and tetragonal phases indicates the formation of BCZT ceramic at the morphotropic phase boundary (MPB). The coexistence of tetragonal and orthorhombic phases at room temperature was also reported in many reports [16, 28]. The data of XRD was analyzed by Rietveld refinement [29, 30], which was achieved using a combination of tetragonal symmetry with space group P4mm and orthorhombic symmetry with space group Pmm2 (χ2 = 1.86, Rp = 17.1 and Rwp = 13.5). Structural information and phase composition are regrouped in Table 1.
Figure 2 shows the surface morphology of the sample of the BCZT ceramic sintered at 1420 °C. The grains of the ceramic show an irregular morphology and a uniform distribution. The average grain size is 45 µm. The SEM shows that the ceramic displays a dense morphology due to the high-sintering temperature. The latter facilitates the mass transfer and pore removal resulting in tightly packed grains and a high density of 5.70 g/cm3, which is ~ 95% of the theoretical density.
3.2 Dielectric properties
Figure 3a shows the thermal variations of the real part of the dielectric permittivity (\(\varepsilon^{\prime}_{{\text{r}}}\)) as a function of the frequency for BCZT ceramic; the inset shows the dielectric losses (tgδ). The results show that \(\varepsilon^{\prime}_{{\text{r}}}\) increases rapidly and then decreases with increasing measurement temperature. This behavior is the same as a conventional ferroelectric material [31]. Two dielectric anomalies, TO–T and TC, were observed around 300 and 352 K, corresponding to the phase transitions from orthorhombic to tetragonal and tetragonal to cubic, respectively. This result agrees well with DSC data, revealing two endothermic peaks at the same transition temperature (TO–T and TC) (Fig. 3b). The response of space charge polarization induced by the creation of lattice vacancies, such as cation or oxygen vacancies, could cause a high \(\varepsilon^{\prime}_{{\text{r}}}\) at a low frequency [26]. However, as frequency increases, space charge polarization become more limited, resulting in a decrease in \(\varepsilon^{\prime}_{{\text{r}}}\) [26]. At 1 kHz, the maximum of \(\varepsilon^{\prime}_{{\text{r}}}\) was 17,841 around 352 K. The dielectric loss tgδ was less than 0.06, which may be attributed to the dense microstructure and the lower electron diffusion in the grain boundaries [33]. Compared to BCZT synthesis by solid-state and sintered at 1350 °C, the maximum of \(\varepsilon^{\prime}_{{\text{r}}}\) was 6330 around 388 K [32], which is lower than our results. The increase in \(\varepsilon^{\prime}_{{\text{r}}}\) could be caused by increasing density, which is essential for the electrocaloric effect.
3.3 Ferroelectric properties
Figure 4 show the hysteresis loops at different temperatures at an electric field of 55 kV/cm for BCZT ceramic. The measurements were carried out at different temperatures and at a constant frequency of 10 Hz. At 303 K, the P–E loop reveals a closed and saturated loop similar to a conventional ferroelectric behavior [33]. The maximal polarization (Pmax) was 25.75 µC/cm2, the remnant polarization (Pr) was 15.15 µC/cm2, and the coercive field (Ec) was found to be 6.41 kV/cm. It is also observed that the hysteresis loops showed typical characteristics of ferroelectric to paraelectric phase transition with nonlinear to linear behavior [34]. As the temperature increases, the hysteresis loops narrow until linear behavior is achieved, and there is a continuous decrease of Pr, Ec, and Pmax due to the thermal agitation [35]. This behavior is a typical feature of paraelectric ferroelectric phase transitions. Pr and Pmax's value is higher than that of BCZT ceramic prepared by solid-state reaction (Pmax = 16.01 µC/cm2–Pr = 10.98 µC/cm2) [32] due to a good homogeneity in grain size and uniform grain microstructure of the samples prepared by sol–gel. Indeed, the proportion (f) of grains' contribution to polarization for ferroelectric materials can be described by [36]:
where d is the grain size and Ga is a constant representing the grain anisotropy energy density. Therefore, the ferroelectric properties were proportional to grain size. Thus, with the increasing grain size, the contribution of grain to polarization increases, giving rise to the enhancement of ferroelectricity [36]. Besides, a relatively lower Ec was found compared to ceramic prepared by solid-state (Ec = 9.30 kV/cm) [32]. The large grain of ferroelectric ceramics is beneficial to a fast ferroelectric domain switching resulting in a reduced coercive electric field.
3.4 Energy storage performances
The energy storage properties of BCZT ceramic were deduced from the P–E hysteresis loops as a function of temperature. Nonlinear dielectrics possess some energy dissipation. Therefore, the total energy density (Wtotal), recoverable energy density (Wrec), and energy storage efficiency (η) could be calculated using the equations [37]:
P–E loops' upper and lower branches represent the discharge and charge process, respectively. Wrec could be determined by integrating the area between the polarization axis and the upper branch curve of the unipolar P–E hysteresis loop, Wrec represents the energy released during the discharging process. In contrast, Wloss produced by the domain reorientation is determined by integrating the P–E loop area and corresponds to the dissipation in dielectrics. Wtotal can be calculated by adding Wrec and Wloss as schematically presented in Fig. 5, and it corresponds to the total energy density during the charging process.
3.5 Electric-field-dependent energy storage properties at room temperature
Using Eqs. (2–4), the obtained values of total energy density Wtotal, energy storage density Wrec, energy loss density Wloss, and energy storage efficiency η as a function of electric field have been illustrated in Fig. 6. Wtotal, Wrec, and Wloss display a sharp linear rise with the increasing applied electric field at room temperature. Wtotal, Wrec, and Wloss increased from 0.10 to 0.55 J/cm3, 0.01 to 0.12 J/cm3 and 0.08 to 0.43 J/cm3 respectively, with the increasing applied electric field from 5 to 55 kV/cm. However, the value of η increases steadily with increasing the electric field. From 5 to 55 kV/cm, η increased from 15.10 to 24.12%. These results indicate that higher applied electric fields can induce more polarization, enhancing the energy storage properties [38].
3.6 Temperature-dependent energy storage properties
Another essential aspect of energy storage applications is the high-temperature stability of dielectric ceramics. To gain insight, the temperature dependence of energy storage properties of BCZT ceramic has been studied. Figure 7 shows the variation of Wtotal, Wrec, Wloss, and η for BCZT ceramic as a function of temperature. On the one hand, Wrec increase with the increase in temperature. This behavior may be due to the enhancement in the mobility of domain walls with a rise in temperature [38]. On the other hand, a decrease in Wloss values with increased temperature is observed. This behavior can be attributed to ease in the reorientation of domains with rising temperature [39]. As a result, energy storage efficiency is enhanced. At the maximum electric field of 55 kV/cm, the maximum efficiency value is η = 88.05% at 324 K with Wrec = 0.24 J/cm3. These results are comparable to others reported in the literature under a different electric field (Table 2). Using the same synthesis method and the temperature of synthesis, Mezzourh et al. [16] found a recovered energy density Wrec of 0.02 J/cm3 and storage efficiency η of 63.65% under 7.7 kV/cm in BCZT ceramic. On the other hand, by using an electric field of 20 kV/cm, Cai et al. [40] reported a recovered energy density of 0.06 J/cm3 and storage efficiency of 85.8% in BCZT ceramic elaborated by the solid-state method with almost the same grain size (44.37 µm) as our samples, which is lower than our results. For BCZT synthesis by sol–gel hydrothermal and at 60 kV/cm, the obtained Wrec was 0.36 J/cm3 and η = 67.25% with the grain size of 22.1 µm, as stated by Hanani et al. [41].
Total energy density (Wtotal), energy storage density (Wrec), energy loss density (Wloss), and energy storage efficiency (η) as a function of temperature for BCZT ceramic at 55 kV/cm Fig. 8 Comparison of the thermal evolution of Wtotal obtained experimentally and using Landau–Ginzburg–Devonshire (LGD) theory at 55 kV/cm for BCZT ceramic
In comparison, BCZT ceramic under 55 kV/cm exhibited lower energy storage performances. Therefore, it is important to note that the energy-storage properties depend on the method of synthesis as well as the grain size. The better energy density may be due to smaller grain sizes and more uniform grain microstructure.
3.7 Temperature-dependent energy storage density using landau phenomenological approach
More insight into calculations of the total energy density Wtotal can be obtained based on the Landau–Ginzburg–Devonshire (LGD) phenomenological theory, describing the macroscopic phenomena in ferroelectric materials near the phase transition. The total energy density Wtotal can be calculated by considering the simple Landau-type free energy model [48]:
where a, b and c are the temperature-dependent coefficients. In ferroelectrics, \(a\left( T \right) = a_{1} \left( {T{-}T_{{\text{c}}} } \right)\) where Tc is the curie temperature. At equilibrium, the first deviation of free energy should equal zero \(\left( {\partial F{/}\partial P = 0} \right)\) [45], which thus leads to:
The electric field versus polarization (E–P) data for all considered temperatures can be fitted by Eq. (6), which allows the extraction of the a, b and c parameters for each temperature. These coefficients are important for energy storage because they express the energy density, according to the Landau model [45], where Pmax is the polarization at Emax.
Figure 8 compare the temperature-dependence of the estimated Wtotal obtained via LGD theory with that deduced from the experimental results under 55 kV/cm in BCZT ceramic. Both methods show the same trend, Wtotal decreases with increasing temperature. At room temperature, the Wtotal obtained by LGD was 0.51 J/cm3, which is lower than that found experimentally (0.55 J/cm3). However, both methods lead to the same values at the paraelectric phase transition. Note, the coefficients a, b and c are temperature-dependent in relaxor ferroelectrics, which could be the main reason for the difference between the results obtained by the theoretical calculation and the experimental result [46].
3.8 Indirect electrocaloric measurements
The electrocaloric effect (ECE) is universally known as the reversible entropy and temperature change when an electric field is applied or removed [47]. An indirect method based on Maxwell’s relation between the entropy (S) and the dielectric polarization (P) is used to estimate the adiabatic temperature change ∆T [48]:
∆T under an applied electric field is calculated by the following equation:
where P is polarization, ρ is the density of the sample, and Cp denotes the specific heat capacity of the material. E1 and E2 are initial and final applied electric fields, respectively. E1 is taken as zero in the present case and E2 corresponds to the saturation field value. The value of polarization gradient \(\left( {\partial P/\partial T} \right)_{E}\) was found from the derivation of P versus T data [39].
Temperature change alone does not provide sufficient data to determine the global EC performance. Thus, the isothermal entropy change (∆S) is an important parameter to evaluate the relative heat extraction capacity of the material. ∆S is calculated by the following equation:
Figure 9a, b shows the variation of ∆T and ∆S as a function of temperature at different applied electric fields for BCZT ceramic. It has been observed that ∆T and ∆S increase with the increase in the electric field. This behavior is as per Eqs. (9) and (10). ∆T and ∆S curves, which correspond to a fixed applied electric field, exhibit a maximum around Tc due to the diffusion behavior of our sample near the ferroelectric-to-paraelectric phase transition and multiphase coexistence [49]. From Eqs. (9) and (10), it is evident that materials having a more significant polarization gradient as well as a higher saturation field (E2) will show larger ∆T. At ∆Emax of 55 kV/cm, ∆Tmax was 2.32 K, and ∆Smax was 1.98 J/Kg/K for BCZT ceramic with a thickness of 400 µm. The maximum peak is near transition temperature for lower electric fields. However, the peak position of ∆ Tmax and ∆ Smax moves gradually toward the higher temperature with increasing the intensity of the applied electric field. This peak shift relates to the diffused nature of phase transition, which might be due to a change in domain configuration or field-induced transition on the application of higher electric fields [38].
However, comprehensive conclusions cannot be drawn alone with adiabatic temperature change (∆T) values about the overall caloric performance of a ferroelectric material. Thus, the electrocaloric responsivity (∆T/∆E), i.e., the ratio of adiabatic temperature change and the field change, is an important parameter to evaluate the ECE behavior of the material [50]. At 384 K, the maximum electrocaloric responsivity ζmax is 0.42 K mm/kV at 55 kV/cm for BCZT ceramic. The EC effect compared for different materials, electric fields, and thickness are gathered in (Table 3). We can observe that the same material with the same composition shows different ECE. Certainly, for the BCZT with 1 mm of thickness and synthesized by solid-state, ζmax reached 0.23 K mm/kV at 25 kV/cm [32], which is lower than our results. On the other hand, with the same ceramic thickness of 1 mm, the ECE of BCZT at 27 kV/cm was higher than many other lead-free ceramics. Indeed, Patel et al. [34] elaborated the (Ba0.85Ca0.15)(Zr0.1Ti0.88Sn0.02)O3 ceramics by solid oxide reaction, revealing that the ECE response reached high values of 0.84 K and 0.26 K mm/kV for ∆ Tmax and ζmax, respectively, under 32 kV/cm at 347 K. As well as (Ba0.85Sr0.15)(Zr0.1Ti0.9)O3 ceramics fabricated by conventional solid-state reaction route, which Δ Tmax = 0.5 K and ζmax = 0.16 K mm/kV at 347 K at 30 kV/cm, as stated by Patel et al. [51]. At an electric field of 55 kV/cm, BCZT exhibited better ECE properties compared to those found in 0.98(BaTiO3)–0.02 Bi(Mg0.5 Ti0.5)O3 ceramics with 2 mm of a thickness (Δ Tmax = 1.21 K and ζmax = 0.22 K mm/kV at 416 K) by Li et al. [52]. The synthesis conditions (such as calcination and sintering), chemical doping, grain form, the number of coexisting phases, the ceramic thickness, and the applied external electric field are all factors that contribute to these differences.
It is crucial to remember that measurements' frequency and temperature interval can affect the ECE values derived from indirect measurements utilizing PE hysteresis loops. It can also be affected by Cp, which is, in approximation, held constant. For that reason, refrigeration capacity (RC) is evaluated to look at the overall material’s performance. RC is calculated by the following equation:
Figure 10 shows the variation of RC as a function of temperature at 55 kV/cm for BCZT ceramic. RC increases with increasing electric field. RC shows a maximum around Tc due to phase transition, as discussed before. At 55 kV/cm, RCmax was estimated to be 4.59 J/kg for BCZT with a thickness of 400 µm. The obtained value was higher than many other lead-free ceramics [35, 52].
Another important measure in evaluating the EC performance is the coefficient of performance (COP) as calculated by the following equation, where Q is the isothermal heat.
Figure 10 displays the variation of COP as a function of temperature at 55 kV/cm for BCZT ceramic. The COP reveals the same behaviors as ∆T, ∆S and RC. Around Tc, COP exhibits a maximum. At 55 kV/cm, COPmax was 12.38 for BCZT ceramic. The obtained value is higher than that obtained by other literature [41, 53]. Nanocrystalline ceramics of [(k0.5na0.5)nbo3]0.97-[lisbo3]0.03 show a COP of 8.14 under an electric field of 40 kV/cm as reported by Kumar et al. [54]. Whereas Hao et al. [55] report a COP of 2.9 in Pb0.97La0.02Zr0.87Sn0.08Ti0.07O3 antiferroelectric thick film. Attending a strong electric field by reducing the thickness of ceramics can improve the EC parameters. The high values of ΔT, ΔS, ζ, RC and COP make BCZT ceramic with low thickness a promising candidate for eco-friendly electrocaloric applications.
The temperature change, ΔT, can also be calculated using Landau–Ginzburg–Devonshire phenomenological theory. The free energy can be definite as Eq. (5) in terms of electric polarization. The entropy change is obtained from Eq. (13) [56].
The EC response can be given using \(\Delta T = - \left( {T\Delta S/C_{{\text{p}}} } \right)\) [56] as follows:
where Cp is the specific heat capacity of the material at given field E. Since ΔT ≪ T, Eq. 14 can be simplified to:
Figure 11 compare the temperature-dependence of the estimated ΔT obtained via LGD theory with that deduced from the experimental results under 55 kV/cm in BCZT ceramic. Both methods show the same behavior, ΔT increase with temperature increasing, reach a maximum around Tc, and then decrease. This comportment is due to the diffusion behavior of our sample near the ferroelectric-to-paraelectric phase transition [49], as discussed before. At room temperature, the ΔT obtained by LGD was 2.33 K, which is lower than that found experimentally (2.11 K). However, the same values at the paraelectric phase transition were observed for both methods. The difference between the results obtained by the theoretical calculation and the experimental result may be due to the coefficients a, b and c, which are temperature-dependent in relaxor ferroelectrics [56].
4 Conclusion
Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramic sintered at 1420 °C for 4 h was successfully prepared by the sol–gel method. The XRD showed the coexistence of two tetragonal and orthorhombic phases at room temperature. The SEM revealed a large grain size of ~ 45 µm. The ceramic thickness was reduced to achieve high-energy storage and a large electrocaloric effect in bulk ceramics. The BCZT ceramic with a thickness of 400 µm showed both interesting energy storage properties and a significant electrocaloric effect. The recoverable energy density Wrec was 0.24 J/cm3, and Wtotal was 0.27 J/cm3 with an efficiency coefficient of ~ 88% at 423 K under a 55 kV/cm electric field. On the other hand, BCZT show an outstanding temperature change ∆T = 2.32 K and significant electrocaloric responsivity ζ = 0.421 K mm/kV, high refrigeration capacity RC = 4.59 J/kg and interesting coefficient of performance COP = 12.38. The total energy density Wtotal and the temperature change ∆T were also calculated by exploiting the Landau-Ginzburg-Devonshire (LGD) theory. The theoretical result matched the experimental findings. These characteristics suggest that this eco-friendly BCZT ceramic could be a prospective candidate for dielectric energy storage and electrocaloric applications.
Data availability
Not applicable.
Code availability
Not applicable.
References
Y. Xu, Ferroelectric materials and their applications (Elsevier, New York, 2013)
R.E. Cohen, Origin of ferroelectricity in perovskite oxides. Nature 358(6382), 136–138 (1992). https://doi.org/10.1038/358136a0
S. Zhang, F. Li, High performance ferroelectric relaxor-PbTiO3 single crystals: Status and perspective. J. Appl. Phys. 111(3), 031301 (2012). https://doi.org/10.1063/1.3679521
L. Wu, C.-C. Wei, T.-S. Wu, C.-C. Teng, Dielectric properties of modified PZT ceramics. J. Phys. C: Solid State Phys. 16(14), 2803–2812 (1983). https://doi.org/10.1088/0022-3719/16/14/020
H.-B. Park, C.Y. Park, Y.-S. Hong, K. Kim, S.-J. Kim, Structural and dielectric properties of PLZT ceramics modified with lanthanide ions. J. Am. Ceram. Soc. 82(1), 94–102 (1999). https://doi.org/10.1111/j.1151-2916.1999.tb01728.x
P.K. Panda, B. Sahoo, PZT to lead free piezo ceramics: a review. Ferroelectrics 474(1), 128–143 (2015). https://doi.org/10.1080/00150193.2015.997146
Y. Huan, X. Wang, J. Fang, L. Li, Grain size effect on piezoelectric and ferroelectric properties of BaTiO3 ceramics. J. Eur. Ceram. Soc. 34(5), 1445–1448 (2014). https://doi.org/10.1016/j.jeurceramsoc.2013.11.030
K. Sakayori et al., Curie temperature of BaTiO3. Jpn. J. Appl. Phys. 34(9S), 5443 (1995). https://doi.org/10.1143/JJAP.34.5443
X.M. Chen, T. Wang, J. Li, Dielectric characteristics and their field dependence of (Ba, Ca)TiO3 ceramics. Mater. Sci. Eng. B 113(2), 117–120 (2004). https://doi.org/10.1016/j.mseb.2004.04.003
T. Badapanda, S. Chaterjee, A. Mishra, R. Ranjan, S. Anwar, Electric field induced strain, switching and energy storage behaviour of lead free Barium Zirconium Titanate ceramic. Physica B 521, 264–269 (2017)
D. Xue, Y. Zhou, H. Bao, J. Gao, C. Zhou, X. Ren, Large piezoelectric effect in Pb-free Ba(Ti, Sn)O3–x(Ba, Ca)TiO3 ceramics. Appl. Phys. Lett. 99(12), 122901 (2011). https://doi.org/10.1063/1.3640214
P. Julphunthong, S. Chootin, T. Bongkarn, Phase formation and electrical properties of Ba(ZrxTi1−x)O3 ceramics synthesized through a novel combustion technique. Ceram. Int. 39, S415–S419 (2013). https://doi.org/10.1016/j.ceramint.2012.10.105
X.W. Wang et al., Effects of calcining temperature on structure and dielectric and ferroelectric properties of sol–gel synthesized Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics. J. Electron. Mater. 49(1), 880–887 (2020). https://doi.org/10.1007/s11664-019-07769-4
M.B. Abdessalem, S. Aydi, A. Aydi, N. Abdelmoula, Z. Sassi, H. Khemakhem, Polymorphic phase transition and morphotropic phase boundary in Ba1−xCaxTi1−yZryO3 ceramics. Appl. Phys. A 123(9), 583 (2017). https://doi.org/10.1007/s00339-017-1196-7
W. Liu, X. Ren, Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 103(25), 257602 (2009). https://doi.org/10.1103/PhysRevLett.103.257602
H. Mezzourh et al., Enhancing the dielectric, electrocaloric and energy storage properties of lead-free Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics prepared via sol–gel process. Phys. B: Condens. Matter 603, 412760 (2021)
Z. Wang et al., Synthesis, structure, dielectric, piezoelectric, and energy storage performance of (Ba0.85Ca0.15)(Ti0.9 Zr0.1)O3 ceramics prepared by different methods. J. Mater. Sci.: Mater. Electron. 27(5), 5047–5058 (2016). https://doi.org/10.1007/s10854-016-4392-x
M. Maraj, W. Wei, B. Peng, W. Sun, Dielectric and energy storage properties of Ba(1–x)CaxZryTi(1−y)O3 (BCZT): A Review. Materials 12(21), 3641 (2019). https://doi.org/10.3390/ma12213641
Y. Zhang, H. Sun, W. Chen, A brief review of Ba(Ti0.8Zr0.2)O3–(Ba0.7Ca0.3)TiO3 based lead-free piezoelectric ceramics: past, present and future perspectives. J. Phys. Chem. Solids 114, 207–219 (2018). https://doi.org/10.1016/j.jpcs.2017.10.041
X. Ji et al., Structural and electrical properties of BCZT ceramics synthesized by sol–gel process. J. Mater Sci. (2018). https://doi.org/10.1007/s10854-018-8751-7
Y. Yao et al., Large piezoelectricity and dielectric permittivity in BaTiO3−XBaSnO3 system: the role of phase coexisting. Europhys. Lett. (EPL) (2012). https://doi.org/10.1209/0295-5075/98/27008
H. Kaddoussi et al., Indirect and direct electrocaloric measurements of (Ba1−xCax)(Zr0.1Ti0.9)O3 ceramics (x = 0.05, x = 0.20). J. Alloys Compd. 667, 198–203 (2016). https://doi.org/10.1016/j.jallcom.2016.01.159
J. Li et al., Grain-orientation-engineered multilayer ceramic capacitors for energy storage applications. Nat. Mater. 19(9), 999–1005 (2020). https://doi.org/10.1038/s41563-020-0704-x
C. Neusel, H. Jelitto, D. Schmidt, R. Janssen, F. Felten, G.A. Schneider, Thickness-dependence of the breakdown strength: analysis of the dielectric and mechanical failure. J. Eur. Ceram. Soc. 35(1), 113–123 (2015). https://doi.org/10.1016/j.jeurceramsoc.2014.08.028
I. Coondoo, N. Panwar, H. Amorín, M. Alguero, A.L. Kholkin, Synthesis and characterization of lead-free 0.5Ba(Zr0.2Ti0.8)O3–0.5(Ba0.7Ca0.3)TiO3 ceramic. J. Appl. Phys. 113(21), 214107 (2013)
P. Jaimeewong, M. Promsawat, A. Watcharapasorn, S. Jiansirisomboon, Comparative study of properties of BCZT ceramics prepared from conventional and sol-gel auto combustion powders. Integr. Ferroelectr. 175(1), 25–32 (2016). https://doi.org/10.1080/10584587.2016.1199913
Y. Tian, L. Wei, X. Chao, Z. Liu, Z. Yang, Phase transition behavior and large piezoelectricity near the morphotropic phase boundary of lead-free free (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 ceramics. J. Am Ceram. Soc. 96(2), 496–502 (2013)
S. Belkhadir et al., Structural, dielectric and electrocaloric properties of (Ba0.85Ca0.15)(Ti0.9Zr0.1−xSnx)O3 ceramics elaborated by sol–gel method. J. Mater. Sci 30(15), 14099–14111 (2019)
Z. Hanani et al., Enhanced dielectric and electrocaloric properties in lead-free rod-like BCZT ceramics. J. Adv. Ceram. 9(2), 210–219 (2020)
E.K. Abdel-Khalek, D.A. Rayan, A.A. Askar, M.I.A.A. Maksoud, H.H. El-Bahnasawy, Synthesis and characterization of SrFeO3−δ nanoparticles as antimicrobial agent. J. Sol-Gel Sci. Technol. 97(1), 27–38 (2021). https://doi.org/10.1007/s10971-020-05431-8
H. Kaddoussi et al., Room temperature electro-caloric effect in lead-free) Ba(Zr0.1Ti0.9)1−SnO3(x = 0, x = 0.075) ceramics. Solid State Commun. 201, 64–67 (2015). https://doi.org/10.1016/j.ssc.2014.10.003
S. Merselmiz et al., Enhanced electrical properties and large electrocaloric effect in lead-free Ba0.8Ca0.2ZrxTi1−xO3 (x= 0 and 0.02) ceramics. J. Mater. Sci 31(19), 17018–17028 (2020)
M. Ben Abdessalem, I. Kriaa, A. Aydi, N. Abdelmoula, Large electrocaloric effect in lead-free Ba1−xCaxTi1−yZryO3 ceramics under strong electric field at room-temperature. Ceram. Int. 44(12), 13595–13601 (2018)
S. Patel Sharma, R. Vaish, Enhanced electrocaloric effect Ba0.85Ca0.15Zr0.1Ti0.9–xSnxO3 in ferroelectric ceramics. Phase Transit. 89(11), 1062–1073 (2016)
S. Patel, M. Kumar, Electrocaloric properties of Sr and Sn doped BCZT lead-free ceramics. Eur. Phys. J. Appl. Phys. 91(2), 20905 (2020). https://doi.org/10.1051/epjap/2020200165
Q. Zhang et al., Enhanced piezoelectric response of (Ba, Ca)(Ti, Zr) O3 ceramics by super large grain size and construction of phase boundary. J. Alloy. Compd. 794, 542–552 (2019)
N. Qu, H. Du, X. Hao, A new strategy to realize high comprehensive energy storage properties in lead-free bulk ceramics. J. Mater. Chem. C 7(26), 7993–8002 (2019). https://doi.org/10.1039/C9TC02088H
P. Redhu, A. Hooda Sharma, S. Dahiya, R. Punia, R.P. Tandon, Study of energy storage and electrocaloric behavior of lead-free Fe-doped BCT ceramics. Ferroelectrics 569(1), 136–147 (2020). https://doi.org/10.1080/00150193.2020.1791661
K.R. Kandula, K. Banerjee, S.S.K. Raavi, S. Asthana, Enhanced electrocaloric effect and energy storage density of Nd-substituted 0.92 NBT-0.08 BT lead free ceramic. Phys. Status Solidi (a) 215(7), 1700915 (2018)
W. Cai et al., Synergistic effect of grain size and phase boundary on energy storage performance and electric properties of BCZT ceramics. J. Mater. Sci.: Mater. Electron. 31(12), 9167–9175 (2020)
Z. Hanani et al., Thermally-stable high energy storage performances and large electrocaloric effect over a broad temperature span in lead-free BCZT ceramic. RSC Adv. 10(51), 30746–30755 (2020)
K. Xu Yang, W. Peng, L. Li, Temperature-stable MgO-doped BCZT lead-free ceramics with ultra-high energy storage efficiency. J. Alloys Compd. 829, 154516 (2020)
Q. Zhang et al., Controllable synthesis of (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 submicron sphere by hydroxide co-precipitation method. Ceram. Int. 46(18), 28285–28291 (2020). https://doi.org/10.1016/j.ceramint.2020.07.331
S. Merselmiz et al., High energy storage efficiency and large electrocaloric effect in lead-free BaTi0.89Sn0.11O3 ceramic. Ceram. Int. 46(15), 23867–23876 (2020). https://doi.org/10.1016/j.ceramint.2020.06.163
Z. Jiang, S. Prosandeev, L. Bellaiche, Energy storage in lead-free Ba(Zr, Ti)O3 relaxor ferroelectrics: Large densities and efficiencies and their origins. Phys. Rev. B 105(2), 024102 (2022). https://doi.org/10.1103/PhysRevB.105.024102
Y. Hou, J. Li, J. Ding, T. Ye, R. Liang, Giant electrocaloric response in compositional manipulated BaTiO3 relaxor–ferroelectric system. J. Appl. Phys. 127(6), 064103 (2020). https://doi.org/10.1063/1.5142635
A.S. Mischenko, Q. Zhang, J.F. Scott, R.W. Whatmore, N.D. Mathur, Giant electrocaloric effect in thin-film PbZr0.95Ti0.05O3. Science 311(5765), 1270–1271 (2006). https://doi.org/10.1126/science.1123811
Z. Kutnjak, B. Rožič, R. Pirc, Electrocaloric effect: theory, measurements, and applications, in Wiley Encyclopedia of Electrical and Electronics Engineering. ed. by J.G. Webster (Wiley, Hoboken, 2015), pp. 1–19. https://doi.org/10.1002/047134608X.W8244
B. Zhang et al., Enhanced electrocaloric effect in the Sm and Hf co-doped BaTiO3 ceramics. Ceram. Int. 47(1), 1101–1108 (2021). https://doi.org/10.1016/j.ceramint.2020.08.226
Y. Liu, J.F. Scott, B. Dkhil, Direct and indirect measurements on electrocaloric effect: recent developments and perspectives. Appl. Phys. Rev. 3(3), 031102 (2016). https://doi.org/10.1063/1.4958327
S. Patel, A. Chauhan, R. Vaish, Caloric effects in bulk lead-free ferroelectric ceramics for solid-state refrigeration. Energ. Technol. 4(2), 244–248 (2016)
M.-D. Li, X.-G. Tang, S.-M. Zeng, Q.-X. Liu, Y.-P. Jiang, W.-H. Li, Giant electrocaloric effect in BaTiO3–Bi(Mg1/2Ti1/2)O3 lead-free ferroelectric ceramics. J. Alloys Compd. (2018). https://doi.org/10.1016/j.jallcom.2018.03.102
S. Merselmiz et al., Thermal-stability of the enhanced piezoelectric, energy storage and electrocaloric properties of a lead-free BCZT ceramic. RSC Adv. 11(16), 9459–9468 (2021). https://doi.org/10.1039/D0RA09707A
R. Kumar, S. Singh, Giant electrocaloric and energy storage performance of [(K0.5Na0.5)NbO3](1−x)–[LiSbO3]x nanocrystalline ceramics. Sci Rep 8(1), 3186 (2018). https://doi.org/10.1038/s41598-018-21305-0
X. Hao, Y. Zhao, Q. Zhang, Phase structure tuned electrocaloric effect and pyroelectric energy harvesting performance of (Pb0.97La0.02)(Zr,Sn,Ti)O3 antiferroelectric thick films. J. Phys. Chem. C 119(33), 18877–18885 (2015). https://doi.org/10.1021/acs.jpcc.5b04178
R. Pirc, Z. Kutnjak, R. Blinc, Q.M. Zhang, Electrocaloric effect in relaxor ferroelectrics. J. Appl. Phys. 110(7), 074113 (2011). https://doi.org/10.1063/1.3650906
G. Singh et al., Electro-caloric effect in 0.45BaZr0.2Ti0.8O3–0.55Ba0.7Ca0.3TiO3 single crystal. Appl. Phys. Lett. 102(8), 082902 (2013). https://doi.org/10.1063/1.4793213
H. Tang, X.-G. Tang, M.-D. Li, Q.-X. Liu, Y.-P. Jiang, Pyroelectric energy harvesting capabilities and electrocaloric effect in lead-free Sr Ba1-Nb2O6 ferroelectric ceramics. J. Alloys Compd. 791, 1038–1045 (2019). https://doi.org/10.1016/j.jallcom.2019.03.385
R. Kumar, S. Singh, Enhanced electrocaloric response and high energy-storage properties in lead-free (1−x)(K0.5Na0.5)NbO3−xSrZrO3 nanocrystalline ceramics. J. Alloys Compd. 764, 289–294 (2018). https://doi.org/10.1016/j.jallcom.2018.06.083
Acknowledgements
The authors gratefully acknowledge the generous financial support of CNRST Priority Program PPR 15/2015, the Slovenian Research Agency programs P1-0125, the European Union's Horizon 2020 research and the Ministry of education and science of the Russian Federation Project #13.2251.21.0042.
Funding
CNRST Priority Program PPR 15/2015; The European Union's Horizon 2020 research; The Ministry of education and science of the Russian Federation Project #13.2251.21.0042.
Author information
Authors and Affiliations
Contributions
All authors certify that they have participated sufficiently in the work to take public responsibility for the content. Furthermore, each author certifies that this work has not been and will not be submitted to other journal or published in any other publication before. AL: investigation, methodology, data curation, writing original draft, validation. HM: investigation, methodology, validation. DM: visualization, methodology, writing—review & editing, validation, supervision. MA: formal analysis, validation. LH: visualization, review, editing, validation, supervision. EHC: visualization, review, editing, validation, supervision. IAL: formal analysis, validation. ZK: review & editing, visualization, validation. MEM: visualization, writing—review & editing, validation, supervision. On behalf of all co-authors.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
Not applicable.
Consent to participate
We confirm that all authors mentioned in the manuscript have participated in, read and approved the manuscript, and have given their consent for the submission and subsequent publication of the manuscript.
Consent for publication
We confirm that all the authors mentioned in the manuscript have agreed to publish this paper.
Relevance summary
In this study, we report the successful preparation of lead-free Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramic with reduced thickness to reach a high electric field. The study of their structural, dielectric, and ferroelectric properties were also reported. The energy storage and electrocaloric properties were investigated using the indirect experimental approach based on the Maxwell relation and the Landau-Ginzburg-Devonshire (LGD) theory. We confirm that this work is original and has not been published elsewhere, nor it is not currently under consideration for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakouader, A., Mezzourh, H., Mezzane, D. et al. Improved energy storage and electrocaloric properties of lead-free Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramic. J Mater Sci: Mater Electron 33, 14381–14396 (2022). https://doi.org/10.1007/s10854-022-08362-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08362-y