Abstract
The Zn1−xGaxO (x = 0–0.020) ceramics modified with V2O5 were prepared by solid-state reaction method. The phase composition, microstructure, electrical conductivity, temperature sensitivity, and thermal aging property were investigated. The main phase of prepared ceramics is a hexagonal wurtzite crystal structure with a space group of P63mc (186). Ga2O3 phase was detected in ceramics when the content of Ga-ion x is higher than 0.010. V2O5 acts as sintering aids and electrical stabilizer and enhanced the ceramic sintering ability. The Ga/V-modified ZnO ceramics exhibit typical NTC characteristics and have high temperature sensitivity with material constant of B values ranging from 3659 to 4590 K. The electrical properties and aging characteristics were studied with alternating current impedance spectrum and X-ray photoelectron spectroscopy. The Ga/V-co-modified ZnO ceramics show high electrical stability with resistance change rate (ΔR/R0) less than 1.85% after aged at 150 °C for 1000 h. The increase of resistance by aging mainly came from the grain boundary effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A negative temperature coefficient (NTC) thermistor shows that its resistivity decreases with increase of temperature, especially, is characterized by that its resistivity decreases exponentially with the increase of temperature. NTC thermistors are widely applied in various fields, such as temperature compensation, temperature measurement and control, surge current suppression, and infrared detection. The researches of the ordinary temperature NTC ceramic thermistors are mainly based on spinel structure compounds [1,2,3], perovskite compounds [4,5,6], and semiconductors based on single cationic oxides [7,8,9]. The small polaron hopping model is generally considered to be the conduction mechanism of AB2O4-type spinel compounds, in which the charge carriers hop between octahedral B-sites such as Mn3+–Mn4+ ions in manganate spinel compounds [1, 2, 10, 11]. Meanwhile, both the band conduction and electron-hopping models were suggested for the conduction mechanism of semiconductor-type NTC thermistors [12,13,14]. Based on the above multi-model conduction mechanisms, the room-temperature resistivity (ρ25) and temperature sensitivity (B25/85 value) of semiconductor-type thermistors could be effectively adjusted through appropriate ion doping. As reported by Wang et al. [13], ρ25 from 64.14 Ω·cm to 43.37 kΩ·cm and B25/85 from 335 to 5169 K were obtained in Li/Fe-modified NiO NTC thermistors. Yang et al. reported that adjustable ρ25 (12.38 Ω·cm–190 kΩ·cm) and B25/85 (1112–4376 K) in CuO-based NTC thermistors were prepared by changing the contents of Y2O3 and B2O3 [14].
Besides ρ25 and B25/85 values, electrical stability is also an important property for a NTC thermistor. The electrical stability is normally characterized by resistance change rate (ΔR/R0) through aging treatment with various periods. The electrical stability of NTC thermistor can be improved by element doping and/or optimization of preparation process [7, 15, 16]. Li et al. showed that Na-ion doping reduced the resistivity and ΔR/R0 of Mn1.95Co0.21Ni0.84O4 ceramics [15]. Gao et al. found that the electrical stability of (Zn0.4Ni0.6)1−xNaxO NTC thermistors was greatly enhanced with the addition of Bi-ion and the ΔR/R0 was reduced from 237 (without Bi2O3) to 1.8% (with Bi2O3) [16]. Sb/Mn-co-doped SnO2 ceramics showed high electrical stability, and the ΔR/R0 of Sn0.91Sb0.05Mn0.04O2 was only 0.6% after aging 500 h in air [7].
ZnO is a typical environmental friendly material with a low cost price, non-toxic, and harmless. Due to its wide band gap (− 3.37 eV), ZnO has been widely used in the fields of luminescence, photocatalysis, varistor, and piezoelectricity [17,18,19,20]. In recent years, the researches on ZnO-based thermistors have also been reported. Li/Y/Cr-co-doped ZnO ceramics showed a critical positive temperature coefficient (PTC) phenomenon with a resistivity temperature coefficient as high as 65% K−1 [21]. Li et al. reported that ZnO-based ceramics co-modified with Al-, La-, and Cu-ions showed good performance for NTC thermistors with controllable ρ25 (0.65–3280 kΩ·cm) and B25/85 values (2500–5850 K) and high electrical stability (ΔR/R0 < 2%) [22]. To further develop the application of ZnO-based ceramics for NTC thermistors, Ga-doped ZnO ceramics modified with V2O5 were investigated in this work. The ceramics exhibit typical NTC characteristics with high temperature sensitivity (B25/85 value) and electrical stability.
2 Experimental
Zn1−xGaxO (x = 0, 0.002, 0.004, 0.006, 0.008, 0.010, 0.012, 0.014, 0.016, 0.018, and 0.020, respectively) ceramics were synthesized with conventional solid-state reaction method. Small amount of V2O5 (from 0.125 to 1.0% in mass ratio) was used as sintering aids and electrical stabilizer. Analytical reagent grade (purity > 99%) ZnO, Ga2O3, and V2O5 from the Sinopharm Chemical Reagent Co., Ltd, China were used as raw materials. According to the stoichiometric ratio of nominal formula Zn1−xGaxO, the weighed ZnO and Ga2O3 powders were mixed by ball milling for 1 h and then were calcined at 900 °C for 5 h in air. For each batch, various contents of V2O5 were added into the calcined powder followed by grinding and mixing for 1 h. An appropriate amount of polyvinyl alcohol solution (PVA) solution (Sinopharm Chemical Reagent Co., Ltd, China) was used as binder during granulating. Then pellets with a diameter of 12 mm and a thickness of about 3 mm were obtained. The pellets were sintered at 1150 °C for 5 h followed by 1350 °C for 2 h in air. The surfaces of as-sintered pellets were ground with abrasive paper. Silver paste was painted on both opposite surfaces and then was heated at 600 °C for 10 min to make ohmic electrodes.
Phase composition of the as-sintered Zn1−xGaxO ceramics was identified by X-ray diffraction (XRD, Rigaku D/Max 2500, Japan) with Cu Kα radiation. Fracture surface of broken ceramics was examined by a scanning electron microscopy (SEM, JMS-7900F), and the related elemental distribution was analyzed with energy-dispersive X-ray spectroscopy (EDS, Oxford Ultim Max 65). The possible valence states of elements in ceramics were analyzed by X-ray photoelectron spectroscopy (XPS, K-alpha 1063, UK). The relative density (ρr) of each sample was determined according to the Archimedes method and was calculated by Eq. (1).
where ρM is the measured density and ρL is the theoretical density. The theoretical density ρL (5.676 g/cm3) of the wurtzite ZnO lattice was calculated by the relationship of ρL = (MZn + MO)/NAV, where MZn is the total molar weight of Zn atoms and MO is the total molar weight of O atoms in each mole ZnO lattice, NA is Avogadro constant, and V is a cell volume of ZnO lattice. In order to simplify the comparison of relative density of the studied ceramics, the influence of dopants and possible lattice defects in the ZnO lattice was not regarded, i.e., only the perfect ZnO lattice was taken into account for ρL in this work.
The temperature dependence of resistance (R–T) of each sample was measured by a resistance–temperature test system (ZWX-C, China) in temperature range from 25 to 250 °C. The resistivities were calculated according to the Ohm's law, ρ = RA/h, where R is the measured resistance, A is the electrode area, and h is the sample thickness. Two kinds of methods were used to test the electrical stability of Zn1−xGaxO-based ceramics: repeated R–T measurements and measurement of the resistance change rate after aging treatment at 150 °C. Alternating current (AC) impedance was performed with electrochemical workstation (Gamry reference USA, 600) in frequency range from 1 Hz to 1 MHz. Each impedance spectrum was analyzed by Gamry analyst.
3 Results and discussion
3.1 Phase and microstructure
In this work, different contents of V2O5 (from 0.125 to 1.0% in mass ratio) were added to Zn0.990Ga0.010O ceramics, respectively. The ρr of Zn0.990Ga0.010O ceramics increased from 87.5 to 92.6% with the increase of V2O5 content from 0.125 to 1.0%. The increase of ρr becomes slow when the V2O5 content is more than 0.5%. So 0.5% of V2O5 was selected in the following experiments. The ρr of ZnO, Zn0.994Ga0.006O, and Zn0.980Ga0.020O with 0.5% V2O5 are 90.5%, 93.2%, and 93.6%, respectively.
Figure 1 shows SEM micrographs obtained from the fracture surfaces of as-sintered ceramics of ZnO without V2O5, ZnO with 0.5% V2O5, Zn0.994Ga0.006O with 0.5% V2O5, and Zn0.980Ga0.020O with 0.5% V2O5. It can be seen from Fig. 1a that the ZnO ceramic without V2O5 contains lots of pores. While, the pores in the ceramics with 0.5% V2O5 addition become much less and even vanish, as shown in Fig. 1b–d. These indicate that the addition of a small amount of V2O5 can effectively enhance the sintering ability of ZnO-based ceramics. This should be due to the low melting point of V2O5 (− 690 °C) for which the formation of liquid phase is helpful for the gas overflowing and can accelerate the mass transfer during sintering process.
The elemental distribution mapping of fracture surface of Zn0.990Ga0.010O ceramic was analyzed by EDS, as shown in Fig. 2. Zn, O, Ga, and V elements are almost evenly distributed in the sample and the black area in these mappings might be caused by the roughness of the fracture surface.
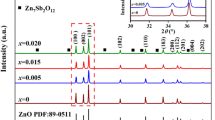
Figure 3 shows XRD patterns of as-sintered Zn1−xGaxO-based ceramics with 0.5% V2O5 (ZnO, Zn0.994Ga0.006O, and Zn0.990Ga0.010O). ZnO and Zn0.994Ga0.006O ceramics are composed of pure hexagonal wurtzite phase with a space group of P63mc (186) (ref. PDF No. 80–0074). While one extra diffraction peak marked by “•” as shown in Fig. 3a can be found in Zn0.99Ga0.010O ceramic and is consistent with the diffraction of Ga2O3 phase (PDF Card of No. 76–0573). These imply that the solid solubility of Ga-ions in ZnO lattice might be x < 0.010 in this work. Figure 3b shows the magnified view of diffraction peaks with 2θ from 30° to 38°. With the increase of Ga-ion concentration, the diffraction peaks shifted toward higher diffraction angles, indicating a reduction of lattice parameters. Refined with Jade 6.0 + PDF 2004 program, the lattice parameters of hexagonal wurtzite phase for each ceramic are obtained as shown in Table 1. The reduction of lattice parameters should result from the substitution of Ga-ions in ZnO lattice for that the ionic radius of Ga3+ ion (0.062 nm) is smaller than that of Zn2+ ion (0.075 nm) [23]. At the same time, some V-cations might also substitute into the ZnO lattice, and the substitution of V-ions also reduced the ZnO-based lattice for the smaller ionic radius of V5+ (0.054 nm) than that of Zn2+ ion.
To explore the possible valence states of elements in as-sintered ceramics, Zn0.990Ga0.010O ceramic was selected for XPS analysis and the results are shown in Fig. 4. Characteristic peaks of Zn, O, Ga, and V can be detected in the full XPS spectrum as shown in Fig. 4a. The narrow spectra for each element were fitted by Avantage 5.52 software and the results are shown in Fig. 4b–e, respectively. As shown in Fig. 4b, the peaks at binding energies of 1021.15 eV and 1044.24 eV correspond to Zn 2p3/2 and 2p1/2, respectively [24], indicating only Zn2+ ions exist in Zn0.990Ga0.010O ceramic.
Figure 4c shows the narrow spectrum of O element. The spectrum of O 1 s can be fitted to be composed of two peaks. One peak locating at 531.33 eV (marked with OD/A) should attribute to defective oxygen (OD) and adsorbed oxygen (OA) and the other peak at 529.89 eV corresponds to lattice oxygen (OL) [25]. According to the areas of fitted peaks, the content ratio of OD/A and OL is [OD/A]/[OL] = 0.961. The peaks of Ga 2p3/2 and 2p1/2 with binding energies of 1117.35 eV and 1144.12 eV, respectively, are shown in Fig. 4c, indicating that Ga-ion is Ga3+ [26]. In Fig. 4e, V 2p3/2 was detected to be composed of peaks at binding energies of 517.02 eV and 515.72 eV, respectively, indicating that V-ions in the ceramics have V5+ and V4+ valences [24]. The content ratio [V5+] / [V4+] in the ceramic is calculated to be 1.80, demonstrating that V5+ is the main valence state of V-ions in Zn0.990Ga0.010O ceramic.
3.2 Electrical properties
The temperature dependence of resistivity in lnρ − 1000/T plots of Zn1−xGaxO ceramics is shown in Fig. 5a and b. The resistivities of Ga-ion-doped ZnO ceramics decrease with the increase of temperature and display typical NTC characteristic. The nearly linear relationship of lnρ − 1000/T follows the Arrhenius law as expressed by Eq. (2).
where ρT is the resistivity at temperature T (in Kelvin), ρ0 is a constant related to material characteristic, Ea is activation energy of conduction, k is the Boltzmann constant, and B is a material constant reflecting the temperature sensitivity of a NTC thermistor. The B value can be calculated by Eq. (3)
where ρ1 and ρ2 are the resistivities at temperatures T1 and T2, respectively. The temperatures of T1 and T2 are normally selected at 298 K (25 °C) and 358 K (85 °C), respectively. So, the B value is often written as B25/85.
Figure 5c shows the plots of Ga-content dependence of lnρ25 and B25/85 in Zn1-xGaxO ceramics. lnρ25 of Zn1−xGaxO ceramics with x ≤ 0.006 decreases with the increase of Ga-content. These should be due to the doping effect of a semiconductor. Ga3+ ions substituted into the ZnO lattice and introduced electron charge carriers. The related defect reaction can be expressed by Eq. (4).
Here, the weakly bound electrons at the donor level are easy to be thermally activated to the conductive band, improving the conductivity of Zn1−xGaxO ceramics. In the meanwhile, the introduced V-ions might also partially substitute into ZnO lattice, and the related defect reaction can be expressed by Eq. (5).
The substituted V-ion can act as donor and introduce electron charge carriers in the ZnO crystal. Combined with V-ions and Ga-ions, too much electrons introduced into the ZnO lattice could lead to aggregation of charge carriers, resulting in the increase of ρ25 when the dopant content was further increased (as shown in Fig. 5c). In the meanwhile, for the solid solubility limit of Ga-ions and V-ions in ZnO, the excessive Ga2O3 and V2O5 might segregate at the grain boundaries and enhance the grain boundary barrier, resulting in high grain boundary resistivity. On the other hand, as shown in Fig. 1, the increase of Ga-ions content in ZnO-based ceramics restrained the grain growing and increased the relative content of grain boundary accordingly. In addition, due to the large difference in porosity among ceramics, pores may have some impact on the conductivity of ceramics. So, the total resistance of ceramics increases with the increase of the content increase of Ga-ions in ZnO-based ceramics.
With the increase of Ga-ion concentration, B25/85 values decrease first and then increase. The minimum B25/85 value of Zn0.990Ga0.010O ceramics is 3659 K when the content of Ga-ion (x) is 0.006. When the content of Ga-ion (x) is more than 0.006, B25/85 values of Zn1−xGaxO ceramics are in the range of 3659–4590 K. The adjustable ρ25 and B25/85 values enhance the application prospect of Zn1−xGaxO ceramics as NTC thermistors.
3.3 Impedance spectrum analysis
To understand the conduction characteristic of Zn1−xGaxO ceramics, alternating current (AC) impedance was measured and analyzed. Figure 6 shows the Nyquist plots of Zn1−xGaxO ceramics with various contents of Ga-ions measured at room temperature. The plots were fitted using equivalent circuit inset as shown in Fig. 6a. Where Rg and Rgb are the resistances corresponding to the grain effect and grain boundary effect, respectively, R0 is the resistance from the measuring system, and CPEg and CPEgb are constant phase components related to internal inhomogeneity or defects. The fitted curves are in good agreement with the measured data. These reveal that the electrical properties of Zn1−xGaxO ceramics originate from both grain effect and grain boundary effect. The fitted resistance from grain effect (Rg), grain boundary one (Rgb), and total one (Rt = Rg + Rgb) of ceramics are shown in Table 2.
Compared with pure ZnO ceramic, both Rg and Rgb decreased with the increase of Ga-ions when x < 0.006 in Zn1-xGaxO and the decrease of Rg is greater than that of Rgb. These should result from the semiconductor doping effect as described in Eq. (4). Conversely, when x > 0.006, both Rg and Rgb increase with the increasing of Ga-ion concentration and the increase of Rgb is more than that of Rg. For solid solubility limit of Ga2O3 in ZnO crystal, the excessive Ga-ions may locate at the grain boundaries as impurity, such as Ga2O3. In the meanwhile, V2O5 might also locate at grain boundaries. The grain boundaries impurities hinder the electron transfer and increase the grain boundary resistance.
3.4 Electrical stability
Electrical stability is an essential property for the commercial application of NTC thermistors. Figure 7 show the plots of temperature dependence of resistivity (lnρ − 1/T plots) tested repeatedly for seven times for Zn0.990Ga0.010O and Zn0.980Ga0.020O ceramics. The lnρ − 1/T plots coincide well with each other, indicating that the Zn0.990Ga0.010O and Zn0.980Ga0.020O ceramics have high electrical repeatability.
Zn1−xGaxO ceramics with silver electrodes were treated at 150 °C for 1000 h to explore their electrical stability characteristics. Figure 8 shows the resistance change rate (ΔR/R0) after aging treatment for different periods. The ΔR/R0 of ZnO ceramic increased continuously during aging and is as high as 67.30% after aging for 1000 h (see in Fig. 8a). Doping with Ga-ions, the ΔR/R0 decrease obviously after aging (see in Fig. 8b, c). The ΔR/R0 of Ga-doped ceramics increase slightly in initial 600 h aging and then the resistance change rate became stable. With 1000 h of aging treatment, the final ΔR/R0 of Zn1−xGaxO is 1.41% for x = 0.002, 1.07% for x = 0.004, 1.28% for x = 0.006, 1.52% for x = 0.008, 1.49% for x = 0.010, 1.59% for x = 0.012, 1.50% for x = 0.014, 1.40% for x = 0.016, 1.68% for x = 0.018, and 1.85% for x = 0.020. These show that Ga-doped Zn1-xGaxO ceramics with V2O5 addition have good electrical stability.
To further explore the aging characteristics of Zn1−xGaxO ceramics, AC impedance spectra were measured for the same samples as shown in Fig. 6 after aging. The related Nyquist plots after aging were analyzed as shown in Fig. 9. The Nyquist plots were fitted with the equivalent circuit inserted in Fig. 6a. The well fitted results indicate that the electrical properties of Zn1−xGaxO ceramics after aging are still composed of grain effect and grain boundary effect. The resistance change rates of grain effect, grain boundary effect, and total effect (ΔRg/Rg0, ΔRgb/Rgb0, and ΔRt/Rt0, respectively) were calculated by comparing the related resistance before and after aging, respectively. The results are shown in Table 3. ΔRt/Rt0 tested by AC impedance method is consistent with the DC-measured results as shown in Fig. 8. Except for ZnO ceramic, Rg decreased (ΔRg/Rg0 < 0) and Rgb increased (ΔRgb/Rgb0 > 0) after aging. These indicate that the aging-induced increase of total resistance mainly came from the grain boundary effect.
To explore the possible origination of aging-induced evolution of electrical properties, the valence states of elements in Zn0.990Ga0.010O ceramic after aging were investigated by XPS analysis. The XPS analysis is shown in Fig. 10. In Fig. 10a, the binding energy peaks of 1021.24 eV and 1044.32 eV correspond to Zn 2p3/2 and 2p1/2, respectively. These indicate that only Zn2+ ions exist in the aged Zn0.990Ga0.010O ceramic. The binding energies of 531.41 eV and 529.90 eV for O 1 s correspond to the peaks of the defective oxygen and/or adsorbed oxygen (OD/A) and lattice oxygen (OL), respectively, in the aged Zn0.990Ga0.010O ceramic as shown in Fig. 10b. Compared with the analyzed results of the sample before aging (see in Fig. 4c), there are tiny deviations in the position of characteristic peaks for OD/A and OL after aging. The ratio of [OD/A]/[OL] after aging is 1.58 and is quite different from that before aging (0.961).
The peaks of the 2p3/2 and 2p1/2 orbital energy spectra of Ga are shown in Fig. 10c. The peaks of binding energies of 1117.48 eV and 1144.25 eV indicate that Ga-element has the valence of Ga3+ in the aged Zn0.990Ga0.010O ceramic. The XPS spectrum of V-cations in Zn0.990Ga0.010O ceramic after aging is shown in Fig. 10d. The analysis shows that the XPS spectrum is composed of peaks with binding energies of 517.01 eV and 515.61 eV, respectively. It indicates that V-ions have V5+ and V4+ kind of valences in the aged Zn0.990Ga0.010O ceramic. The [V5+]/[V4+] ratio in the aged ceramic is 2.92, which is higher than that in the sample before aging ([V5+]/[V4+] = 1.80 as analyzed in Fig. 4e).
According to the analysis of O- and V-XPS spectra as discussed in Figs. 4c and e and 10b and d, both the [OD/A]/[OL] and [V5+]/[V4+] increased after aging treatment. These imply that the aging process should be closely related to oxygen adsorption and the change of V-ionic valence. Oxygen molecules (O2) in the aging environment might adhere on the ceramic surface. The adhered O2 may capture electrons from the ceramic body and turns into the adsorbed oxygen (O2ad) and even undergoes the procedure of \({\text{O}}_{{\text{2 ad}}} \to {\text{O}}_{{\text{2 ad}}}^{ - } \cdot \to {\text{O}}_{{{\text{ad}}}}^{ - } \to {\text{O}}^{2 - }\) [27, 28]. So, the reactive species of \({\text{O}}_{{\text{2 ad}}}^{ - } \cdot\) and oxygen ions (O2−) are formed. This procedure resulted in the increase of OD/A quantity and [OD/A]/[OL] ratio. At the same time, the variation of oxygen species must capture electrons from the ceramic body (both grains and grain boundaries). As in the analysis of XPS spectra, the valence state of V-ions changed during aging. So the captured electrons by OD/A should mainly come from V-ions, i.e., partial V4+ ions were oxidized to V5+ ions, resulting in the higher [V5+]/[V4+] ratio in the aged ceramic.
There might be two sources for the captured electrons by OD/A, directly from grain boundaries or from intragranular crystals. When the electrons were captured from the intragranular crystals, the segregation degree of excessive charge carriers induced by excessive doping Ga- and V- ions reduced, as discussed in Fig. 5c, resulting in the slight decrease of resistivity of the grain effect. These are in agreement with the results as analyzed in Fig. 9 and Table 3. When the OD/A-captured electrons come from the grain boundaries, the electron should originate from the V-ions for that vanadium oxides as sintering aids might locate at the grain boundaries. The defect reaction of \(V^{4 + } - e^{\prime} \to V^{5 + }\) took place and increased the [V5+]/[V4+] ratio. This process enhanced the Schottky barrier at grain boundaries and increased the resistivity of the grain boundary effect.
4 Conclusion
0.5% (mass ratio) of V2O5 can enhance the sintering ability of Ga-doped ZnO (Zn1−xGaxO) ceramics. V2O5-modified Zn1−xGaxO exhibits typical NTC characteristics and has high temperature sensitivity with material constant of B25/85 from 3659 to 4590 K. The prepared ZnO-based NTC ceramics show high electrical stability with resistance change rate (ΔR/R0) less than 1.85% after aged at 150 °C for 1000 h. The aging-induced resistance change rate is mainly due to the increase of grain boundary resistance. The aging process is proposed to include the following factors: the adsorbed oxygen captures electrons from the ceramics, the quantity of defect oxygen, and [V5+]/[V4+] ratio increases in the ZnO-based NTC ceramics.
References
K. Park, Microstructure and electrical properties of Ni1.0Mn2−xZrxO4 (0≤x≤1.0) negative temperature coefficient thermistors. Mater. Sci. Eng. B 104, 9–14 (2003)
C. Ma, H. Gao, TEM and electrical properties characterizations of Co0.98Mn2.02O4 NTC ceramic. J. Alloys Compd. 749, 853–858 (2018)
W. Hao, H. Zhang, C. Gao, B. Li, Z. Ma, Z. Li, Influence of V/Ni-doping on electrical properties and aging stability of ZnFe2O4-based NTC ceramics. J. Mater. Sci. Mater. Electron. 32, 17800–17809 (2021)
M. Chen, H. Zhang, T. Liu, H. Jiang, A. Chang, Preparation, structure and electrical properties of La1-xBaxCrO3 NTC ceramics. J. Mater. Sci. Mater. Electron. 28, 18873–18878 (2017)
J. Wang, H. Zhang, D. Xue, Z. Li, Electrical properties of hexagonal BaTi0.8Co0.2O3−δ ceramic with NTC effect. J. Phys. D 42, 235103 (2009)
F. Guan, Z. Dang, S. Huang, J. Wang, I. Milisavljevic, D. Carloni, X. Cheng, Y. Wu, LaCr1-xFexO3 (0 ≤ x ≤ 0.7): A novel NTC ceramic with high stability. J. Eur. Ceram. Soc. 40, 5597–5601 (2020)
G. Jiang, Z. Li, C. You, W. Hao, Z. Ma, H. Zhang, Temperature sensitivity and electrical stability of Sb/Mn co-doped SnO2 ceramics. J. Mater. Sci. Mater. Electron. 32, 16945–16955 (2021)
X. Wang, Z. Li, W. Yan, P. Wang, H. Zhang, Electrical properties of Nb/Al-doped CuO-based ceramics for NTC thermistors. Process. Appl. Ceram. 14, 47–55 (2020)
Z. Guo, J. Shao, H. Lin, M. Jiang, S. Chen, Z. Li, Electrical conductivity & temperature sensitivity of ceramics based on NiO simple oxides for NTC applications. J. Mater. Sci. Mater. Electron. 28, 11871–11877 (2017)
Q. Wang, W. Kong, J. Yao, A. Chang, Fabrication and electrical properties of the fast response Mn1.2Co1.5Ni0.3O4 miniature NTC chip thermistors. Ceram. Int. 45, 378–383 (2019)
J. Wang, J. Zhang, Structural and electrical properties of NiMgxMn2−xO4 NTC thermistors prepared by using sol–gel derived powders. Mater. Sci. Eng. B 176, 616–619 (2011)
Z. Yang, H. Zhang, Z. He, B. Li, Z. Li, Influence of B3+- and Na+-ions on electrical property and temperature sensitivity of NiO-based ceramics. J. Mater. Sci. Mater. Electron. 30, 3088–3097 (2019)
J. Wang, H. Zhang, X. Sun, Y. Liu, Z. Li, Electrical properties and temperature sensitivity of Li/Fe-modified NiO-based ceramics as NTC thermistors. J. Mater. Sci. Mater. Electron. 27, 11902–11908 (2016)
B. Yang, H. Zhang, J. Zhang, X. Zhang, Z. Li, Electrical properties and temperature sensitivity of B-substituted CuO-based ceramics for negative temperature coefficient thermistors. J. Mater. Sci. Mater. Electron. 26, 10151–10158 (2015)
H. Li, I.P.L. Thayil, X. Ma, X. Sang, H. Zhang, A. Chang, Electrical properties and aging behavior of Na-doped Mn1.95Co0.21Ni0.84O4 NTC ceramics. Ceram. Int. 46, 24365–24370 (2020)
C. Gao, Z. Li, L. Yang, D. Peng, H. Zhang, Investigation of electrical and aging properties of Bi-modified (Zn0.4Ni0.6)1-xNaxO ceramic thermistors. J. Eur. Ceram. Soc. 41, 4160–4166 (2021)
H. Zeng, G. Duan, Y. Li, S. Yang, X. Xu, W. Cai, Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: defect origins and emission controls. Adv. Funct. Mater. 20, 561–572 (2010)
Y. Zhu, L. Zhou, Q. Jiang, One-dimensional ZnO nanowires grown on three-dimensional scaffolds for improved photocatalytic activity. Ceram. Int. 46, 1158–1163 (2020)
M. Ha, S. Lim, J. Park, D.S. Um, Y. Lee, H. Ko, Bioinspired interlocked and hierarchical design of ZnO nanowire arrays for static and dynamic pressure-sensitive electronic skins. Adv. Funct. Mater. 25, 2841–2849 (2015)
Z.L. Wang, J. Song, Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 312, 242–246 (2006)
S. Li, H. Zhang, S. Leng, Z. Yang, J. Shao, Z. Li, Characterization of temperature induced resistivity jump in Li/Y/Cr co-doped ZnO ceramics. J. Mater. Sci. Mater. Electron. 29, 10969–10975 (2018)
P. Li, H. Zhang, C. Gao, G. Jiang, Z. Li, Electrical property of Al/La/Cu modified ZnO-based negative temperature coefficient (NTC) ceramics with high ageing stability. J. Mater. Sci. Mater. Electron. 30, 9598–19608 (2019)
J. Fan, S. Leng, Z. Cao, W. He, Y. Gao, J. Liu, G. Li, Colossal permittivity of Sb and Ga co-doped rutile TiO2 ceramics. Ceram. Int. 45, 1001–1010 (2019)
M.C. Biesinger, L.W.M. Lau, A.R. Gerson, R.S.C. Smart, Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu, and Zn. Appl. Surf. Sci. 257, 887–898 (2010)
Y. Niu, X. Yin, C. Sun, X. Song, N. Zhang, Adjusting surface oxygen vacancies prompted perovskite as high performance cathode for solid oxide fuel cell. J. Alloys Compd. 865, 158746 (2021)
R.J. Iwanowskia, M.H. Heinonenb, I. Prackac, J. Kachniarza, XPS characterization of single crystalline SrLaGa3O7:Nd. Appl. Surf. Sci. 283, 168–174 (2013)
J. Chen, Z. Chen, X. Zhang, X. Li, L. Yu, D. Li, Antimony oxide hydrate (Sb2O5·3H2O) as a simple and high efficient photocatalyst for oxidation of benzene. Appl. Catal. B 210, 379–385 (2017)
G. Zou, Y. Xu, S. Wang, M. Chen, W. Shangguan, The synergistic effect in Co-Ce oxides for catalytic oxidation of diesel soot. Catal. Sci. Technol. 5, 1084–1092 (2015)
Acknowledgements
This work is supported by the research funds from the National Natural Science Foundation of China (No. 51767021), Research and development program in key areas of Guangdong Province, China (No. 2019B090913002), and the General Project of Scientific Research Project of Hunan Provincial Department of Education Scientific, China (No. 19C0342).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any financial interest or personal relationship with any other people or organization that could inappropriately influence (bias) the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, B., Li, Z., Zhang, S. et al. Electrical properties of Ga/V-modified ZnO ceramic thermistors. J Mater Sci: Mater Electron 32, 28792–28806 (2021). https://doi.org/10.1007/s10854-021-07264-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07264-9