Abstract
High edge energy storage with large life-span stable materials have become the most significant and major requirement in near future. Bismuth sulfide (Bi2S3) nanoparticles (NPs) was effectively synthesized by utilizing bismuth diethyldithiocarbamate (Bi[DTC]3) complex as single-source antecedent. The synthesized Bi2S3 NPs were affirmed by structural, morphological and thermal analysis with various analytical studies. The electrochemical performance of Bi2S3 NPs reveals that, exhibit redox behavior from cyclic voltammetry analysis, the charge–discharge analysis was examined by the galvanostatic chronopotentiometry. Bi2S3 NPs exhibit excellent supercapacitor behavior with value of the specific capacitance 470 Fg−1 at current density 0.5 Ag−1 and the retentivity of 79% after 1000 cycles. The Bi2S3 NPs were further utilized as photocatalytic discoloration of Congo Red (CR) dye in aqueous medium under UV light irradiation. Which shows, excellent catalytic behaviour, up to 98% of CR dye degrade within 150 min and it is exhibit good catalytic stability and reusability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the recent decade, broad ranging attempts were made to grow use of new phase grid storage system with maximum power and energy density to be used in crossover hybrid car and versatile hardware. Energy storage devices are considered as a promising nominee for securing and conveying a moderately high measure of energy in brief timeframes, indicating magnificent reversibility and lifelong execution [1]. Other than the carbon materials, for example, carbon nanofibers, actuated carbons and graphene oxides change metal mixes, for example, metal sulfides or metal oxides moreover hydroxides are considered as appealing potential terminal equipment for utilize in supercapacitors because of the large-capacitance esteems, which transfer into the upgraded energy density [2,3,4,5].
The presentation of the semiconductor materials into the respectable metal nanostructures totally modifies their electronic and optical properties; along these lines, positions them as most chased materials utilized in field impact transistors, photocatalytic response, photo detectors and furthermore in supercapacitors (broadly utilized in buyer and printed hardware, flight, transportation, and related innovations) [6,7,8,9]. In meantime; metal sulfides are significant product of semiconductor and have also been utilized to create supercapacitor electrodes [10]. On account of metal sulfides, it has been recognized as a fantastic pseudo capacitive (redox) material at room temperature with its high conductivity and redox properties. The metal sulfides like, Bismuth sulfide [11], Nickel sulfide [12], Zinc sulfide [13], Cadmium sulfide [14], Cobalt sulfide [15] is proposed with different composite stages and has a widespread scope of energy storage applications [16, 17].
Metal sulfides are attractive in wide group of functional materials because of their wide applications within electronic, catalytic, sensor, optics, biomedical sectors and energy storage conversion [18,19,20] with few synthesis reports are available like, co precipitation, hydrothermal, microwave irradiation and sonochemical techniques [21]. In addition, preparation of Bi2S3 NPs by various methods has been employed such as chemical vapor deposition method, electrochemical deposition, sonochemical system, organometallic complex decomposition. Hydrothermal technique has been valid to bismuth sulfide nano ribbons and nanotubes preparation [22]. Bi2S3 nanomaterials is a prominent semiconducting material and it has drawn significant interest in nonlinear optical device, thermoelectric device, optical modulators, sensors, and optoelectronic devices, electronics, and well as FT-IR spectroscopy due to its potential applications. Bi2S3 (Bismuth sulfide) has a direct band gap semiconductor with a high coefficient of absorption, the larger band gap energy 1.3–1.7 eV [23,24,25,26]. This material provides possible applications for thermoelectric cooler system as well [10].
In recent days, many researchers concentrate on semiconducting photocatalysts to accomplish the needs regarding organic pollutions, photochemical water splitting technique [27]. Photocatalytic technology is an upcoming growth in oxidation innovation, which uses to stimulate electrons to provide more oxidizing methods. It can mineralize organic molecules into carbon dioxide and water and reduces organic pollutants from water completely [28, 29]. Metal sulfides are introduced to gathering a deeply effective performance for photocatalytic process, since photo generated charge carrier can rapidly move to the surface of the catalysts, or redox organic molecule. For example, bismuth sulfide (Bi2S3) nanomaterials were used as a photocatalyst to degrade congo red dye, and the reaction process occurs under UV light conditions [27, 30]. Bi2S3 additionally belongs to the category of nontoxic semiconductor materials. Moreover, semiconducting materials for optoelectronics shows high absorption coefficient (104 cm−1) range [31]. This material is a proper photocatalytic behavior for the enhancement of electron movement.
In this present work, the single-source precursor (Bi[DTC]3) have been used for preparation of Bi2S3 NPs and HDA as capping and shape directing agent by solvothermal method. As-prepared Bi2S3 NPs have been successfully examined with electrochemical super capacitive behaviour for energy storage application. In addition, the Bi2S3 NPs have been utilized for the effective photocatalytic discoloration of the CR dye in aqueous medium under UV light irradiation. Moreover, stability and reusability also studied.
2 Experimental part
2.1 Materials and methods
Bismuth(III) nitrate [Bi(NO3)3·5H2O], Sodium diethyldithiocarbamate [C5H10NNaS2·3H2O], N, N-Dimethylformamide (DMF) [C3H7NO], And potassium hydroxide [KOH], Hexadecylamine (HDA) [C16H35N] and ethanol [C2H5OH], methanol [CH3OH] all chemical were received from Sigma Aldrich Pvt. Ltd with AR Grade. Congo red textile dye was purchased from local market with commercial grade. The metal complex synthesis was performed in DI water.
2.2 Preparation of Bi[DTC]3 (single source precursor) complex
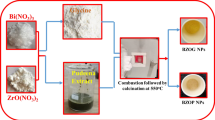
In typical preparation, equal molar ratio of bismuth(III) Nitrate (4.85 g) and Sodium diethyldithiocarbamate [Na(DTC)] (4.50 g) was separately dissolved with 100 ml of deionized (DI) water with 1 h continuously stirred and mixed together with drop by drop. The yellowish green colour precipitation was formed. Then, it was allowed to continuous stirring for 2 h. After, homogeneous mixing the yellow colour precipitate has been separated and washed several time by DI water and ethanol by centrifugal force. The resulting material was continuously desiccated at 60 °C for 12 h. After cooling to the room temperature Bi[DTC]3 complex were collected for further use [16].
2.3 Preparation of bismuth sulfide (Bi2S3) NPs from Bi[DTC]3 complex
The prepared Bi[DTC]3 complex as precursor and Hexadecylamine (HDA) as capping agent and shape directing agent were utilized in 1:1 weight ratio and dispersed in 100 ml of N, N-Dimethylformamide (DMF). Then the above mixture is heated at 120 °C for 1 h by solvothermal method. Finally, dark colour precipitate was collected and washed with methanol for remove un-reactant. The final product (Bi2S3 NPs) was collected and dried in oven at 60 °C for overnight and used to confirm with different analytical methods are shown in Fig. 1.
2.4 Instrumentation
Crystalline nature of the prepared Bi2S3 NPs was examined by Powder X-ray diffractometer using Bruker instrument of model D8 advance ECO XRD frameworks with SSD160 1D Detector which has Cu-Kα radiation of frequency 1.5406 Å. The surface morphological were studied by scanning electron microscope instrument ZEISS EVO 18 which was outfitted with BRUKER X-Flash 6130 equipped with energy dispersive X-beam spectrometer to confirm the elements of Bi2S3 NPs. An instrument Shimadzu IR Trace-100 spectrometer was utilized to record the infrared range of arranged unadulterated Bi2S3 test by KBr pellet approach. The Raman ridiculous investigation was recorded on laser Raman microscope (Raman-11,500 mm spectrometer Nano photon Corporation Japan). Higher Resolution Transmission Electron Microscope (HR-TEM) was utilized to trace the structural morphologies of the prepared sample.
2.5 Working electrode preparation
The electrochemical reaction of the Bi2S3 electrodes was investigated in 1 M KOH electrolyte by utilizing three-electrodes system electrochemical work station CHI6008E. The electrode material of Bi2S3 was organized utilizing 0.01 mm thick and 1cm2 nickel foil for electrochemical execution investigation. At first, the outside of Ni foil was wind-swept with emery sheet and took into consideration sonication, at that point cleaned with (CH3)2CO. Bi2S3 material, activated carbon, polyvinylidene fluoride (PVDF) cover was blended in with the weight proportion of 80:10:10 and ground well for blending homogeneous blend. What’s more, a drop of N-methyl-2-pyrrolidone (NMP) was added to the blend to make it as slurry and afterward it was applied as, a slight film on nickel foil. It was dried at 80 °C during 12 h further the electrode was used to analysis of electrochemical studies.
2.6 Photocatalytic experiments
The photodegradation experiment was performed using Bi2S3 NPs as active catalyst for the effective photo discoloration commercial Congo Red dye under UV light (λ = 365 nm) irradiation. The experiment was studied by with help of HEBER Photo reactor (Model—HML-MP 88). 50 mg of Bi2S3 NPs was used as catalyst in aqueous medium. CR dye was used of 5 × 10–5 M of concentration. Every 15 min. 1.5 ml of reaction solution was reserved for monitor the absorption value of CR dye was used for degradation. A control experiment was performed without catalyst for CR dye degradation.
3 Results and discussion
3.1 X-ray diffraction analysis
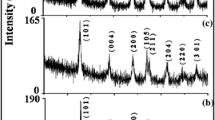
The XRD analysis for the as-prepared Bi2S3 NPs showed in Fig. 2. The indexed diffraction peaks are noticed at 2θ values 15.61, 17.54, 22.35, 23.51, 24.85, 28.51, 31.6, 35.45, 39.68, 45.27, 46.42, 48.93, 52.4, 58.94, 62.41, 64.72, 69.34, 71.85 and 84.75 correspond to hkl value (0 2 0), (1 2 0), (2 2 0), (1 0 1), (1 3 0), (2 3 0), (2 2 1), (2 4 0), (1 4 1), (0 0 2), (4 3 1), (0 2 2), (1 3 2), (4 6 0), (4 3 2), (5 6 0), (1 6 2), (5 4 2), and (4 7 2), respectively, The positions and comparative intensities of the considerable number of peaks are in acceptable understanding, with those are orthorhombic crystalline formation for Bi2S3 NPs and they are indexed to standard JCPDS card number 89-8964. The all-around coordinates with the average cell parameters morals (a = 11.13 Å, b = 11.29 Å, and c = 3.981 Å). The standard grain sizes are determined from the expansion of the X-ray diffraction peaks utilizing the Scherrer method.
where, D—standard grain size, λ—X-ray wavelength (0.15405 nm) and θ and β—diffraction angle as well as full-width at half maximum in radians of the experimentally observed peak correspondingly [9, 21]. The average grain sizes of prepared Bi2S3 NPs were ~ 54.7 nm.
3.2 FTIR spectrum
Figure 3 shows the FT-IR spectra of as-prepared Bi2S3 nanoparticles were recorded to confirm the prepared nanoparticles via its vibration and their intermolecular interactions. In Fig. 3, a small weak vibration peak appeared around 554 cm−1 due to rocking vibration of bismuth metal [32]. The broad peaks found in the range of 1300–1370 cm−1 due to interaction between sulfur-atmospheric carbon and oxygen bonding. In the range of 2842–2913 cm−1 observed two bands of N–H and OH stretching vibration [33,34,35]. Apart from these no other peaks were found in FTIR spectrum it clearly evident for free from impurities.
3.3 Raman spectrum
Raman spectroscopy is an effective inorganic tool to identify materials in structural approach [36]. Therefore, the Bi2S3 NPs were recorded Raman spectrum at room temperature in the range of 0–2000 cm−1 is shown in Fig. 4. The spectrum has four distinct vibrational peaks of approximately 260, 306, 420, and 967 cm−1 the order coincides with the Bi2S3 characteristics peaks, which are stable with the previously reported significance in the research. A strong peak occurred at an altitude of 967 cm−1 due to the high surface to volume ratio of surface phonon modes [37, 38].
3.4 Thermal stability of Bi[DTC]3 metal complex
Figure 5 shows the TG–DTA curve of the bismuth diethyldithiocarbamate Bi[DTC]3 complex, the complex was tested under Nitrogen atmosphere. From the thermo gravimetric graph some miles weight occurred due to solvent evaporation and second weight loss starting from 119.7 °C onwards due to decomposition of unreacted sodium dithiocarbamate. In the range of 192 °C starts a major weight loss due to Bi[DTC]3 complex at the point the metal complex fully converted in to metal sulfides Bi2S3 up to 41.8% weight loss shows in this region due to single-source precursor converted in metal sulfides in this region further after heating the heat exchange from 195 to 317.3 °C, this might be because of the evaporation of the sulfur. The final decomposition stage about 3.7% at the temperature range 503.5 °C and No weight loss about above 503.5 °C. This could be additionally distinguished by the observable three endothermic top about 149 °C, 283.7 °C, and 479.5 °C, respectively, in the DTA curves. Apart from this curve no weight loss were found the remaining residue is confirmed as metal sulfides (Bi2S3).
3.5 SEM and EDAX with mapping analysis
Surface morphological analysis and shape of the as-prepared Bi2S3 nanoparticles were examined by SEM images as shown in Fig. 6. From the morphological studies uniform rod-spherical shaped Bi2S3 NPs are found in Fig. 6a, b. It is clearly shows the HDA capped Bi2S3 NPs uniformly distributed throughout the sample. The purity of synthesized bismuth sulfide nanoparticles is declared since the energy dispersive X-ray spectrum. The appeared Fig. 7a an energy dispersive X-ray spectrum along with Fig. 7b uncovers mapping images of synthesized bismuth sulfide (Bi2S3) nanoparticles. The inset showed in Fig. 7a as the mass level of Bi and S is resolute by EDAX (energy dispersive X-ray) analysis. An EDAX study shows that the all-around cleaned the last thing is commonly made out of Bismuth (Bi) and Sulfur (S), through cannot be present any extra unwanted components Fig. 7a. It has the in accumulation the average atomic gain of Sulfur (S) was especially elevated from the energy dispersive X-ray image 59.5% and Bismuth (Bi) peak contained in the range of 40.5%.
3.6 HR-TEM analysis
The structural and morphology of the prepared Bi2S3 nanoparticles were characterized by high resolution transmission electron microscope. Figure 8a–d shows the nanostructured Bi2S3 nanoparticles with mixed morphologies like nanoplates, nanorods and spherical in shaped NPs and this shows, more number of spherical shape nanoparticles were aggregate and to form rod shape structure Fig. 8a, b. The average size of the nanoparticles observed around ~ 20 nm thickness to micro meter range. This nanoscale Bi2S3 NPs may lead to enhance the photocatalytic and electro catalytic activity due to high surface to volume ratio. This result coincides with SEM and XRD analysis. Hexadecylamine did the vital role for capping and shape directing agent. The atomic lattice fringes are clearly observed in an HR-TEM micrograph and a d-spacing value of 0.540 nm range are shown in Fig. 8c. Figure 8d displays the selected area electron diffraction (SAED) pattern of the Bi2S3 nanoparticles. From the SAED pattern clearly shows concentrical diffraction rings of orthorhombic crystalline structure and Bi2S3 nanoparticles verified the polycrystalline nature preferably distinct single crystal.
3.7 XPS analysis
The surface chemical oxidation state and binding energy of the spherical shaped Bi2S3 NPs were probed by XPS studies, from this result (Fig. 9). Figure 10 no impurities peaks were observed in the full survey trace excluding Bi, S, O and C. The atomic sensitivity factor of bismuth and sulfur ratio is around 2:3 according to quantification of the Bi4f and S2s peak surface area, signifying the stoichiometric composition of Bi2S3 and this result coincided with EDAX studies. Figure 10a shows the two higher resolution spectra of Bi4f7/2 and Bi4f5/2 spin orbit doublet are traced at 157.8 and 163.1 eV, respectively [39]. The small peak appeared at 161.5 eV for S2p owing to the primary valance state of sulfur [40]. Fig. 10b shows the strong peak found at 228.2 eV corresponding to the binding energy (B.E) of S2s [1]. The C1s peak is found at 284.5 eV and all spectra are corrected with carbon spectrum as a reference is shown in Fig. 10c. The oxygen element may present due to adsorption or contamination of atmosphere on the Bi2S3 surface (Fig. 10d).
3.8 Cyclic voltammetry analysis
The electrochemical performances of the Bi2S3 modified electrode material have been evaluate by the cyclic voltammetry (CV) results. The above mentioned method was using to analyse by three-electrode system with 1 M KOH as electrolyte solution. Figure 11a shows that the cyclic voltammetry curves of the Bi2S3 electrode material was studied at various scan rates (1–100 mV s−1) in the potential windows ranging from 0 to 0.6 (V) Vs (Ag/AgCl). The CV curves revealed high oxidation and reduction peaks have been present in the potential range at 0.52 (V) and 0.33 (V), respectively. The redox behavior of CV results directly exhibit pseudo capacitive nature of the electrode material. The anodic and cathodic peaks in the CV curves were mainly due to the oxidation and reduction of OH− ions and Bi2S3 electrode material. The redox responses of Bi2S3 matrix was mainly induced by the possible reversible conversion, though the scan rate increases from 1 to 100 mV s−1 the shape of CV curves was maintained, representing the excellent rate performance of the electrode material. The redox equation of the Bi2S3 electrode material becomes
Furthermore, the redox peaks shift to negative or positive due to increments of scan rates, which exhibit the internal diffusion resistance in the electrode material [41].
The Randles–Sevcik plot is shown in Fig. 11b after the completion of CV test. It was explain the linear increase of specific peak current for anode and cathode side (i.e., Redox) with respect to the square root of scan rate. From the Randles graph, value of R2 was seen at 0.97405 for anodic (oxidation) and 0.97091 for cathodic (reduction) process, respectively. This exact parameter was because of the limited diffusion process of static reversible faradaic reaction [42, 43].
The specific capacitance value of the Bi2S3 NPs working electrodes has been calculated from the CV curves and GCD curves using the formulas (3) and (4).
where, ∫Idv (A) is the integral area under the cyclic voltammetry curves, v is the scan rate (mV s−1), m (g) is the mass of active electrode material, and ΔV (V) is the potential difference
where, m (g) is the mass of the active electrode material, ΔV (V) is the potential difference, Δt (s) is the discharge time, and I (A) is the constant discharge current.
The calculated specific capacitance values of scan rates 1–100 mV s−1 are shown in Fig. 11c. The individual values of specific capacitance and different scan rates are given in Table 1. At minimum scan rate 1 mV s−1, active electrode surface area is high for involving in electrochemical reaction. The increasing scan rates to restrict the electrolyte ions towards moving to the electrode active site, which reflects the decreasing specific capacitance [44].
3.9 Chronopotentiometry
Galvanostatic charging discharge (or) chronopotentiometry was analysis the different current density range (0.5–10 Ag−1) of Bi2S3 electrode material. Figure 12a shows charge discharge curve it was indicates the ability of the Bi2S3 electrode. On the basis, the specific capacitances increase with due to increase discharging time. The charge/discharge curve was interpreted as due to these redox reactions occurs interface between the electrode and electrolyte. The higher current densities have higher internal resistance drop, its closely relative to the charge transfer resistance [45].
a Galvanostatic charge–discharge curves at different current densities, b specific capacitance vs current density, c EIS spectra, d Bode plot for before and after 1000 cycles, e Ragone plot related to power densities and energy densities of Bi2S3 NPs, f specific capacitance variation over 1000 charge–discharge cycles at 5 Ag−1, and retentivity plot of Bi2S3 NPs
Figure 12b explains the variation between specific capacitance and current density of Bi2S3 electrode material. The Eq. 4 is to calculate the value of specific capacitance from current density. The calculate value of specific capacitance of Bi2S3 electrode with various current density is shown in Table 2. In addition, increasing specific capacitance was obtained at lower current density. Correspondingly, the surface interfaces and higher inside activates accessible charge/discharge at lower current densities. Moreover, the mixed morphology like spherical and rod shaped nanoparticles could provide more active sites for the redox process of the active materials with greater interfacial contact between the electrode and electrolyte. Thus increased contact area could play a significant role to accessibility of the composite materials to KOH ions and shorten the ion migration pathway during the charging discharging process [17]. Form this study, Bi2S3 electrode material conclude that for 0.5 Ag−1 current density and high specific capacitance be 470 Fg−1, this Csp value is higher than as previous report, Vadivel (290 Fg−1) [17], Liu (233 Fg−1) [46], Liang (270 Fg−1) [47] and Noordeen (152.2 Fg−1) [48], Raut (289 Fg−1) [49] Ma (185.7 Fcm−2) [50], respectively. Table 3, shows the comparison of specific capacitance for the same electrode materials with various preparation methods.
The electrochemical impedance spectrum of Bi2S3 was studied in the 1 Hz–1 MHz. The impedance performance of Bi2S3 was shown before and after 1000 cycles in Fig. 12c. The corresponding Niquiest plot was fitted with Z view software. The Niquiest plot consist three regions namely low, high and mid frequency regions. In addition, the phase angle as a function of frequency is mentioned as a Bode plot is shown in Fig. 12d. This reveals the solution resistance and charge transfer resistance of the Bi2S3 NPs. It was evalued value of phase angle near to − 70° in frequency frame of impedance study shows the perfect capacitive behavior Bi2S3 electrode material [44].
The impedance parameter is mentioning Table 4. The solution resistance of the Bi2S3 becomes 0.89 Ω and charge transfer resistance have 1.96 Ω. The absorption of OH− ions enhanced to flux vacancy of the cations from the metal electrolyte toward metal sulfide surfaces. The interfaces metal exchanged cations [51]. The equivalent circuit is shown in Fig. 12c. The high frequency region semicircle reveals the sluggish redox reaction kinetics, charge transfer resistance of the Bi2S3 electrode its become 1.96 Ω. The Warburg regions exhibit the diffusion of the electrode material due to ions in the electrolyte. Obviously, the low frequency region slope shows the fast absorption of the ion mobility in the electrolyte on the surface of the electrode [52].
The Ragone plot of Bi2S3 electrode was calculated from the three-electrode analysis [45]. The power density and energy density figure is shown in Fig. 12e Bi2S3 of the electrode materials. The Bi2S3 electrode material shows a higher energy density 16.31 Wh kg−1 (with power density 125 Wkg−1) and power density of 2500 Wkg−1 (with energy density 8.3 Wh kg−1). In the term, the acquired value of energy density is beyond that previous reported like Bi2S3:PbS (13.36 Wh kg−1) [53], and Co3So4 (27.77 Wh kg−1) [45]. The cycle performance and electrode stability was analyzed from the charge discharge method up to 1000 cycles in Fig. 12f. The cycle test was linearly decreasing, its exhibit stability of the electrode material. Finally Bi2S3 test holds for 79% of original value of the capacitance. The impedance spectrum of Bi2S3 was after 1000 cycle exhibit the electrode stability in the electrolyte. This represents the best cathodic electrode material for supercapacitor devices.
3.10 Photodegradation studies
In order to study photocatalytic activities for the prepared Bi2S3 nanoparticles was utilized against commercial Congo Red (CR) textile dye under UV–Vis. light irradiation. At first 50 mg of Bi2S3 catalyst was loaded in100 ml of 1 × 10–5 M of CR dye aqueous solution [54]. A control reaction was carried out under dark room condition for 15 min with photocatalyst to attain equilibrium condition between CR dye and Bi2S3 nanocatalyst with exposed λ = 365 nm UV light irradiation continuously up to 150 min. 3 ml of reaction solution was withdrawn with every 15 min. to monitor the absorbance intensity of CR dye at 498 and 364 nm using UV–Vis. Spectrometer. Finally, the nano catalyst were collected using centrifuge machine and washed with DI water followed by methanol and dried at 70 °C for 4 h to reuse the catalyst. The same procedure will followed by further consecutive testing.
Figure 13A shows the UV–Vis. Absorption spectra of CR at 50 mg of Bi2S3 NPs loaded on 100 ml of aqueous dye. The spectra shows visibly and gradually reduced the CR dye intensity with respect of UV exposed time. The same experiment was carried out without catalyst named as blank with similar irradiation time. From the UV absorbance spectra was obviously conclude the role of catalyst on CR dye medium. Figure 13B shows the concentration (C/C0) plot of CR with and without catalyst. In the case of bank (without catalyst) the colour was not change after 150 min. UV irradiation and it is periodically monitored with UV-Spectrometer after removal of Bi2S3 catalyst. In-opposite periodically CR dye intensity was reduced in presence of as-prepared and Calcinated Bi2S3 samples as shown in Fig. 13B. The CR dye exhibits two major peaks in the region of 364 and 498 nm respective to the naphthalene (aromatic) rings and π–π∗ transitions of (–N=N–) azo group. The aqueous CR solution λ(Max) = 498 nm gradually suppressed under UV light irradiation in the presence of Bi2S3 catalyst. In the case of 364 nm gradually decrease with slight shifting towards lower wavelength. It May indicate that azo bonds are more vulnerable to degradation than the aromatic rings by hydroxyl radicals [55]. Hence it is proved the single-source precursor fabricated spherical shaped Bi2S3 NPs have excellent photocatalytic performance under UV light irradiation.
A Photocatalytic degradation of Congo Red (CR) dye using Bi2S3 as photocatalyst, B degradation profiles of CR in the presence of Bi2S3 NPs and hierarchical nanostructures. C Photodegradation calibration plot, D recycle efficiency of the photocatalyst, photocatalytic efficiency of the reused Bi2S3 NPs for the degradation of CR dye
The degradation efficiency can calculate by the Eq. (5),
where, ‘C0’ is initial concentration of dye and ‘C’ is the concentration after irradiation time (t). Moreover, the calibration linear plot are fitted with Irradiation time and ln(C/C0) and compared as shown in Fig. 13C. The degradation rate constant (k) were calculated from slop of linear fit line, according to first order kinetic act ln(C/C0) = kt [56, 57]. The R2 value and rate constant (K) of Bi2S3 NPs was found R2 = 0.99 and k = 0.0127. The results of rate constant and linear regression coefficient (R2) suggest that the Bi2S3 sample revealed superior photo catalytic performance. Possible mechanism of photodegradation are given below,
The Bi2S3 NPs was exposed under UV light irradiation which resulted in the electrons (e−) excitation from valance band to conduct band while leaving holes (h+) in the valance band. The e− and generated O2 are formed as radical and h+ in the valance band were absorbed the water molecule then produced OH., H+ radical which is lead to facilitate to degrade the CR dye molecules as shows in the above equations.
3.11 Catalyst stability and reusability studies
The stability and reusability of the prepared catalyst are very important in catalysis application. Because it can reduce the overall expenditure for the waste water treatment. In this connection, the prepared Bi2S3 NPs were examined used as catalyst among five successive cycles of photodegradation were carried out using the same catalyst other than fresh solution of CR dye in aqueous medium. After each reuse the catalyst was washed with methanol and dried at 80 °C for 3 h. The % of degradation was 97.3, 94.8, 92.4, 90.6 and 86.4 for 1–5th cycles, respectively. It was noticed that good and constant decrement of the photocatalyst efficiency up to 4th cycle and after 4th cycle an immediate drop will occurred as shown in Fig. 13D. Hence it is concluded, the spherical, nanoplates and nanorods shaped nanoparticles were highly stable up to four cycles. These experimental results suggested that, the Bi2S3 NPs have excellent stability and reusability and practically applicable to degrade environmental water contamination.
4 Conclusion
Bi2S3 NPs has been successfully accomplished using solvothermal method. The synthesized Bi2S3 nanoparticle was characterized for XRD plot indicated that the crystalline nature was orthorhombic crystalline structure. Raman and FT-IR spectral studies have clarify the presence of functional group in the synthesized nanomaterials. Furthermore, we in addition inspected, the morphology of Bi2S3 nanoparticle was capture by SEM result of identical, nanoparticle-like morphology with a spherical shape. Photocatalytic discoloration of CR dye was investigated using Bi2S3 NPs under UV and visible light irradiation. The sample was fully degraded among 150 min of irradiation in presence of UV–visible light, and the restriction for the stability of photocatalytic degradation were superior. The Bi2S3 NPs was water purify steady up to 5th cycles it will be utilized to degrade the CR dye degradation under UV–Vis. light. In addition, The Bi2S3 NPs electrode material was exhibits high specific capacitance at 470 Fg−1 and current density value at 0.5 Ag−1. Likewise, they performed great cyclic stability of 79% at after 1000 cycles. Finally the prepared Bi2S3 NPs material was perfect energy storage and photocatalytic applications.
References
A. Moyseowicz, J. Solid State Electrochem. 23, 1191 (2019)
A. Moyseowicz, G. Gryglewicz, Compos. Part B Eng. 159, 4 (2019)
Z. Wu, Y. Sun, Y. Tan, S. Yang, X. Feng, K. Müllen, J. Am. Chem. Soc. 134, 19532 (2012)
S. Yang, X. Feng, L. Wang, K. Tang, J. Maier, K. Müllen, Angew. Chem. Int. Ed. 49, 4795 (2010)
A. Śliwak, N. Díez, E. Miniach, G. Gryglewicz, J. Appl. Electrochem. 46, 667 (2016)
M.P. Motaung, J. Osuntokun, D.C. Onwudiwe, Mater. Sci. Semicond. Process. 99, 92 (2019)
B. Weng, M. Qi, C. Han, Z. Tang, Y. Xu, ACS Catal. 9, 4642 (2019)
D.C. Onwudiwe, P.A. Ajibade, Int. J. Mol. Sci. 12, 5538 (2011)
J. Arumugam, A.D. Raj, A.A. Irudayaraj, Mater. Today Proc. 5, 16094 (2018)
S.V.P. Vattikuti, A.K.R. Police, J. Shim, C. Byon, Sci. Rep. 8, 4194 (2018)
X. Chen, Q. Wang, L. Wang, F. Gao, W. Wang, Z. Hu, Biosens. Bioelectron. 66, 216 (2015)
A. Singh, A.J. Roberts, R.C.T. Slade, A. Chandra, J. Mater. Chem. A 2, 16723 (2014)
F. Amirian, M. Molaei, M. Karimipour, A.R. Bahador, J. Lumin. 196, 174 (2018)
P. Devendran, T. Alagesan, K. Pandian, Asian J. Chem. 25, S79 (2013)
H. Rao, W. Sun, S. Ye, W. Yan, Y. Li, H. Peng, Z. Liu, Z. Bian, C. Huang, ACS Appl. Mater. Interfaces 8, 7800 (2016)
P. Devendran, T. Alagesan, A. Manikandan, S. Asath Bahadur, M. Krishna Kumar, S. Rathinavel, K. Pandian, Nanosci. Nanotechnol. Lett. 8, 478 (2016)
S. Vadivel, A.N. Naveen, V.P. Kamalakannan, P. Cao, N. Balasubramanian, Appl. Surf. Sci. 351, 635 (2015)
M.D. Regulacio, M.-Y. Han, Acc. Chem. Res. 43, 621 (2010)
X. Xin, M. He, W. Han, J. Jung, Z. Lin, Angew. Chem. Int. Ed. 50, 11739 (2011)
H. Zhong, Z. Bai, B. Zou, J. Phys. Chem. Lett. 3, 3167 (2012)
E. Sathiyaraj, S. Thirumaran, Chem. Phys. Lett. 739, 136972 (2020)
H. Zhou, S. Xiong, L. Wei, B. Xi, Y. Zhu, Y. Qian, Cryst. Growth Des. 9, 3862 (2009)
P.K. Panigrahi, A. Pathak, J. Nanopart. 2013, 1 (2013)
A. Begum, A. Hussain, A. Rahman, Mater. Sci. Appl. 02, 163 (2011)
R. Chen, M.H. So, C.-M. Che, H. Sun, J. Mater. Chem. 15, 4540 (2005)
J. Chao, S. Xing, J. Zhao, C. Qin, D. Duan, Y. Zhao, Q. He, RSC Adv. 6, 55676 (2016)
Z. Fang, Y. Liu, Y. Fan, Y. Ni, X. Wei, K. Tang, J. Shen, Y. Chen, J. Phys. Chem. C 115, 13968 (2011)
S.G. Kumar, K.S.R.K. Rao, Appl. Surf. Sci. 391, 124 (2017)
Q. Hao, C. Xie, Y. Huang, D. Chen, Y. Liu, W. Wei, B.-J. Ni, Chin. J. Catal. 41, 249 (2020)
G. Zhao, Y. Zheng, Z. He, Z. Lu, L. Wang, C. Li, F. Jiao, C. Deng, Trans. Nonferr. Met. Soc. China 28, 2002 (2018)
B. Li, Y. Zhang, R. Du, L. Gan, X. Yu, Langmuir 32, 11639 (2016)
J. Arumugam, A. Dhayal Raj, A. Albert Irudayaraj, J. Mater. Sci. Mater. Electron. 28, 3487 (2017)
S. Ranjitha, S. Vadivel, J. Marimuthu, S. Natarajan, IJSR 20 (2014)
M. Salavati-Niasari, Z. Behfard, O. Amiri, Asia-Pac. J. Chem. Eng. 9, 16 (2014)
P. Nazari, R. Dowlatabadi-Bazaz, M.R. Mofid, M.R. Pourmand, N.E. Daryani, M.A. Faramarzi, Z. Sepehrizadeh, A.R. Shahverdi, Appl. Biochem. Biotechnol. 172, 570 (2014)
J. Ota, S.K. Srivastava, J. Phys. Chem. C 111, 12260 (2007)
S. Sharma, N. Khare, Adv. Powder Technol. 29, 3336 (2018)
S.R. Kadam, R.P. Panmand, R.S. Sonawane, S.W. Gosavi, B.B. Kale, RSC Adv. 5, 58485 (2015)
S. Wang, W. Li, H. Song, C. Mao, Z. Zhang, H. Peng, G. Li, Inorg. Chem. Front. 6, 1275 (2019)
H.-P. Jiao, X. Yu, Z.-Q. Liu, P.-Y. Kuang, Y.-M. Zhang, RSC Adv. 5, 16239 (2015)
X. Zhai, J. Gao, X. Xu, W. Hong, H. Wang, F. Wu, Y. Liu, J. Power Sources 396, 648 (2018)
B. Chameh, M. Moradi, S. Kaveian, Synth. Met. 260, 116262 (2020)
S. Ezhil Arasi, P. Devendran, R. Ranjithkumar, S. Arunpandiyan, A. Arivarasan, Mater. Sci. Semicond. Process. 106, 104785 (2020)
B. Pandit, G.K. Sharma, B.R. Sankapal, J. Colloid Interface Sci. 505, 1011 (2017)
S.J. Patil, J.H. Kim, D.W. Lee, J. Power Sources 342, 652 (2017)
K.L. Liu, F. Chen, Y. Liu, D. Li, W.D. Shi, CrystEngComm 19, 570 (2017)
K. Liang, C. Wang, X. Xu, J. Leng, H. Ma, Phys. Lett. A 381, 652 (2017)
A.K. Noordeen, S. Sambasivam, S. Chinnasamy, J. Ramasamy, T. Subramani, J. Inorg. Organomet. Polym. Mater. 28, 73 (2018)
S.S. Raut, J.A. Dhobale, B.R. Sankapal, Physica E Low-Dimens. Syst. Nanostruct. 87, 209 (2017)
L. Ma, Q. Zhao, Q. Zhang, M. Ding, J. Huang, X. Liu, Y. Liu, X. Wu, X. Xu, RSC Adv. 4, 41636 (2014)
Z. Grubać, M. Metikoš-Huković, J. Electroanal. Chem. 565, 85 (2004)
E. Miniach, G. Gryglewicz, J. Mater. Sci. 53, 16511 (2018)
B. Pandit, S.A. Pande, B.R. Sankapal, Chin. J. Chem. 37, 1279 (2019)
P. Devendran, T. Alagesan, T.R. Ravindran, K. Pandian, Curr. Nanosci. 10, 302 (2014)
D. Malwal, P. Gopinath, Catal. Sci. Technol. 6, 4458 (2016)
K. Ravichandran, R. Mohan, B. Sakthivel, S. Varadharajaperumal, P. Devendran, T. Alagesan, K. Pandian, Appl. Surf. Sci. 321, 310 (2014)
M. Thiruppathi, P. Senthil Kumar, P. Devendran, C. Ramalingan, M. Swaminathan, E.R. Nagarajan, J. Alloys Compd. 735, 728 (2018)
Acknowledgements
Author C. S and P. D have thankfully acknowledged Kalasalingam Academy of Research and Education (KARE) for given University Research Fellowship (URF) and providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sambathkumar, C., Manirathinam, V., Manikandan, A. et al. Solvothermal synthesis of Bi2S3 nanoparticles for active photocatalytic and energy storage device applications. J Mater Sci: Mater Electron 32, 20827–20843 (2021). https://doi.org/10.1007/s10854-021-06596-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06596-w