Abstract
Lead-free films of Bi4-xNdxTi3O12 were deposited on Pt(111)/Ti/SiO2/Si(100) substrate via spin-coating methods. It is shown that there are no secondary phases in the films of Bi4-xNdxTi3O12 and clear interfaces between the films of Bi4-xNdxTi3O12 and substrates when the films are annealed at 700 °C. And the films of Bi4-xNdxTi3O12 also exhibit a blue light emission peak at 437 nm and a yellow light emission peak at 580 nm. There are narrower band gaps, greater values of dielectric constant and lower values of dielectric loss when the concentration of Nd3+ varies from 0 to 0.85. And the films of Bi4-xNdxTi3O12 possess the minimum of band gap energy (2.67 eV). Moreover, the films of Bi4-xNdxTi3O12 exhibit minimal leakage current density and maximal remanent polarization, which is highly beneficial for the potential applications in multi-functional devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the ferroelectricity in Rochelle salt crystals was discovered by French Valasek in 1920, ferroelectric materials have become a research hotspot [1] and been widely used in transducers, filters, resonators, ferroelectric memories and other fields [2,3,4,5,6]. Up to date, the widely used ferroelectric materials are lead-based because of their stable and excellent ferroelectric properties [7,8,9,10]. However, lead-based ferroelectric materials undesirably bring about harm to human beings and pollution to environment during the preparation and application processes. As a result, researchers have been looking for alternative lead-free ferroelectric materials with good ferroelectricity to rival the lead-based ferroelectrics such as PbZrxTi1-XO3 [11].
Bismuth titanate (Bi4Ti3O12), a typical lead-free multiferroics, has drawn widespread attention owing to its high Curie temperature and excellent fatigue resistance [12,13,14]. Unfortunately, its remnant polarization is rather low because of the evaporation of Bi3+, which gives rise to the increase in concentration of oxygen vacancies and the deterioration of ferroelectricity [15]. Thus, more and more researchers have been trying to inhibit the evaporation of Bi3+ through doping of rare elements in Bi4Ti3O12 [16,17,18]. M. Chen [19] reported that the substitution of Pr3+ for Bi3+ will decrease the space charge density which improves ferroelectric properties of Bi4Ti3O12 materials. And C. P. Cheng [20] reported that the doping of Dy3+ will enhance the ferroelectricity of Bi4Ti3O12. U. Chon [21] and X. Y. Mao [22] pointed that the doping of Nd3+ in Bi4Ti3O12 can also enhance the ferroelectric properties. The reason is that the substitution of Nd3+ for Bi3+ causes obvious structural distortion along C axis, thus resulting in a large polarization along C axis [21, 23] besides inhibiting evaporation of Bi3+. Surprisingly, Bi4Ti3O12 materials doped with rare earth elements possess obvious photoluminescence [24, 25]. R. Bokolia [26] reported that Er3+-doped Bi4Ti3O12 materials possess photoluminescence besides ferroelectricity. And K. Ruan [27] maintained that Eu3+-doped films of Bi4Ti3O12 possess good photoluminescence. Although, up to date, ferroelectricity of Nd3+-doped films of Bi4Ti3O12 has been investigated [28, 29], there are a few reports on investigating photoluminescence of Nd3+-doped films of Bi4Ti3O12 [30, 31]. Therefore, it is necessary to investigate not only ferroelectricity but also photoluminescence of Nd3+-doped films of Bi4Ti3O12 systematically.
In this work, the films of Bi4-xNdxTi3O12 were prepared on the substrates (Pt(111)/Ti/SiO2/Si(100)) via the sol–gel and spin-coating method. And impact of the concentration of Nd3+ in Bi4-xNdxTi3O12 films on the ferroelectric properties, dielectric properties, leakage current densities and photoluminescence was investigated. Photoluminescence and ferroelectric properties were studied in detail.
2 Experimental
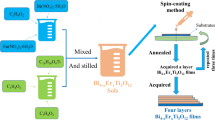
The films of Bi4-xNdxTi3O12 (when x = 0, 0.25, 0.45, 0.65, 0.85) were deposited on the Pt(111)/Ti/SiO2/Si(100) substrate. And precursors were fabricated by sol–gel method. Neodymium oxide (Nd2O3), bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) and tetrabutyl titanate (Ti(OC4H9)4) were selected as starting materials. 2-methoxyethanol (C3H8O2) and acetic acid (C2H4O2) were used as the solvents. Acetylacetone (C5H8O2) was used to stabilize the solution. Firstly, certain proportion of Nd2O3 and Bi(NO3)3·5H2O was dissolved in C2H4O2 according to the stoichiometry of Bi4-xNdxTi3O12. Thus, solution A was obtained. And excessive Bi(NO3)3·5H2O (8 wt%) was used to compensate volatilization of Bi3+ during annealing process. Then, Ti(OC4H9)4 was dissolved in C3H8O2, and a small quantity of stabilizer (C5H8O2) was dripped into C3H8O2. Then, solution B was obtained. Then, the above two solutions were mixed to get a sol. And the sol was deposited on the substrates to get monolayered films of Bi4-xNdxTi3O12 via spin-coating technology. Subsequently, the films of Bi4-xNdxTi3O12 were pre-annealed and annealed in tube furnace. At last, the above-mentioned process needs to be repeated three times to acquire four-layered films of Bi4-xNdxTi3O12. The flowchart of preparation is displayed in Fig. 1.
The phase constitutions of the films of Bi4-xNdxTi3O12 were carried out by X-ray diffraction (X 'Pert Pro MPD, PANalytical, Holland). The morphologies of the surface and cross-section were investigated though FESEM (SU8020, Hitachi, Japan). Ag dot electrodes with thickness of 100 nm and diameter of 1 mm were deposited on the surface of the films through vacuum evaporation coater (HFJS-DZ300, JieShuo, P. C. China). P-E hysteresis loops of the films were determined by Precision LC Unit (Radiant Precision LC, Radiant Technologies, USA). The leakage current densities were determined by semiconductor tester (4200-SCS, Keithley, USA). Absorption spectrum of films was obtained by the UV spectrophotometer (Cary-5000, Agilent, USA). Photoluminescent spectrum of the films was measured via fluorescence spectrophotometer (F4500, Hitachi, Japan).
3 Results and discussion
3.1 Phase constitution
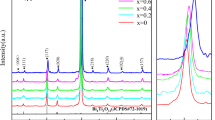
The XRD patterns of the five films of Bi4-xNdxTi3O12 are displayed in Fig. 2. As shown in Fig. 2a, it is obvious that the diffraction peaks of the films of Bi4-xNdxTi3O12 are composed of those from Bi4Ti3O12 (JCPDS No.72–1019) and from Pt on the top layer of the substrates without any from impurity phases [32, 33]. It indicates that Nd3+ has dissolved into the crystal lattice of Bi4Ti3O12 and pure films of Bi4-xNdxTi3O12 have been prepared [34, 35]. And the sharp peaks imply good crystallinity [26]. As shown in Fig. 2b, for all the five films, the diffraction peaks at about 30.0° shift slightly to a greater angle. This is mainly due to the fact that the radius of Nd3+ (1.11 Å) is less than that of Bi3+ (1.17 Å) [22, 28]. The replacement of Bi3+ with Nd3+ will lead to reduction in lattice parameters and crystal plane spacing, which give rise to shift of the diffraction peaks to a greater angle according to Bragg equation.
3.2 Microstructure
The FESEM morphologies of surface of the films are illustrated in Fig. 3. It is seen that, for the films of Bi4-xNdxTi3O12 (x being 0 and 0.25, respectively), there are obvious cracks and pores on the surfaces of these samples. However, for the other films, a few pores can be found. For five films, grain boundaries are not be found. And there are polygonal particles without plate-like particles. And the average particle sizes of five films of Bi4-xNdxTi3O12 are listed in Table 1. With the increase in concentration of Nd3+, the particle sizes decrease and the relative density of the films of Bi4-xNdxTi3O12 increases.
The FESEM morphologies of cross-section of the films of Bi4-xNdxTi3O12 are shown in Fig. 4. All the five films exhibit layered structure. The interfaces between the films of Bi4-xNdxTi3O12 and substrates are flat. And there are no inter-layers between the films and substrates, implying that no diffusion exists between the films of Bi4-xNdxTi3O12 and substrates. It can be observed that the thicknesses of the films of Bi4-xNdxTi3O12 are all around 200 nm, which is greater than that of pure films of Bi4-xNdxTi3O12. The difference in thicknesses for the films of Bi4-xNdxTi3O12 with different concentration of Nd3+ is mainly due to the preparation of films by spin-coating technology.
3.3 Ferroelectric properties
The hysteresis loops of the films of Bi4-xNdxTi3O12 are displayed in Fig. 5. The results obtained from Fig. 5 are listed in Table 1. The films of Bi4-xNdxTi3O12 seem to exhibit good ferroelectricity. The values of remnant polarization (Pr) and saturated polarization (Ps) of the films of Bi4-xNdxTi3O12 are great than those of un-doped film of Bi4Ti3O12 which indicates that doping of Nd3+ is beneficial for the improvement of the ferroelectric properties. It is seen that, firstly, the values of Pr increase to 17.25 µc·cm−2 and then decrease gradually with increasing concentration of Nd3+. The films of Bi3.55Nd0.45Ti3O12 possess the maximal value of Pr (17.25 µc·cm−2) which is greater than those films of Bi4Ti3O12 in Ref. [28] (10.5µc·cm−2), Ref. [36] (10.5µc·cm−2), Ref. [38] (10 µc·cm−2) and Ref. [29] (14.3 µc·cm−2). And it is also significantly greater than un-doped film of Bi4Ti3O12 in Ref. [38] (4.8 µc·cm−2).
In addition, it is worth noting that volatilization of Bi3+ during annealing process inevitably brings about increased oxygen vacancies and degraded ferroelectric properties of the films [32, 34]. The doping of a small amount of Nd3+ will inhibit the volatilization of Bi3+ and reduce the concentration of oxygen vacancies. As shown in Fig. 5, the values of Pr increase. However, with the further increase in the concentration of Nd3+, particle size decreases and the values of Pr reduce correspondingly owing to the pinning effect [33, 37]. Therefore, the film of Bi3.55Nd0.45Ti3O12 possesses the maximal value of Pr.
3.4 Leakage current densities
The leakage current densities of the films of Bi4−xNdxTi3O12 are displayed in Fig. 6. Obviously, when the values of applied electric field (E) are below 100 kV·cm−1, the leakage current densities increase rapidly with increasing E. However, when the values of E are greater than 100 kV·cm−1, the leakage current densities increase slowly and remain constant finally. Compared with the film of Bi4Ti3O12, Nd3+-doped films possess lower leakage current densities, indicating that doping of Nd3+ is beneficial for the reduction in leakage current densities. The reason is that volatilization of Bi3+ during the annealing process induces higher concentration of oxygen vacancies [39]. And the substitution of Nd3+ for Bi3+ inhibits the volatilization of Bi3+ effectively, thus reducing oxygen vacancies and leakage current densities. The data summarized from Fig. 6 are shown in Table 1. Among the five films, the film of Bi3.55Nd0.45Ti3O12 possesses the minimum of leakage current density (1.02 × 10–6 A·cm−2), which is close to the data from Ref. [28] (2 × 10–6 A·cm−2) and lower than those from Ref. [40] (1.15 × 10–5 A·cm−2) and Ref. [29] (2.0 × 10–5 A·cm−2). It should be noted that leakage current density of the films of Bi3.55Nd0.45Ti3O12 is about one order lower than that of the film of Bi4Ti3O12 (1.85 × 10–5 A·cm−2).
3.5 Dielectric properties
Variation of dielectric constant (ε) and dielectric loss (tanδ) with frequency is shown in Fig. 7. As shown in Fig. 7a, at lower frequency (< 10,000 Hz), the values of ε decrease rapidly with increasing frequency. Nevertheless, at higher frequency (> 10,000 Hz), the values of ε decrease slowly and remain nearly constant. The high values of dielectric constant at low frequency are due to the fact that the charges were trapped at interface states, which follows the alternating current variations [30]. And the space charge effect is suppressed at a higher frequency [30], thus the values of dielectric constant are greater at low frequency than those at high frequency. It is clearly seen from Fig. 7b that, with increasing frequency, the values of tanδ remain nearly unchanged when frequency is smaller than 10,000 Hz and increase rapidly when frequency is above 10,000 Hz. The significant increase in dielectric loss at high frequency may be ascribed to Maxwell–Wagner interfacial polarization or space charge polarization [31]. At the same frequency, the values of ε increase and the values of tanδ decrease with the increasing concentration of Nd3+. The structural inhomogeneity arising from Nd3+ doping breaks the translational symmetry which is not conducive to the coupling of electric dipoles. Thus, the concentration and the coupling of the dipoles decrease [41]. And dielectric constant increases due to the doping of Nd3+. The replacement of Bi3+ with Nd3+ inhibits the formation of oxygen vacancies [31]. And decrease in the values of dielectric loss with the increase in doping concentration of Nd3+ may be attributed to the reductions of concentrations of oxygen vacancy [42]. This indicates that doping of Nd3+ enhances the dielectric properties of the films effectively.
3.6 Photoluminescence
Excitation spectra of the films of Bi4−xNdxTi3O12 are shown in Fig. 8a. Obviously, there is a distinct excitation peak at about 294 nm which is close to the value of wavelength in Ref. [43] (292 nm). This position of wavelength corresponds to the energy level transition of 4I9/2 → 2H11/2 [43, 44]. The emission spectra of the films of Bi4−xNdxTi3O12 are illustrated in Fig. 8b, showing that there is a blue light emission peak at 437 nm and one yellow light emission peak at 580 nm which are close to the values of wavelength in Ref. [43] (434 nm and 582 nm). These may be due to the energy level transitions of 4G9/2 → 4F9/2 and 4G7/2 → 4F9/2, respectively [43, 44]. The values of wavelength of the emission peaks remain almost constant under different doping concentration of Nd3+. With increasing concentration of Nd3+, the emission intensities increase firstly and then decrease. The emission intensity reaches the maximum when the concentration of Nd3+ is equal to 0.45, which indicates quenching concentration is about 0.45. Concentration quenching mechanism is highly relevant to the critical interaction distance between neighboring activators in the host lattice [45, 46].
Next, we give an intuitive description on the luminescence mechanism of the films of Bi4−xNdxTi3O12 based on the possible partial energy level diagram of Nd3+, as shown in Fig. 8c. It can be clearly seen that Nd3+ was excited from ground state (4I9/2) to excited state (2H11/2) when excited at a deep ultraviolet light (DUV, 294 nm). Then, Nd3+ was relaxed from excited state of 2H11/2 to the energy level of 4G9/2 and 4G7/2 via non-radiative-relaxation [44, 46]. Finally, the electronic transitions from energy level of 4G9/2 and 4G7/2 to the energy level of 4F9/2 give rise to a blue light emission peak at 437 nm and a yellow light emission peak at 580 nm, respectively.
The diagrams of Commission Internationale de L’Eclairage (CIE) chromaticity coordinate of the films of Bi4−xNdxTi3O12 are shown in Fig. 9. It can be found that CIE chromaticity coordinates of Bi3.75Nd0.25Ti3O12, Bi3.55Nd0.45Ti3O12, Bi3.35Nd0.65Ti3O12 and Bi3.15Nd0.85Ti3O12 are (0.2568, 0.2545), (0.2402, 0.2552), (0.2509, 0.2610), (0.2499, 0.2688), respectively. The CIE coordinates are located in the blue region of CIE diagram, as a contrast, CIE coordinates of nanometer powder prepared in Ref. [30] are in the green-yellow region.
The ultraviolet (UV) -visible (Vis) -near infrared (NIR) absorption spectra of the films of Bi4−xNdxTi3O12 are shown in Fig. 10a. It can be seen that the films exhibit obvious absorptions from 350 to 500 nm. In general, for the indirect transitions, the band gap energy (Eg) can be obtained via following formula [47, 48]:
where α is absorption coefficient from the absorption spectrum, A is constant and hν is energy of a photon, respectively. Variations of (αhν)1/2 with hν of the films are shown in Fig. 10b. The values of Eg are determined from the intersections of the curve tangent and the abscissa [49, 50], as shown in Table 1. Apparently, the values of Eg decrease gradually with the increasing concentration of Nd3+, which is beneficial for enhancing visible light catalytic activity [47, 49]. It is reported that the doping of Nd3+ gives rise to the distortions of TiO6 octahedra [42], which is a dominating reason for reducing band gap energy [31]. Therefore, the value of Eg decreases with the increase in Nd3+ concentration. The value of Eg of the film of Bi3.15Nd0.85Ti3O12 is 2.67 eV, which is smaller than that reported in Ref. [47] (2.82 eV). The narrowed band gaps imply the potential application in light catalytic fields as smaller Eg is beneficial for enhancing visible light catalytic activity [47, 49].
4 Conclusions
The films of Bi4−xNdxTi3O12 doped with different concentrations of Nd3+ were deposited on the substrate via spin-coating technology. The as-prepared films of Bi4−xNdxTi3O12 are pure and have a few pores and cracks on the surfaces of the films. The interfaces between the films of Bi4−xNdxTi3O12 and the substrates are clear and flat. The film of Bi3.55Nd0.45Ti3O12 possess the maximum of Pr (17.25 µc·cm−2) and the minimum of leakage current density (1.02 × 10–6 A·cm−2). With the increasing concentration of Nd3+, the values of ε of the films increase and the values of tanδ decrease. The films of Bi4−xNdxTi3O12 exhibit a blue light emission peak at 437 nm and a yellow light emission peak at 580 nm. With increasing concentration of Nd3+, the values of Eg decrease gradually. For the film of Bi3.15Nd0.85Ti3O12, the value of band gap energy is 2.67 eV. The photoluminescence and ferroelectric properties of the film of Bi3.55Nd0.45Ti3O12 are, on the whole, good and helpful for the applications in multi-functional devices.
References
B. Fugiel, S. Komraus, T. Kikuta, Influence of side electric potential on hysteresis loop parameters and electric permittivity in the rochelle salt. Phys. B 407, 3956–3959 (2012)
W.T. Chen, A.E. Gurdal, S. Tuncdemir, J. Gambal, C.A. Randall, Introducing an extremely high output power and high temperature piezoelectric bimorph energy harvester technology based on the ferroelectric system Bi(Me)O3-PbTiO3. J. Appl. Phys. 128, 144102 (2020)
M.Z. Koohi, A. Mortazawi, Negative piezoelectric based electric-field-actuated mode-switchable multilayer ferroelectric FBARs for Selective control of harmonic resonances without degrading keff. IEEE. T. Ultrason. Ferr. 67, 1922–1930 (2020)
J. Wang, M. Park, S. Mertin, T. Pensala, F. Ayazi, A. Ansari, A film bulk acoustic resonator based on ferroelectric aluminum scandium nitride films. J. Microelectromech. S. 29, 741–747 (2020)
X. Qiao, W. Geng, D. Zheng, X. Chou, Robust in-plane polarization switching in epitaxial BiFeO3 films. J. Alloy. Compd. 852, 156988 (2020)
P. Pandey, S.H. Wan, K.R. Udayakumar, T.S. Moise, A.C. Seabaugh, Programming-pulse dependence of ferroelectric partial polarization: insights from a comparative study of PZT and HZO capacitors. IEEE. T. Electron. Dev. 67, 4482–4487 (2020)
R. Indergand, A. Vidyasagar, N. Nadkarni, D.M. Kochmann, A phase-field approach to studying the temperature-dependent ferroelectric response of bulk polycrystalline PZT. J. Mech. Phys. Solids. 144, 104098 (2020)
S.A. Sharko, A.I. Serokurova, N.N. Novitskii, V.A. Ketsko, A.I. Stognij, Continuous ferrimagnetic Y3Fe5O12 layers on the ceramic PbZr0.45Ti0.55O3 substrates. Ceram. Int. 46, 22049–22056 (2020)
B. Gao, Z.H. Yao, D.Y. Lai, Q.H. Guo, W.G. Pan, H. Hao, M.H. Cao, H.X. Liu, Unexpectedly high piezoelectric response in Sm-doped PZT ceramics beyond the morphotropic phase boundary region. J. Alloy. Compd. 836, 155474 (2020)
P. Kumar, C. Prakash, Synthesis, dielectric and ferroelectric properties of Sm3+ modified PZTFN ceramics. Mater. Chem. Phys. 251, 123061 (2020)
H. Wang, H. Yuan, X. Liu, K. Wu, D. Lin, Achieving high energy-storage properties in Bi0.5Na0.5TiO3-based lead-free ceramics under low electric fields. Ceram. Int. 47, 1344–1352 (2020)
M.A. Wederni, D.B. Jemia, H. Rahmouni, R.J. Martin-Palma, R. Chtourou, Structural, morphological, vibrational, and impedance properties of ytterbium modified bismuth titanate. Chem. Phys. Lett. 755, 137787 (2020)
M. Alguero, M. Perez-Cerdan, P.R.D. Real, J. Ricote, A. Castro, Novel aurivillius Bi4Ti3-2xNbxFexO12 phases with increasing magnetic-cation fraction until percolation: a novel approach for room temperature multiferroism. J. Mater. Chem. C. 836, 12457–12469 (2020)
C.B. Long, H.Q. Fan, W. Ren, J.Y. Zhao, Double polarization hysteresis and dramatic influence of small compositional variations on the electrical properties in Bi4Ti3O12 ceramics. J. Eur. Ceram. Soc. 39, 4103–4112 (2019)
R. Nie, J. Yuan, Q. Chen, J. Xing, J. Zhu, W. Zhang, Crystal distortion and electrical properties of Ce-doped BIT-based piezoelectric ceramics. J. Am. Ceram. Soc. 102, 5432–5442 (2019)
S. Ma, X.W. Cheng, T. Ali, Influence of tantalum on mechanical, ferroelectric and dielectric properties of Bi-excess Bi3.25La0.75Ti3O12 thin film. Appl. Surf. Sci. 463, 1141–1147 (2019)
C.Y. Yau, R. Palan, K. Tran, R.C. Buchanan, Mechanism of polarization enhancement in La-doped Bi4Ti3O12 films. Appl. Phys. Lett. 86, 032907 (2005)
Y.Y. Wu, X.H. Wang, L.T. Li, Ferroelectric and dielectric properties of La/Mn co-doped Bi4Ti3O12 ceramics. Chinese. Phys. B. 19, 037701 (2010)
M. Chen, Z.L. Liu, Y. Wang, C.C. Wang, X.S. Yang, K.L. Yao, Ferroelectric properties of Pr6O11-doped Bi4Ti3O12. Solid. State. Commun. 130, 735–739 (2004)
C.P. Cheng, M.H. Tang, Z. Ye, Y.C. Zhou, Ferroelectric properties of dysprosium-doped Bi4Ti3O12 thin films crystallized in various atmospheres. T. Nonferr. Metal. Soc. 16, S33–S36 (2006)
U. Chon, J.S. Shim, H.M. Jang, Compositional dependence of ferroelectric properties of highly c-axis oriented Bi4-xNdxTi3O12 film capacitors. Solid. State. Commun. 129, 465–468 (2004)
X.Y. Mao, F.W. Mao, X.B. Chen, Ferroelectric and dielectric properties of Bi4-xNdxTi3O12 ceramics. Integr. Ferroelectr. 79, 155–161 (2006)
Y. Ahn, J.Y. Son, Mixed grains and orientation-dependent piezoelectricity of polycrystalline Nd-substituted Bi4Ti3O12 thin films. Ceram. Int. 42, 13061–13064 (2016)
F. Gao, H.F. Liu, F. Ren, K.T. Wang, X.S. Li, Y.B. Wang, C.L. He, Y.Z. Wei, Tunable structure and intensive upconversion photoluminescence for Ho3+-Yb3+ codoped bismuth titanate composite synthesized by sol-gel-combustion (SGC) method. Cream. Int. 46, 3015–3022 (2020)
F.M. Yang, B. Jia, T. Wei, C.Z. Zhao, Q.J. Zhou, Z.P. Li, M.R. Du, M.C. Wang, Y.Y. Liu, C.Y. Xie, Reversible regulation of upconversion luminescence in new photochromic ferroelectric materials: Bi4-xErxTi3O12 ceramics. Inorg. Chem. Front. 6, 2756–2766 (2019)
R. Bokolia, O.P. Thakur, V.K. Rai, S.K. Sharma, K. Sreenivas, Dielectric, ferroelectric and photoluminescence properties of Er3+ doped Bi4Ti3O12 ferroelectric ceramics. Ceram. Int. 41, 6055–6066 (2015)
K. Ruan, A. Gao, W. Deng, X. Chen, D. Bao, Orientation dependent photoluminescent properties of chemical solution derived Bi4-xEuxTi3O12 ferroelectric thin films. J. Appl. Phys. 104, 036101 (2008)
D.P. Song, J. Yang, J.X. Sun, L.Y. Chen, J.K. Lee, Controlling the crystallization of Nd-doped Bi4Ti3O12 thin-films for lead-free energy storage capacitors. J. Appl. Phys. 127, 224102 (2020)
Y.Q. Gong, H.Y. Chen, S.H. Xie, X.J. Li, Effects of Nb Content on the ferroelectric and dielectric properties of Nb/Nd-Co-doped Bi4Ti3O12 thin films. J. Electron. Mater. 47, 1792–1797 (2018)
A. Marikani, V. Selvamurugan, G. Mangamma, S. Ravi, R. Krishnasharma, P.V. Chandrasekar, M. Kamruddin, D. Madhavan, Ferroelectric, dielectric, and optical properties of Nd-substituted Bi4Ti3O12 nanoparticles synthesized by sol-gel method. Prog. Nat. Sci. 26, 528–532 (2016)
M.S. Alkathy, F.L. Zabotto, M.H. Lente, J.A. Eiras, Octahedral distortion and oxygen vacancies induced band-gap narrowing and enhanced visible light absorption of Co/Fe co-doped Bi3.25Nd0.75Ti3O12 ferroelectrics for photovoltaic applications. J. Phys. D. Appl. Phys. 53, 465106 (2020)
X.D. Li, Z.N. Chen, L.S. Sheng, Large enhancement of piezoelectric properties and resistivity in Cu/Ta co-doped Bi4Ti3O12 high temperature piezoceramics. J. Am. Ceram. Soc. 102, 7366–7375 (2019)
S.K. Badge, A.V. Deshpande, Effect of vanadium doping on structural, dielectric and ferroelectric properties of bismuth titanate (Bi4Ti3O12) ceramics. Ceram. Int. 45, 15307–15313 (2019)
X. Li, L. Zhu, P. Huang, Z. Chen, W. Bai, L. Li, F. Wen, P. Zhen, W.L. Wu, Y. Zheng, Reduction of oxygen vacancy concentration and large enhancement of electrical performances in Cu/Sb co-doped Bi4Ti3O12 high temperature piezoelectric ceramics. J. Appl. Phys. 127, 044102 (2020)
Q. Zhou, B.J. Kennedy, C. Howard, Structural studies of the ferroelectric phase transition in Bi4Ti3O12. Chem. Mater. 15, 5025–5028 (2003)
Y. Liu, L. Fan, W. Yi, C. Yan, J. Ma, Q. Ji, Q. Lin, Microstructure and ferroelectric properties of bi-excess Bi4Ti3O12 thin films grown on Si and Pt/Ti/SiO2/Si substrates. Ferroelectrics 54, 144–149 (2020)
X. Du, W. Huang, S.K. Thatikonda, N. Qin, D. Bao, Improved ferroelectric and dielectric properties of Sm, La co-doped Bi4Ti3O12 multifunctional thin films with orange-red emission. J. Mater. Sci.-Mater. El. 30, 13158–13166 (2019)
D. Coathup, Z. Li, X. Zhu, H. Yan, R. Zhang, H. Ye, Dielectric and ferroelectric properties of BTFCO thin films. J. Electroceram. 43, 92–95 (2019)
X. Hu, A. Garg, Z.H. Barber, Structural and electrical properties of samarium-substituted bismuth titanate ferroelectric thin films on Pt/TiOx/SiO2/Si substrates. Thin Solid Films 484, 188–195 (2005)
G. Xia, H. Tan, Ren, Effect of Fe substitution on microstructure and properties of bismuth titanate thin films. Ceram. Int. 42, 1267–1271 (2016)
M.A. Islam, M.S. Islam, Synthesis and Characterization of La and Nd Co-doped bismuth titanate ferroelectric ceramics. Int. J. Appl. Ceram. Tec. 12, E191–E196 (2015)
D. Zhou, H.S. Gu, Y.M. Hu, Z.L. Qian, Z.L. Hu, K. Yang, Y.N. Zou, Z. Wang, Y. Wang, J.G. Guan, W.P. Chen, Raman scattering, electronic, and ferroelectric properties of Nd modified Bi4Ti3O12 nanotube arrays. J. Appl. Phys. 107, 094105 (2010)
V.R. Prasad, M. Seshadri, S. Babu, Y.C. Ratnakaram, Concentration-dependent studies of Nd3+-doped zinc phosphate glasses for NIR photoluminescence at 105 μm. Luminescence 32, 443–451 (2017)
M. Balestrieri, S. Colis, M. Gallart, G. Schmerber, A. Dinia, Photoluminescence properties of rare earth (Nd, Yb, Sm, Pr)-doped CeO2 pellets prepared by solid-state reaction. J. Mater. Chem. C. 3, 7014–7021 (2015)
M. Pinatti, T.M. Mazzo, R.F. Gonçalves, J.A. Varela, E. Longo, I.L.V. Rosa, CaTiO3 and Ca1-3xSmxTiO3: photoluminescence and morphology as a result of hydrothermal microwave methodology. Ceram. Int. 42, 1352–1360 (2016)
S. Yang, J. Yao, Y. Quan, Monitoring the charge-transfer process in a Nd-doped semiconductor based on photoluminescence and SERS technology. Light.-Sci. Appl. 9, 117 (2020)
Y. Chen, C.H. Nie, Y.L. Bai, S.F. Zhao, The photovoltaic spectral response regulated by band gap in Zr doped Bi4Ti3O12 thin films. J. Mater. Sci.-Mater. El. 26, 5917–5922 (2015)
H. He, Z. He, Z. Jiang, J. Wang, T. Liu, N. Wang, A controllable photoresponse and photovoltaic performance in Bi4Ti3O12 ferroelectric thin films. J. Alloy. Compd. 694, 998–1003 (2017)
Y. Chang, C.S. Tu, P.Y. Chen, Raman vibrations and photovoltaic conversion in rare earth doped (Bi0.93RE0.7)FeO3 (RE=Dy, Gd, Eu, Sm) ceramics. Ceram. Int. 42, 834–842 (2016)
S. Sharma, J.M. Siqueiros, O.R. Herrera, Structural, dielectric, ferroelectric and optical properties of Er doped BiFeO3 nanoparticles. J. Alloy. Compd. 853, 156979 (2021)
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (52072103).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Y., Hu, K., Shi, M. et al. Effect of concentration of Nd3+ on the photoluminescence and ferroelectric properties of Bi4-xNdxTi3O12 films. J Mater Sci: Mater Electron 32, 15653–15664 (2021). https://doi.org/10.1007/s10854-021-06117-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06117-9