Abstract
Carbon quantum dots (CQDs) were synthesized by coffee grounds as the carbon source under hydrothermal carbonization treatment for the first time. The obtained turn-on CQDs showed a strong emission at the wavelength of 460 nm, with an optimum excitation of 370 nm, and enrichment of the surface functional groups (–OH, –NH2). Sodium cyclamate in an aqueous solution and the turn-on CQDs could form a core–shell structure by hydrogen bonding and van der waals bonding to enhance the fluorescent emission. With such a design, turn-on CQD-based fluorescence detection techniques towards sodium cyclamate could be realized. Under optimal conditions, a linear relationship was found in the range of 2.8–56 µmol/L sodium cyclamate with the detection limit (3δ/k) of 3.16 µmol/L which is lower than the national standard detection limit of 49.7 µmol/L. The sensitive method has a great application prospect in detection of sodium cyclamate in real samples owing to its low detection limit, simplicity and rapidity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sodium cyclamate is one of the common food additives [1,2,3]. In GB 2760-2014, the dosage of sodium cyclamate is in the range of 0.65–8.0 g/kg [4]. Daily intake value of sodium cyclamate was 7 mg/(kg bw) [5]. The use of excessive range and limit of sodium cyclamate can damage the microfilaments and microtubules of osteoblasts, inhibit bone cells activity and the expression of bone morphogenetic protein-2 [6] and even damage the structure of DNA [2]. Various techniques including gas chromatography [1, 7], liquid chromatography [8] and liquid chromatography-mass spectrometry [9] [10,11,12,13] have been established for the detection of sodium cyclamate. However, new methods with low detection cost, high sensitivity and short response time are always being established.
Carbon quantum dots (CQDs) are a new kind of nanomaterials, which use natural resources or waste materials (papaya, watermelon, tomato, aloe vera, peach, etc.) as carbon sources in order to reduce the environmental pollution [14,15,16,17,18,19]. Although CQD-based fluorescence detection techniques towards food additives have been the subject of great progress, most mechanisms are based on the principle of fluorescence turn-off [20,21,22,23,24]. By contrast, strategies based on fluorescence turn-on are more sensitive and attractive owing to their better ability to resist background interference and lower signal-to-noise ratio [25,26,27]. Nevertheless, rational synthesis of fluorescence turn-on CQDs for specific food additives detection still faces great challenges due to a lack of in-depth exploration of sensing mechanisms. To the best of our knowledge, the fabrication and design of a new fluorescence turn-on CQDs for detection of the sodium cyclamate have not been reported yet.
Coffee grounds are rich in carbon sources and nitrogen sources, so it can be used as a high-quality carbon source for the production of turn-on CQDs [28,29,30]. Up to now, fluorescence turn-on CQDs for detection of the sodium cyclamate have been scarcely reported. In this work, coffee grounds as carbon source to synthesize turn-on CQDs by one-step hydrothermal method could detect sodium cyclamate in food with high efficiency and are environment-friendly. In the concentration range of 2.8–56 μmol/L, sodium cyclamate was linear with the turn-on CQDs of coffee grounds, and the detection limit was 3.16 μmol/L.
2 Experimental
2.1 Chemicals and materials
Coffee grounds were obtained from Shanghai, China. Sodium cyclamate, Sucrose, Sorbic acid, FeCl3·6H2O, CuSO4 and other interfering metals were obtained from Aladdin Reagent Company. All chemicals were at least of analytical grade and used without further purification. Ultrapure water was supplied by a Millipore System throughout the whole experiment.
2.2 Instrumentation
The fluorescence spectra of CQDs were recorded using a fluorescence spectrophotometer(F-4600). X-ray powder diffraction (XRD) patterns of the CQDs were recorded with a D/MAX-IIIC (Japan) operating with Cu Kα radiation with a scanning angle (2θ) range from 5° to 80°. The microstructures of the samples were observed by scanning electron microscopy (SEM) (Inspect F50) and transmission electron microscopy (TEM) (HT7700). FT-IR was carried out with a Germany Bruker Company TENSOR 27 by means of KBr pellet with the wavelength range of 4000–400 cm−1.
2.3 Synthesis of CQDs
The hydrothermal treatment of coffee grounds to prepare CQDs is shown in Fig. 1. Firstly, coffee grounds were washed with the mixture of ultrapure water and ethanol, and dried at 80 °C for 12 h. Then, coffee-ground powder in varying weights (0.25 g, 0.125 g, 0.0625 g) was dispersed in 40 mL ultrapure water. After ultrasonication for 30 min, the dispersed solution was transferred into a steel kettle and reacted at the constant temperature (120 °C, 160 °C, 200 °C) for a fixed time (6 h, 10 h, 14 h). The final brown hydrothermal products were centrifuged at 6000 rpm for 5 min to wipe out the large particles. Finally, the CQDs solution was purified in a 500-Da dialysis bag in ultrapure water, and then the remaining solution was put into the oven to obtain solid CQDs powder. Each experiment has three parallel experiments, in order to prevent the experimental results from being discovered accidentally.
2.3.1 Effects of the reaction concentration
The effect of coffee-ground concentration was investigated. As displayed in Fig. 2a, comparing the fluorescence intensity of the three concentrations of CQDs under the same conditions, we can clearly conclude that the CQDs of 3.125 g/L coffee grounds have the best fluorescence intensity.
2.3.2 Effects of the reaction time
The effect of the reaction time was investigated. As displayed in Fig. 2b, the fluorescence intensity of CQDs in 6 h was similar to 12 h, but the fluorescence intensity of CQDs in 10 h was significantly higher. Therefore, the best reaction time is 10 h.
2.3.3 Effects of the reaction temperature
The effect of reaction temperature on the fluorescence intensity of the system was also studied. As depicted in Fig. 2c, the intensity of this system reached maximum and was stable at 120 °C. And the fluorescence intensity of CQDs at 120 °C is much higher than that at 160 °C and 200 °C.
All in all, the best fluorescence properties of CQDs synthesized from coffee grounds were obtained under optimal conditions (3.125 g/L, 120 °C and 10 h).
2.4 Fluorescence sensing of sodium cyclamate
0.0561 g of sodium cyclamate was dissolved in 100 mL ultrapure water as standard reserve solution. The concentration gradient of sodium cyclamate was set at 0, 2.8, 9.4, 11.8, 28, 56 and 112 μmol/L. 1 mL of the as-prepared CQDs solution was added in the corresponding pH buffer solution (pH 2.18, 3.25, 4.23, 5.39, 6.08, 7.01, 7.61, 8.53, 9.45,10.94). The final fluorescence spectra of the tested samples were recorded under the excitation of 370 nm.
3 Results and discussion
3.1 Structure analysis and characterization
3.1.1 XRD and FT-IR
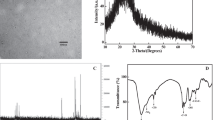
The structures of the obtained CQDs are detected by the XRD and FT-IR. As shown in Fig. 3a, the primary XRD peaks of the obtained CQDs showed a broad diffraction peak at 23.5°, which was associated with the diffraction of the (002) crystal plane, as a graphite structure.
The surface groups of CQDs measured via FT-IR spectroscopy are shown in Fig. 3b. It can be confirmed that a broad peak at about 3310 cm−1 resulted from the vibrations of O–H and N–H, and abundant hydroxyl and amino groups are integrated on the surface of CQDs, promoting water solubility of CQDs. The peak at 2941 cm−1 was associated with the stretching vibrations of –CH3 and –CH2. A characteristic peak at about 1670 cm−1 belonged to the typical vibration of the C=O group. Other two peaks were observed at around 1395 and 1365 cm−1, which resulted from the characteristic vibrations of C–N and C–O–C, respectively.
3.1.2 TEM
The morphology of the as-prepared CQDs was characterized via TEM. As seen in Fig. 4a, CQDs exhibited a spherical structure and a well-dispersed state. The size distribution revealed that the size of CDs ranged from 1.9 to 5.9 nm with the average diameter of about 4.0 nm (Fig. 4b).
3.2 Spectral properties of CQDs
The spectral properties of CQDs are shown in Fig. 5a. The absorption band observed at 320 nm was attributed to the π–π* transition of the C=C bond. With the increase of excitation wavelength from 340 to 400 nm, emission peaks showed red-shift from 440 to 470 nm. When the excitation of CQDs is at 370 nm, CQDs exhibited the maximum fluorescence emission intensity at 460 nm in Fig. 5b.
3.3 Fluorescent response towards sodium cyclamate
Under optimum conditions, a calibration curve was drawn by conducting a series of similar experiments at various concentrations of sodium cyclamate and the results are depicted in Fig. 6. Calibration curve was linear in the range of 2.8–112 μmol/L with the correlation coefficient of 0.992. Fluorescence sensing between CQDs of coffee grounds and sodium cyclamate belongs to the “turn on” type. This synergistic system may be due to the formation of cyclohexyl aminosulfonic acid from cyclohexyl aminosulfonate in aqueous solution, and then sodium cyclamate and the CQDs form a core–shell structure by hydrogen bonding and van der waals bonding, which stabilizes or even strengthens the morphology of the surface groups of CQDs in the original solution and reduces unnecessary energy transfer.
3.4 Effect of pH
In the 100 mL mixed solution (phosphoric acid, acetic acid, boric acid, concentration of 0.04 mol/L), the corresponding pH buffer solution was obtained by adding 0.2 mol/L NaOH. Ten buffer solution values of 2.18, 3.25, 4.23, 5.39, 6.08, 7.01, 7.61, 8.53, 9.45 and 10.94 were set up, and the fluorescence intensity of CQDs with and without sodium cyclamate was measured at each fixed value, and the results were shown in Fig. 7.
3.5 Fluorescence emission studies with analyte
From Fig. 8, it can be seen that the fluorescence sensing between the CQDs of coffee grounds and sodium cyclamate belongs to “turn on” type, and the CQDs of coffee grounds and copper ions are “turn off” type. However, there is no fluorescence sensing response for other metal ions, indicating that the CQDs of coffee grounds are highly selective for the detection of sodium cyclamate.
3.6 Calculation of detection limit
Firstly, fluorescence intensity of ten times CQDs solution and the first addition of sodium cyclamate solution were measured respectively, and the results were shown in Table 1. The average fluorescence intensity of F0 was calculated and the data were calculated using formula (1) to get the standard deviation value S0. The difference of fluorescence intensity was obtained from the formula ΔF = F − F0. At the same time, the sensitivity S was obtained from formula (2) in which ΔC is the concentration change of enhancer in the whole solution system after adding sodium cyclamate. Finally, the detection limit (DL = 3.16 μmol/L) was obtained by formula (3) [31]. The latest National Food Safety Standard for the determination of sodium cyclamate (GB 5009.97-2016) is 0.010 g/kg. The concentration of dilute solution is approximately regarded as the concentration of aqueous solution 1 kg/L, while the DL of the national standard is 49.7 μmol/L after conversion. The DL of our method is far lower than that of the national standard method.
4 Conclusion
A new type of CQDs using coffee grounds as carbon source was synthesized by one-step hydrothermal method, which was used to detect the sodium cyclamate with highly sensitive and selective. Under optimum conditions, the calibration curve was linear in the range of 2.8–112 mmol/L with the correlation coefficient of 0.992, and the DL of sodium cyclamate was 3.16 μmol/L. The CQDs of coffee grounds can be used for the rapid and selective detection of sodium cyclamate and serve as a reference for food safety supervision and detection.
References
S.-B. Yu, B.-H. Zhu, F. Lv, S.-X. Li, W.-X. Huang, Food Chem. 134, 2424–2429 (2012)
Y. Hu, M.-Y. Xie, X.-Y. Wu, Spectrochim. Acta A 220, 117085–117091 (2019)
M. Behrens, K. Blank, W. Meyerhof, Cell Chem. Biol. 24, 1199–1204 (2017)
GB2760-2014, National standard for food safety Standard for use of food additives. (Standards Press of China, Beijing, 2014)
P. Cao, N. Ma, J. Liang, X.-D. Wang, H.-B. Xu, Chin. J. Food Hyg. 28, 111–114 (2016)
Z.-H. Chen, G.-Y. Chen, K. Zhou, P. Zhang, X.-L. Ren, X.-F. Mei, Biochem. Biophys. Res. Commun. 508, 507–511 (2019)
M. Hashemi, A. Habibi, N. Jahanshahi, Food Chem. 124, 1258–1263 (2010)
I. Casals, M. Reixach, J. Amat, M. Fuentes, L. SerraMajem, J. Chromatogr. A. 750, 397–402 (1996)
Z.-Q. Huang, J.-Y. Ma, B. Chen, Y. Zhang, S.-Z. Yao, Anal. Chim. Acta 555, 233–237 (2005)
Y.-T. Wang, B. Li, X.-J. Xu, H.-B. Ren, J.-Y. Yin, H. Zhu, Y.-H. Zhang, Food Chem. 303, 125404–125415 (2020)
X.-M. Li, S.-Q. Li, H.-M. Li, J. Wang, Q. Luo, X. Yin, Food Chem. 342, 128331–128339 (2021)
D.M. Cárdenas-Soracá, V. Singh, E. Nazdrajić, T. Vasiljević, J.J. Grandy, J. Pawliszyn, Talanta 211, 120714–120723 (2020)
S.-G. Xia, D. Yin, Y.-L. Chen, Z.-C. Yang, Y. Miao, W.-F. Zhang, S. Chen, W.-D. Zhao, S.-S. Zhang, Can. J. Chem. 97, 344–351 (2019)
X.-D. Tang, H.-M. Yu, B. Bui, L.-Y. Wang, C. Xing, S.-Y. Wang, M.-L. Chen, Z.-Z. Hu, W. Chen, Bioact. Mater. 6, 1541–1554 (2021)
C. Karami, M.A. Taher, M. Shahlaei, J. Mater. Sci. Mater. Electron. 31, 5975–5983 (2020)
Y. Dong, Y.-D. Zhang, S.-M. Zhi, X.-Y. Yang, C. Yao, ChemistrySelect 6, 123–130 (2021)
M. Masteri-Farahani, N. Mosleh, J. Mater. Sci. Mater. Electron. 30, 21170–21176 (2019)
G.K. Kanthi, A. Tejaswini, H.-F. Wu, B. Venkateswararao, J. Anil, R.A. Kumar, G. Gangaraju, Colloids Surf. B 197, 111362–111369 (2021)
X.-F. Lin, M.-G. Xiong, J.-W. Zhang, C. He, X.-M. Ma, H.-F. Zhang, Y. Kuang, M. Yang, Q.-T. Huang, Microchem. J. 160, 105604–105620 (2021)
M. Galal, A.F.A. Hakiem, B. Fathalla, A.M. Abdel-Megied, Food Chem. 343, 128539–128548 (2021)
L.-F. Liu, Q. Hu, H.-J. Sun, J. Han, Y.-N. Pan, Z.-Q. Yang, J. Food Compos. Anal. 94, 103639–103647 (2020)
L.-J. Ren, X.-X. Hang, Z.-R. Qin, P. Zhang, W. Wang, Y.-T. Zhang, L.Y. Jiang, Optik 208, 163560–163566 (2020)
X. Zhou, Q. Qu, L. Wang, L. Li, S.-L. Li, K. Xia, J. Nanopart. Res. 22, 20–33 (2020)
Y.-N. Sun, M. Zhang, B. Bhandari, C.-H. Yang, Food Rev. Int (2020). https://doi.org/10.1080/87559129.2020.1818255
Y.-W. Rong, S. Ali, Q. Ouyang, L. Wang, B. Wang, Q.-S. Chen, Food Chem. 351, 129215–129223 (2021)
S. Palanisamy, L.-F. Chen, S.-C. Tzou, Y.-M. Wang, Sens. Actuator B 310, 127839–127847 (2020)
J.-X. Xu, J.-W. Xiong, Y.-L. Qin, Z.-Z. Li, C.-Q. Pan, Y.-P. Huo, H.-L. Zhang, Mater. Chem. Front. 4, 3338–3348 (2020)
D. Kim, H. Choi, C. Lee, Biomass Convers. Biorefinery (2020). https://doi.org/10.1007/s13399-020-01171-5
I.J. Buerge, T. Poiger, M.D. Muller, H.R. Buser, Environ. Sci. Technol. 37, 691–700 (2003)
H. Rumana, N.R. Khayyam, M. Irshad, S. Veena, J. Energy Storage 33, 102113–102123 (2021)
H.-Y. Du, G. He, T.-H. Liu, L.-P. Ding, Y. Fang, J. Photochem. Photobiol. A 217, 356–362 (2011)
Acknowledgements
This study was financially supported by the scholars' science and technology activities project of Sichuan province in 2018 and supported by Sichuan Science and Technology Program (2021JDRC0105).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Du, H., Xu, Y. et al. A turn-on fluorescent sensor based on coffee-ground carbon dots for the detection of sodium cyclamate. J Mater Sci: Mater Electron 32, 13581–13587 (2021). https://doi.org/10.1007/s10854-021-05933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05933-3