Abstract
In the recent days, rare-earth doped phosphors have attracted an immense attraction in luminescence field for various solid-state lighting and electronic display device applications. However, need of efficient red phosphor is a prevalent challenge in this field. Herein, we report a bright red light emitting Na+ co-doped SrAl2O4:Eu3+ phosphor which is synthesized by modified sol–gel technique. These nanophosphors show excellent red emission due to characteristic 5D0 → 7Fj (j = 1, 2, 3, 4) transitions of the doped Eu3+ ions excited by 394 nm. Photoluminescence studies of both Eu3+ doped SrAl2O4 and Na+ co-doped SrAl2O4:Eu3+ has shown more than fourfold increase in the intensity for optimum Na+ addition. The improved decay time obtained from decay measurement has revealed that the co-doped Na+ has successfully reduced the nonradiative transitions. Thus, addition of monovalent Na+ to Eu3+ doped SrAl2O4 was found to be effective to address the charge imbalance problem along with significant enhancement in luminescence intensity and decay time. The results suggest that this nanophosphor could be a major candidate in the rapidly increasing field of solid-state lighting applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ever-increasing demand of energy-efficient solid-state lighting, especially in high quality displays and household lights have propelled the scientific society towards developing high quality phosphors. These phosphors are widely used in various fields like white light emitting diodes (WLEDs), plasma display panels (PDPs), and field emission displays (FEDs) [1,2,3]. Among these, white LEDs have gathered considerable attraction as fourth-generation lighting source due to its superiority over present incandescent and fluorescent lamps [4,5,6]. An emerging route of preparing WLEDs consists of utilizing ultraviolet (UV) emission of InGaN chips to excite different phosphors. A suitable mixing of three elementary colors, i.e. red, green, and blue from proper UV-excited phosphors can generate the required white color [7,8,9,10]. However, the poor efficiency of the red part with respect to the blue and green counterpart is a major disadvantage of this method. Thus, the preparation of a suitable and efficient red phosphor excited by UV light is still a major challenge in this field.
Rare-earth (RE) ions doping as luminescent centers in proper host material has always been the most practiced way to obtain outstanding phosphor materials [11,12,13,14]. Among various RE ions present, trivalent europium (Eu3+) ion is the most utilized one regarding its characteristic red emission (5D0 → 7F2) under UV excitation [15,16,17,18]. As the transfer of absorbed energy from the host material to RE ions is essential for realizing bright luminescence, wide band gap, and low phonon losses from the host are essential for excellent luminescence properties of the phosphor [19, 20]. In this regard, alkaline earth aluminate materials have gathered significant attention in the recent days [21,22,23,24]. Among them, SrAl2O4 is a widely studied material due to its stable structure, chemical resistance, low toxicity, and excellent optical properties [25,26,27,28,29]. Owing to wide band gap (~ 6.6 eV) [30, 31], SrAl2O4 does not absorb in the entire visible spectra, which theoretically eliminates the probability of re-absorption of the emitted lights from the RE ions. The low phonon energy (~ 470–480 cm−1) [32] of the host also reduces the chances of non-radiative losses.
From application point of view, the nanophosphors can be coated on a substrate with considerably greater homogeneity and controlled thickness than the bulk phosphor. Another well-recognized phenomenon is that the photoluminescence (PL) intensity of RE doped phosphors increases with increasing dopant concentration up to a certain concentration only [7]. Beyond this optimum concentration, some impurity phases are created or concentration quenching occurs. Thus, designing an efficient phosphor with enhanced PL intensity within the critical concentration is a difficult problem to solve.
In order to address the aforementioned demands, co-doping monovalent alkali metal ions (Li+, Na+, K+) with the RE doped phosphors has been studied rigorously in recent years [33,34,35]. Although Eu3+ doped SrAl2O4 has been studied thoroughly, to the author’s best knowledge, there is no report on Na+ co-doped SrAl2O4:Eu3+ nanophosphors synthesized by sol–gel route. Herein, we have reported Na+ co-doped SrAl2O4:Eu3+ nanophosphors synthesized via modified citrate–nitrate type sol–gel method. Extensive study of the PL property of both SrAl2O4:Eu3+ and Na+ co-doped SrAl2O4:Eu3+ nanophosphors over a range of Na+ concentration has shown more than four times enhancement in PL intensity with optimum Na+ co-doping. Thus, these results may offer an attractive alternate for addressing the challenges regarding red LEDs in day-to-day solid-state lighting applications.
2 Experimental sections
2.1 Synthesis of SrAl2O4:Eu3+ and Na+ doped SrAl2O4:Eu3+ nanophosphors
The targeted SrAl2O4: x% Eu3+ (x = 0, 1, 2, 3, 4) and SrAl2O4: 3% Eu3+, y% Na+ (y = 1, 2, 3) nanophosphors were prepared using a modified sol–gel method [35]. The chemicals used were Sr(NO3)2 (Merck 99.9%), Al(NO3)3 ·9H2O (Merck, 99.9%), Eu2O3 (Sigma-Aldrich 99.99%), HNO3, NaNO3 (Merck 99.9%), citric acid (Merck 99.9%), and ethylenediamine (Merck 99.9%). Eu(NO3)3 was prepared by dissolving Eu2O3 in HNO3 in proper stoichiometry. All chemicals were analytical grade and used without further purification. For synthesis, stoichiometric amount of Sr(NO3)2, Al(NO3)3 ·9H2O, Eu(NO3)3 and NaNO3 were taken in 35 mL DI water and the solution was magnetically stirred for 1 h. Then, the aqueous citric acid solution was added drop by drop to the above metal nitrate solution in molar ratio of Cit3−/(Sr2+ + Al3+) = 1. This solution was magnetically stirred for 30 min to get a homogeneous and transparent solution. pH of this solution was adjusted to 8 by ethylenediamine. The solution was slowly evaporated using a hot water bath, which converted the solution to a highly viscous colloidal gel. In subsequent steps, the gel was dried in oven at 120 °C for overnight and then it was calcined at 500 °C for 4 h to get rid of the organics. Finally, the obtained porous material was finely ground using mortar pestle and the powder was annealed at 1000 °C for 3 h in a muffle furnace to get the ultrafine Eu3+ doped and Na+ co-doped SrAl2O4 powders. The Eu3+ doped SrAl2O4 nanophosphors were synthesized following the same procedure, but NaNO3 was not added this time.
2.2 Characterization
For crystal structure and phase detection, the synthesized nanophosphors were analyzed using Bruker D8 ADVANCED ECO diffractometer with monochromatic Cu Kα radiation (λ = 1.5406 Å). The generator power was 40 kV and current was 25 mA for the experiment. The FTIR studies were performed using a Shimadzu IRAffinity-1S FTIR spectrophotometer. The microstructural analysis was done by HRTEM (FEG-TEM, JEOL-JEM 2100F). The photoluminescence properties were studied by Edinburgh FLS 980 Spectrofluorometer with Xenon lamp as the excitation source. CIE chromaticity coordinates of the nanophosphors were determined using 1931 CIE chromaticity theory.
3 Results and discussion
3.1 Structural, microstructural, and compositional analysis
3.1.1 XRD analysis
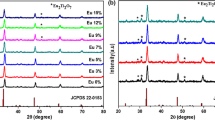
The X-ray diffractogram of SrAl2O4:Eu3+ and Na+ co-doped SrAl2O4:Eu3+ nanophosphors are represented in Fig. 1a and c, respectively. The diffraction patterns reveal that all the obtained nanophosphors were monoclinic with P21 space group (JCPDS card number 34–0379). Six major peaks of SrAl2O4 were found at 2θ positions 20.06°, 22.81°, 28.56°, 29.32°, 29.73° and 35.09° corresponding (011), (120), (\({\bar{2}}11\)), (220), (211) and (031), respectively. Doped Eu3+ occupies the Sr2+ sites in SrAl2O4 structure. Substitution of smaller of Eu3+ (0.95 Å) ion in Sr2+ (1.18 Å) leads to the shrinkage of the unit cell which is in accordance with the observed higher angle shifting of the characteristic peak near 2θ = 20.06° as shown in Fig. 1b. There was no trace of excess Eu3+ in SrAl2O4:Eu3+ up to 4% Eu3+ which indicates proper incorporation of Eu3+ in the host. Due to comparable sizes of Sr2+ and Na+ (1.18 and 1.02 Å, respectively), Na+ also goes to Sr2+ sites in the SrAl2O4 lattice.
As presented in Fig. 1c, XRD patterns of Na+ co-doped SrAl2O4:Eu3+ shows that there was no trace of excess Na+ up to 3% Na+ addition. This co-doped Na+ occupies the vacant Sr2+ sites, which solves the charge imbalance problem created by doping of Eu3+ in Sr2+ sites. Initially, the co-doping of Na+ leads to increased crystallinity of the nanophosphor. But this enhancement is only up to a certain Na+ concentration. Above this concentration, decrease in crystallinity with increasing Na+ is observed due to defects introduced by excess Na+ ions incorporated.
3.1.2 Microstructural Study
Figure 2 represents the TEM micrograph of the synthesized Na+ co-doped SrAl2O4:Eu3+ nanophosphor. As presented in Fig. 2a–b, an interconnected chain-like morphology is observed for the synthesized phosphors. Figure 2c shows that the average chain diameter was found to be in the range of 100–150 nm. Figure 2d clearly shows the array of atoms present in the phosphor. A portion of the atoms belong to the (011) plane with an interplanar spacing of 0.44 nm as indicated in Fig. 2d.
3.1.3 FTIR study
As shown in Fig. 3, vibration stretching frequencies between 700 and 1000 cm−1 are related to Sr–O–Al and Al–O bond vibrations. Sr–O anti-symmetric stretching vibrations are present in frequencies between 500 and 650 cm−1. Thus, the formation of the polyhedrons confirmed by the metal-oxide stretching frequencies indicates the formation of SrAl2O4 [27].
3.2 Spectroscopic analysis
The PL excitation and emission spectra of both SrAl2O4: Eu3+ and Na+ co-doped SrAl2O4: Eu3+ nanophosphors were studied. PL excitation spectra of the 3% Eu3+ doped SrAl2O4 and 3% Eu3+, 2% Na+ doped SrAl2O4 nanophosphors are presented in Fig. 4, which were recorded by monitoring the emission of Eu3+ at 616 nm.
As shown in Fig. 4, the excitation spectra have a broad peak at 285 nm and other narrow peaks were observed between 300 and 420 nm. In SrAl2O4, the 2p orbitals of O2− form the valence band and the conduction band is composed of 4d orbitals of Sr2+ [26, 30]. The broad peak at 285 nm corresponds to the charge transfer band (CTB) due to transfer of electrons from O2− (2p) orbital to the empty states of the 4f6 orbital of Eu3+ (Eu3+—O2−transition). The excitation spectra also contain some other transitions between 300 and 420 nm arising from f–f transitions of the Eu3+ ions in the host lattice. These peaks, resulting from the direct excitation of the Eu3+ ground state to the higher levels in the 4f6 configuration, can be assigned as 7F0 → 5D4 (361 nm), 7F0 → 5L7 (382 nm), 7F0 → 5L6 (394 nm), and 7F0 → 5D3 (413 nm) transitions. Among these sharp peaks, the 394 nm wavelength peak exhibits the highest peak intensity. From the excitation spectra, it is also prominent that due to Na+ co-doping the intensity of the excitation spectra increases, which in turn enhances the intensity of PL emission. The strong excitation peak at 394 nm would enable this nanophosphor to be potentially used in UV-LEDs.
Room temperature PL emission spectra of SrAl2O4:Eu3+ and Na+ co-doped SrAl2O4:Eu3+ nanophosphors, excited by 394 nm wavelength, are represented in Fig. 5a and b, respectively. In this configuration, the crystal field exerted by coordinating O2− splits the energy levels of Eu3+. This splitting leads to formation of sharp and intense emission lines between 580 and 710 nm for both Eu3+ doped and Eu3+ doped, Na+ co-doped samples. Four sharp peaks present in the emission spectra at 587, 616, 649 and 701 nm occur due to the transitions 5D0 → 7Fj, j = 1,2,3,4 of the Eu3+ ions, respectively. The peak at 587 nm (5D0 → 7F1) is due to magnetic dipole transitions while the peak at 616 nm (5D0 → 7F2) is due to electric dipole transitions [34]. For Eu3+ doped samples, quenching of PL intensity was observed after 3% Eu3+ doping. This can be ascribed to concentration quenching by interaction of the Eu3+ ions with each other [34]. As shown in Fig. 5a and b, for the Eu3+ doped samples, the sample with the highest PL intensity is SrAl2O4:3%Eu3+ and for Na+ co-doped SrAl2O4:Eu3+ phosphors, the sample with the highest PL intensity is SrAl2O4:3%Eu3+,2% Na+. These two samples are referred to optimized samples.
3.2.1 Photoluminescence mechanism
Figure 6 is a schematic representation of the energy levels of Eu3+ and possible mechanism for photoluminescence in the synthesized phosphor. In SrAl2O4, O2− ions form the valence band and Sr2+ ions form the conduction band [30]. A charge transfer band (CTB) is formed within the valence and conduction band due to electronic charge transfer from the 2p orbitals of O2− ions to 4f orbitals of Eu3+ ions. The excitation process consists of two main pathways, one is the direct transfer of valence band electrons to the CTB and another is transferring the electrons to higher energy levels of Eu3+. The first process takes place at 285 nm and the second one at 394 nm. In both processes, the excited electrons quickly jump to the lowest excited level (5D0) of Eu3+ via nonradiative transition, and then it makes 4 transitions to the lower energy levels. These four transitions give rise to four emission lines in the emission spectra at 587 nm, 616 nm, 649 nm, and 701 nm.
3.2.2 Effect of Na+ doping
Emission spectra for different concentrations of co-doped Na+ in SrAl2O4:3%Eu3+ is presented in Fig. 5b. There is no significant change in the position of the emission peak, but the emission intensity has increased significantly with increasing Na+ concentration from 1 to 2% and the intensity is greatly enhanced than that of 3% Eu3+ doped SrAl2O4 nanophosphor. As shown in Fig. 5b, the luminescence intensity increased gradually with the increase in Na+ concentration from 1 to 2% and then it decreased. From Fig. 5b, it can be seen that for optimum co-doped sample (2% Na+) the emission intensity obtained was more than four times than the highest emission of optimum Eu3+ doped sample.
This can be ascribed to several reasons. Charge compensation is most important among them. When Eu3+ is doped in SrAl2O4 matrix, it substitutes the Sr2+ and this creates an extra positive charge for each substitution. To maintain the overall electroneutrality of the crystal, incorporation of two Eu3+ ions leads to elimination of three Sr2+ ions and formation of one Sr2+ vacancy as shown by Kröger–Vink notation:
These cation vacancies reduce the luminescence intensity due to increase in non-radiative transition from Eu3+ luminescence centre to these Sr2+ vacancies. This problem can be effectively overcome by incorporating monovalent alkali metal ions by balancing the charge and number of sites simultaneously. Two Sr2+ sites are occupied by one Eu3+ and one Na+. This can be represented by following notation:
But volume imbalance is created due to dissimilar ionic radii of host (Sr2+) ion and Na+ ion. Volume imbalance problem is not very significant up to a certain doping concentration of Na+ ion. Beyond 2% Na+ lattice distortion takes place and subsequently the luminescence intensity decreases due to decrease in crystallinity. Another important parameter that affects emission intensity SrAl2O4:Eu3+, Na+ nanophosphors is the formation of oxygen vacancies which are created by the replacement of Sr2+ by Na+. Nature of the surrounding ions strongly regulate CT energy. As shown in the excitation spectra, broad band in the range of 250–310 nm has been observed due to the electron transfer from the filled 2p orbitals of the O2− ions to the empty 4f shell of the Eu3+ ions (charge transfer transition). The created oxygen vacancies act as the sensitizer for the Eu3+ ions and cause a very efficient energy transfer to Eu3+ ions. As a result, emission intensity of Eu3+ gets enhanced due to effective energy transfer from host to activator. However, the amount of oxygen vacancies in the crystal becomes significant enough after 2% Na+. As a result, the overall crystallinity decreases and the luminescence intensity decrease. The electric dipole transition at 616 nm maintains its dominance over 587 nm for both Eu3+ doped and Eu3+ doped Na+ co-doped sample because Sr2+ is located at a low symmetry site.
Figure 7 indicates the CIE plot of both SrAl2O4: 3%Eu3+ and SrAl2O4: 3%Eu3+, 2% Na+ for comparison. The co-ordinates for both cases are shown in Table 1.
An increase in x coordinate with simultaneous decrease in y coordinate in the chromaticity diagram confirms the improved redness with addition of Na+ ions. This improvement in redness makes it more suitable for its application in display and lighting purposes.
3.2.3 Decay study
The room temperature decay profile for the 616 nm emission of Eu3+ excited at 394 nm UV radiation was also studied as represented in Fig. 8.
The decay curves of the 5D0 → 7F2 transition for Eu3+ doped and Eu3+, Na+ co-doped nanophosphors were fitted into a bi-exponential and mono-exponential function, respectively. The bi-exponential decay equation (Eq. 3) and the monoexponential decay equation (Eq. 4) are given below
where I0 is the initial luminescence intensity, I(t) is the luminescence intensity at time t, τ1, and τ2 represent components of luminescence decay time, A1 and A2 are the weighting parameters. For mono-exponential function, τ is the luminescence decay time. Eq. 5 was used to determine the average decay time of bi-exponential decay.
The average decay time for 616 nm from 3% Eu3+ doped nanophosphor is found to be 396 μs and the decay time for 3% Eu3+, 2% Na+ co-doped nanophosphor is found to be 559 μs. For the Eu3+ doped samples, the biexponential PL decay characteristics in our system may be arising due to non-uniform distribution of Eu3+ in the SrAl2O4 matrix [36]. For co-doped samples, the single exponential decay behaviour indicates that the coordination environment of the Eu3+ ion in SrAl2O4 matrix is homogeneous [37]. Enhancement in decay time after Na+ codoping is an indication of reduction in the surface defects and nonradiative centers in the phosphor system [34]. A microsecond order decay time for all the samples confirms the suitability of the materials for utilizing in lighting applications.
4 Conclusions
In summary, a range of Eu3+ doped SrAl2O4 and Eu3+ doped, Na+ co-doped SrAl2O4 was successfully synthesized via facile sol–gel method. These nanophosphors have shown bright red emission under 394 nm excitation due to 5D0 → 7Fj (j = 1, 2, 3, 4) of Eu3+ substituted in the SrAl2O4 matrix. The intensity of red emission from Eu3+ doped SrAl2O4 was enhanced successfully via incorporation of Na+ ions as evident in the corresponding photoluminescence emission spectra. This enhancement is attributed to the charge compensation and increase in crystallinity through addition Na+ which subsequently reduces the amount of non-radiative centers in SrAl2O4 matrix. Photoluminescence decay study showed that the Na+ co-doped sample has higher decay time which also supports the reduction of nonradiative centers in these phosphors. The amount of Eu3+ and Na+ was optimized to be 3% and 2%, respectively (i.e. SrAl2O4:3%Eu3+ and SrAl2O4:3%Eu3+, 2%Na+). Finally, the addition of Na+ was found to be a successful method of improving the red emission of Eu3+ doped SrAl2O4 which can be possibly utilized in solid-state lighting applications.
References
M. Shang, C. Li, J. Lin, How to produce white light in a single-phase host? Chem. Soc. Rev. 43, 1372–1386 (2014)
Y. Zhang, Z. Wu, D. Geng et al., Full color emission in ZnGa2O4: simultaneous control of the spherical morphology, luminescent, and electric properties via hydrothermal approach. Adv. Funct. Mater. 24, 6581–6593 (2014)
X. Li, Y. Zhang, D. Geng, CaGdAlO4:Tb3+/Eu3+as promising phosphors for full-color field emission displays. J. Mater. Chem. C 2, 9924–9933 (2014)
K. Li, S. Liang, M. Shang et al., Photoluminescence and energy transfer properties with Y+SiO4 substituting Ba+PO4 in Ba3Y(PO4)3: Ce3+/Tb3+, Tb3+/Eu3+ phosphors for w-LEDs. Inorg. Chem. 55, 7593–7604 (2016)
K. Li, M. Shang, H. Lian et al., Recent development in phosphors with different emitting colors via energy transfer for FEDs and UV/n-UV w-LEDs. J. Mater. Chem. C 4, 5507–5530 (2016)
S. Liang, M. Shang, H. Lian et al., Deep red MGe4O9:Mn4+(M = Sr, Ba) phosphors: structure, luminescence properties and application in warm white light emitting diodes. J. Mater. Chem. C 4, 6409–6416 (2016)
B. Samanta, A.K. Dey, P. Bhaumik et al., Controllable white light generation from novel BaWO4:Yb3+/Ho3+/Tm3+ nanophosphor by modulating sensitizer ion concentration. J. Mater. Sci.: Mater. Electron. 30, 1068–1075 (2019)
C. Yang, X. Li, Q. Liu et al., Tunable white light emission of rare earth ions doped single matrix SrAl2Si2O8 phosphors. J. Mater. Sci.: Mater. Electron. 31, 1057–1064 (2020)
Y. Liu, G. Liu, J. Wang et al., Single-component and warm-white-emitting phosphor NaGd(WO4)2: Tm3+, Dy3+, Eu3+: synthesis, luminescence, energy transfer, and tunable color. Inorg. Chem. 53, 11457–11466 (2014)
N. Guo, Y. Huang, M. Yang et al., A tunable single-component warm white-light Sr3Y(PO4)3: Eu2+, Mn2+ phosphor for white-light emitting diodes. Phys. Chem. Chem. Phys. 13, 15077–15082 (2011)
A. Santra, K. Panigrahi, S. Saha et al., Enhancement of radiative transitions in Sm3+ activated CaTiO3 nanophosphor by modulating co-activator concentration. J Mater. Sci.: Mater. Electron. 30, 6311–6321 (2019)
S. Mondal, J. Sarkar, S. Panja et al., Tuning visible emission from red to yellow of PbWO4: Yb3+/Ho3+ nanophosphor by modulating activator ion concentration. J. Phy. Chem. Sol. 129, 442–447 (2019)
D. Geng, G. Li, M. Shang et al., Nanocrystalline CaYAlO4:Tb3+/Eu3+ as promising phosphors for full-color field emission displays. Dalton Trans. 41, 3078–3086 (2012)
A.K. Dey, B. Samanta, P. Bhaumik et al., Low-temperature synthesis of thermally stable BaWO4:Yb3+:Ho3+ nanophosphors: tuning visible emission by controlling activator ion concentration. J. Lumin. 211, 251–257 (2019)
B.K. Gupta, D. Haranath, S. Saini et al., Synthesis and characterization of ultra-fine Y2O3:Eu3+ nanophosphors for luminescent security ink applications. Nanotechnology 21, 55607 (2010)
R. Yadav, A.F. Khan, A. Yadav et al., Intense red-emitting Y4Al2O9:Eu3+phosphor with short decay time and high color purity for advanced plasma display panel. Opt. Express 17, 22023–22030 (2009)
M. Saraf, P. Kumar, G. Kedawat et al., Probing highly luminescent europium-doped lanthanum orthophosphate nanorods for strategic applications. Inorg. Chem. 54, 2616–2625 (2015)
A. Kumar, G. Kedawat, P. Kumar et al., Sunlight-activated Eu2+/Dy3+ doped SrAl2O4 water resistant phosphorescent layer for optical displays and defence applications. New J. Chem. 39, 3380–3387 (2015)
B. Shao, Y. Feng, M. Jiao et al., Two-step synthetic route to GdOF:Ln3+ nanocrystals with multicolor luminescence properties. Dalton Trans. 45, 2485–2491 (2016)
C. Li, P. Ma, P. Yang et al., Fine structural and morphological control of rare earth fluorides REF3 (RE=La–Lu, Y) nano/microcrystals: microwave-assisted ionic liquid synthesis, magnetic and luminescent properties. CrystEngComm 13, 1003–1013 (2011)
X.Y. Chen, C. Ma, X.X. Li et al., Novel necklace-like MAl2O4:Eu2+, Dy3+ (M = Sr, Ba, Ca) phosphors via a CTAB-assisted solution-phase synthesis and postannealing approach. J. Phys. Chem. C 113, 2685–2689 (2009)
R. Wiglusz, T. Grzyb, Sol−Gel synthesis of micro and nanocrystalline BaAl2O4:Eu3+ powders and their luminescence properties. Opt. Mater. 36, 539–545 (2013)
B. Qu, B. Zhang, L. Wang et al., Mechanistic study of the persistent luminescence of CaAl2O4:Eu. Nd. Chem. Mater. 27, 2195–2202 (2015)
K. Panigrahi, S. Saha, S. Sain et al., White light emitting MgAl2O4: Dy3+, Eu3+ nanophosphor for multifunctional applications. Dalton Trans. 47, 12228–12242 (2018)
T. Peng, L. Huajun, H. Yang et al., Synthesis of SrAl2O4:Eu, Dy phosphor nanometer powders by sol–gel processes and its optical properties. Mater. Chem. Phys. 85, 68–72 (2004)
F. Clabau, X. Rocquefelte, S. Jobic et al., Mechanism of phosphorescence appropriate for the long-lasting phosphors Eu2+-doped SrAl2O4 with codopants Dy3+and B3+. Chem. Mater. 17, 3904–3912 (2005)
D.P. Bisen, R. Sharma, Mechanoluminescence properties of SrAl2O4:Eu2+ phosphor by combustion synthesis. Luminescence 31, 394–400 (2016)
B.C. Jamalaiah, M. Jayasimhadri, Tunable luminescence properties of SrAl2O4: Eu3+ phosphors for LED applications. J. Mol. Struct. 1178, 394–400 (2018)
M. Nazarov, M.G. Brik, D. Spassky et al., Crystal field splitting of 5d states and luminescence mechanism in SrAl2O4:Eu2+ phosphor. J. Lumin. 182, 79–86 (2017)
J. Hölsä, T. Laamanen, M. Lastusaari et al., Electronic structure of the SrAl2O4:Eu2+ persistent luminescence material. J. Rare Earths 27, 550–554 (2009)
V. Vitola, D. Millers, K. Smits et al., The search for defects in undoped SrAl2O4 material. Opt. Mater. 87, 48–52 (2019)
H. Terraschke, M. Suta, M. Adlung et al., SrAl2O4:Eu2+(Dy3+) nanosized particles: synthesis and interpretation of temperature-dependent optical properties. J. Spec. (2015). https://doi.org/10.1155/2015/541958
S. Saha, S. Das, U.K. Ghorai et al., Charge compensation assisted enhanced photoluminescence derived from Li-codoped MgAl2O4:Eu3+ nanophosphors for solid state lighting applications. Dalton Trans. 42, 12965 (2013)
S. Saha, S. Das, U.K. Ghorai et al., Controlling nonradiative transition centers in Eu3+ activated CaSnO3 nanophosphors through Na+ co-doping: realization of ultrabright red emission along with higher thermal stability. J. Phys. Chem. C 119, 16824–16835 (2015)
R. Chatterjee, S. Saha, D. Sen et al., Neutralizing the charge imbalance problem in Eu3+-activated BaAl2O4 nanophosphors: theoretical insights and experimental validation considering K+ codoping. ACS Omega 3, 788–800 (2018)
S.K. Gupta, J.P. Zuniga, M. Abdou et al., Optical properties of undoped, Eu3+ doped and Li+ co-doped Y2Hf2O7 nanoparticles and polymer nanocomposite films. Inorg. Chem. Front. 7, 505–518 (2020)
M. Yu, J. Lin, Z. Wang et al., Fabrication, patterning, and optical properties of nanocrystalline YVO4: A (A = Eu3+, Dy3+, Sm3+, Er3+) phosphor films via sol-gel soft lithography. Chem. Mater. 14, 2224–2231 (2002)
Acknowledgements
UKG acknowledges the Teachers Associateship for Research Excellence (TARE) fellowship and research grant (TAR/2018/000763) of SERB, Govt. of India. UKG also acknowledges Science and Technology and Biotechnology Department, Govt. of West Bengal for proving the financial support [199 (Sanc.)/ST/P/S&T/6G-12/2018]. UKG also acknowledges the central DST-FIST programme (SR/FST/College-287/2015) and DBT star college scheme (BT/HRD/11/036/2019) for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De, A., Dey, A.K., Samanta, B. et al. Enhanced red photoluminescence in chain-like SrAl2O4:Eu3+ nanophosphors: utilizing charge compensation by modulating Na+ co-doping concentration. J Mater Sci: Mater Electron 32, 8648–8656 (2021). https://doi.org/10.1007/s10854-021-05524-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05524-2