Abstract
The current work presents the optical, photoluminescence and ferromagnetic properties of Zn1−xNixO samples with (0.00 ≤ x ≤ 0.20). The samples are well crystallizing in hexagonal wurtzite structure without a significant change in their lattice constants, while the crystallite size, grain size and number of unit cells in ZnO particle are increased. The residual stress is negative (compressive) and almost increases as Ni increases up to 0.10, but it is changed from negative to positive (tensile) at Ni = 0.20. The absorption and reflectance of UV spectra are increased as Ni increases up to 0.20. An absorption band centered at a wavelength of 552 nm is also observed and is gradually shifted by Ni towards longer wavelengths. Interestingly, two direct electronic transitions are found for all samples and two induced bandgaps which are decreased by Ni. While the other optical constants such as linear χ1 and non-linear third-order susceptibilities χ3, dispersion parameters (Ed, Eo), refractive indexes (no, n2), lattice dielectric constant εL, N/m*, optical and electrical conductivities (σopt, σele) are increased by Ni, the dissipation factor (tanδ) is decreased. Although some of the continuous peaks of lower PL intensities are recorded at UV band edges and slightly shifted to longer wave lengths (red shift) as Ni increases, strong UV emission peaks at about 390, 420 and 440 nm are recorded. Finally, the signature of a clear weak ferromagnetism ordering at room temperature, with evaluated magnetic parameters such as magnetization Ms, magnetic moments μ and magneto-crystalline anisotropy factor γ, is recorded for Ni ≥ 0.05 doped samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Many efforts have been made on improving electronic devices to be low cost and more compatible with the well-known current applications [1,2,3,4,5]. Hexagonal wurtzite n-type ZnO is well known for being a conductive oxide with lot of properties such as benefiting wide optical energy gap (3.37 eV), high Urbach energy (60–100 meV), high melting temperature, good chemical stability, inexpensive growth, earth abundance and non-toxicity. These evaluated properties are of interest to highlight ZnO either pure or with additives as an outstanding optoelectronic and optical devices through promising phosphor material for ultraviolet (UV) and photoluminescence emissions [6,7,8,9,10,11].
The charge carries such as electrons are excited from VB due to absorption of photons, and consequently the pairs of e–h are obtained [12,13,14]. The irradiative and Urbach tail processes have been found to be responsible for the chat of light energy above and below the limit of the ZnO gap, respectively. Anyhow, the binding energy of exciton (e–h pairs) of ZnO is given by the amount of photon energy against maximum absorbance or lower reflectance [15, 16].
On the other hand, the ZnO degree of quality through emission from the states of discrete and structural defects could be provided from photoluminescence (PL) [17]. It is usually characterized by green-yellow emission regime according to the vacancies of oxygen. The near band edge (NBE) due to blue emission of PL usually occurs as a result of recombination of e–h pairs [18,19,20,21], while the yellow-orange emission regime is due to interstitial-oxygen and hydroxide-surface states [22]. However, the deep level and oxygen vacancies, during sintering of ZnO sintering, are taken as inevitably feature of reaction method.
Several efforts have been carried out to describe the influence of additives, such as Bi2O3, Cr2O3, Al2O3, MnO, NiO, CoO and Fe2O3, on the properties of ZnO [23,24,25,26,27,28,29,30,31,32]. It is approved that the d and f states of impurities can fill the odd states of ZnO, and consequently a clear ferromagnetism at RT (RTFM) along with evaluated magnetic moment may be obtained [33,34,35,36,37]. This behavior originates also from the interaction between the delocalized free carriers of ZnO with the d-spin localized states of dopants [38,39,40,41,42,43,44,45].
However, structural, mechanical and electrical properties of ZnO varistors doped by Ni have been investigated [46]. It is approved that the potential barrier of ZnO is deformed by 0.025 of Ni and, consequently, the non-linearity disappears. It is also believed that RTFM and valence state produced by Ni are responsible for that barrier deformation. Though mostly Fe, Co and Mn ion-doped ZnO has been studied, only limited studies have been reported on Ni. Although the limit (0.05–0.10) of 3D transition metals is well chosen to avoid their solubility within ZnO, Ni is expected to have a larger solubility due to the relatively smaller Ni ion as compared to Zn at the same tetrahedral fold coordination [10].
In this study, the samples are synthesized by conventional solid-state reaction method due to its simplicity and it is a low-cost method. The structural, optical, photoluminescence and ferromagnetic properties are investigated as a function of the Ni doping concentration. It is shown that addition of Ni up to 0.20 to ZnO reduces the energy loss rate of the oscillation mode of ZnO which support the present samples for the high-frequency applications required for high quality of capacitors. Further, a decrease of bandgaps is obtained by Ni and, consequently, the optical and electrical conductivities are improved. Moreover, the signature of a clear ferromagnetic with an increase for saturated magnetization and effective magnetic moment is obtained. Therefore, addition of Ni to ZnO materials makes them more suitable for optoelectronic applications which highlight the present investigation.
2 Experimental details
Zn1−xNixO samples with x = 0.00, 2.5%, 5%, 10% and 20% are synthesized by using conventional solid-state reaction method. The powders of ZnO and NiO (Aldrich 99.999 purity) are thoroughly mixed and calcined for 12 h in air at 1000 °C, pressed into pellets, sintered at 1000 °C for 10 h, and then quenched to RT. The purity of the phase and structural morphology are tested by XRD and SEM techniques. The reflectance and absorption spectra against wavelength are recorded by Jasco V-570 (Japan) double-beam spectrophotometer. However, PL spectra are obtained using a JASCO PF-6300 WTE spectrofluorometer with S.N. C033560902, 220–750 nm wave length with standard PM tube, light source Xe lamp, Ac220V and class I protect. Finally, the magnetization curves at RT and up to 18,000 G are obtained by using vibrating sample magnetometer (VSM).
3 Results and discussion
3.1 Structural analysis
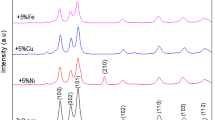
The structural investigation of the samples is carried out using the XRD pattern shown in Fig. 1. As seen in the figure, the common diffraction peaks belong to wurtzite structure of pure and Ni-doped ZnO are clearly observed. The well-known peaks (101), (210) and (220) of little intensities recorded at Ni ≥ 0.05 are belonging to ZnO wurtzite structure. It is noted that the peaks (100), (002) and (111) formed with relatively more increased intensities for Ni samples up to 0.20 as compared to pure ZnO. This indicates that Ni is well substituted for Zn2+ in the unit cell due to the matching of ionic radii of Ni2+ (0.69 Å) as compared to Zn2+ (0.74 Å) at the same tetrahedral fold coordination. The values of lattice parameters a and c listed in Table 1 are slightly changed with Ni and they are consistent with those ideal values of ZnO (a = 3.250 Å, c = 5.206 Å) [47,48,49]. The values of U-parameters, \(U = 0.333\left( \frac{a}{c} \right)^{2} + 0.25\), listed in Table 1, are nearly constant (U = 0.379) as reported [50, 51]. The Zn–O bond length (L), \(L = \left[ {\left( {\frac{{a^{2} }}{3}} \right) + (0.5 - U)^{2} c^{2} } \right]^{\frac{1}{2}}\)[52] are between 1.991 and 1.981 Å for all samples indicating that no more electrons contributed in the bond formation [53,54,55,56].
The crystallite size Dhkl is given by [15, 57, 58]
K = 0.9 for ZnO, λ = 1.5418 Å and β is the half-maximum line width. The average values of D for the most intense diffraction peaks (D100), (D002) and (D111), listed in Table 1, are increased by Ni up to 0.20. They are 28.32, 41.77, 36.30, 38.58 and 37.1 nm for all samples. Although the values of D are not systematic against Ni content, it is generally increased as Ni increases. The inverse behavior is obtained for the dislocation density calculated by, δ = 1/D2 and listed in Table 1 [59, 60]. This behavior indicated that Ni helps the doped ZnO samples to have good crystalline qualities and to be free from the lattice defects [16, 61]. The number of unit cells existent in each particle of ZnO is given by [62, 63]
where Vun and Vuc are the volumes of ZnO as a spheres and ZnO hexagonal unit cell, respectively. The values of n listed in Table 1 are increased by Ni as well as Dhkl behavior (n α D). For more illustration of the effect of Ni on the structure defects of ZnO, the residual stress \(\sigma\) is given by [17, 64, 65]
where = \({S}_{13}\) = 104.2 GPa, \({S}_{33}\) = 213.8 GPa, \({S}_{11}\)= 208.8 GPa, \({S}_{12}\)= 119.7 GPa are the elastic stiffness constants of hexagonal Zn. ε is the lattice strain along c-axis and can be calculated by \(\varepsilon = \frac{{c - c_{ \circ } }}{{c_{ \circ } }}\), where co = 5.2066 Å for the unstrained ZnO hexagonal structure [66, 67]. However, similar values of ε and \(\sigma\) listed in Table 1 indicated that ε is positive and almost decreases as Ni increases up to 0.10, but it is changed from positive to negative at Ni = 0.20. An inverse behavior is obtained for\(\sigma\), which indicated compressive stress for the samples up to Ni = 0.10, but it is changed to tensile stress for Ni = 0.20. Consequently, 0.20 of Ni to ZnO may eliminate the compressive stress and turn it to tensile behavior [68].
The structural morphology shown in the SEM micrographs of Fig. 2 does not show any second phases formed by Ni at the grain boundaries, but the grains are randomly distributed and composed over the matrix structure which makes them higher conductive as compared to ZnO. The average grain size (D) is given by \(D = \frac{1.56\ell }{{MN}}\), M is the magnification, \(\ell\) is the arbitrary length, M is the graph magnification of graph, and N is the counted grain boundaries [69]. The average grain sizes listed in Table 1 are 2.06, 3.09, 2.59, 2.27 and 2.46 μm, respectively, for all pure and doped samples. This means that D is generally increased by Ni, which is in agreement with the behavior of crystallite size Dhkl deduced from XRD analysis.
3.2 Optical constants
Here the optical constants of the investigated samples are determined using the absorbance and reflectance data shown in Fig. 3. As shown in the figure, the absorbance as well as reflectance decreases with increasing the wavelength and become nearly constant at longer wavelengths. The effect of the Ni content on both the absorbance and reflectance is clearly seen where they increase with increasing the concentration of Ni. One can observe the improvement in the optical absorption of ZnO samples by the doping with Ni ions. This result states that Ni doping plays an important role in optimizing the optical properties of ZnO samples for optoelectronic applications based on the materials of high absorption as well as good conductivity. Furthermore, an absorption band is observed in the optical data which is centered at a wavelength of 552 nm (see Fig. 3). In addition, it is observed that the center of the band is shifted towards longer wavelengths with increasing Ni content. This band could be attributed to some structural defects which are formed as result of the substitution of Zn ions by Ni ones. On the other hand, the absorption coefficient \(\alpha\) of the pure and Ni-doped ZnO samples is calculated using the following equations:
where A is the absorbance. The absorption coefficient is among the important optical parameters which is used here in the optical analysis to determine the type of electronic transition and the value bandgap of the ZnO samples as well. This can be achieved by using the following relation [70, 71]:
where G is constant and \({E}_{g}\) is the optical bandgap. From the results, we found that r = 0.54 indicating a direct transition for the considered samples. Therefore, Eq. 5 can be rewritten as
Figure 4 shows \({(\alpha h\nu )}^{2}\) against \(h\nu\) of the pure and Ni-doped ZnO samples. One can see that there are two absorption edges, which confirms that the studied samples possess two electronic transition mechanisms. The intercept of the straight lines in Fig. 4 and inset figure with x axis at \({(\alpha h\nu )}^{2}=0\) gives the value of the direct bandgap (Eg1 and Eg2) for the two transitions of the studied samples (see Table 2) [72]. As shown in Table 2, the bandgaps Eg1 and Eg2 decrease from 3.31 to 2.91 and 2.84 to 2.65 eV, respectively, indicating the great effect of the Ni content on the band structure of ZnO. Such trend of bandgap narrowing is also observed for Co-doped ZnO thin films [73]. This behavior is explained in terms of the proposed model which is used for interpreting the winding and shrinkage of the dopants, and consequently the energy gap is determined by [74, 75]
where \({E}_{g0}\) is the energy gap between the upper limit of VB to the lower limit of CB, while \({E}_{wg}\) is the gap widening defined by the shift of Burstein Moss [76]. This shift is due to that the lowest states of conduction band are populated and this causes a blocking of the conduction band minimum. This phenomenon usually appears for the degenerate semiconductors [77]. The third term \({E}_{Ng}\) in Eq. 7 is the decrease in the gap as a result of Coulomb forces between the electrons in CB and electron impurity scattering [78]. In our present work, the ZnO samples could not degenerate semiconductors and thus the term \({E}_{wg}\) is negligible compared to \({E}_{Ng}\). In this case, increasing the Ni doping concentration will result in an increase of \({E}_{Ng}\) , thereby causing a reduction in the optical bandgap of Ni-doped ZnO samples. Another reason for the reduction of Eg is the formation Sp–3d hybridization by the 3d levels of the Ni2+ ions which are located below the conduction band. Increasing the Ni doping concentration usually decreases the strength of hybridization between Sp and 3d, and therefore the gap may also decrease [79]. On the other hand, the two bandgaps observed for the investigated ZnO samples could be attributed to two electronic transition mechanisms. The first is due to transition from the upper of VB to the upper of CB and the other from VB to the bottom of CB.
In addition to the above-determined optical constants, the refractive index n is also computed for the investigated samples using the following formula [80]:
One can observe in Fig. 5 that n increases by the increase of Ni and λ. This could be caused by increasing the density of states as result of increasing the number of Ni ions in ZnO samples. In addition, a peak is a formed around 528 nm and its maximum is shifted to higher wavelengths with increasing the Ni content.
Determining the refractive index in the present work enabled us to get many optical parameters which are important from the point view of the optoelectronic applications. Among these parameters are the optical \({\sigma }_{\text{opt}}\) and electrical \({\sigma }_{\text{elec}}\) conductivity which are obtained using the relations [80]:
where c is speed of the light and \({\varepsilon }_{0}\) free space permittivity. As indicated in Fig. 6, \({\sigma }_{\text{opt}}\) and \({\sigma }_{\text{elec}}\) generally increase as Ni increases as a result of increasing the free carriers in the host lattice of ZnO.
Fabrication of the capacitors requires materials with specific characteristics. The dissipation factor tan (δ) is among the physical quantities which determine the degree of the capacitors quality using the real and imaginary parts of dielectric constant, \({\varepsilon }_{r} \left({\varepsilon }_{r}={n}^{2}-{k}^{2}\right)\), \({\varepsilon }_{i} \left({\varepsilon }_{i}=2nk\right)\) [81]:
Figure 7 shows the dissipation factor tanδ versus hν and in which tan(δ) decreases with increasing the Ni content and photon energy as well. This refers to that the Ni doping reduces the energy loss rate of the oscillation mode of ZnO samples. It is known that the quality of the capacitor depends on tan(δ). It is inversely proportional to the dissipation factor. Hence, the studied Ni-doped ZnO materials can be potential materials for the fabrication of high-frequency applications which require high-quality capacitors.
Additionally, the real and imaginary parts are used to determine the volume energy (VELF) and surface energy loss functions (SELF) which represent the electrons energy loss as they move through the bulk or surface of the materials, respectively [82]:
The parameters VELF and SELF are plotted versus the photon energy in Fig. 8. It is found that VELF and SELF decrease with increasing the photon energy and Ni as well. It is clear that the doping of ZnO by Ni2+ ions reduce the energy loss of electrons as they travel through the bulk or surface of the ZnO samples. This could be interesting property from the point of view of the practical applications of ZnO.
The refractive index values are analyzed using different models to determine other parameters such as the high-frequency dielectric constant \({\varepsilon }_{L}\) and the dispersion parameters (dispersion Ed and single oscillator energy E0). This was carried out using the following equations [82]:
where e is the electronic charge and N/m* is the ratio of carrier number to the effective mass of the electron. Figure 9a and b represents the plot of n2 versus the wavelength \(\lambda\) and (n2 − 1)−1 versus \((h\nu )^{2}\). The appearance of the straight lines in Fig. 9 confirms the linear relationship of both Eqs. 12a and b. Furthermore, the linear fit procedure of the data in the figure enabled obtaining the values of \({\varepsilon }_{L}\), E0, Ed and N/m* which are listed in Table 2. The first observation in the table is that N/m* is continuously increasing with the Ni content, indicating that the introduction of Ni2+ ions into the ZnO samples produce more free carriers thereby improving the optical and electrical conductivity as mentioned above. Furthermore, the dispersion energy and high-frequency dielectric constant increase but the oscillator energy is reduced with the doping concentration. This reveals the effect of the incorporation of Ni2+ ions on the dispersion parameters which is a good indication of controlling the optical parameters of ZnO by the doping and this is very important for the fabrication of the optical devices. In addition, the non-linear optical parameters for the optoelectronic show that refractive index depends on light intensity which propagates in the material. In this case, the refractive index is called non-refractive index and is given by [83]
where χ(3) is third-order non-linear susceptibility and no is static dielectric constant which are computed by
where χ(1) is the linear susceptibility which is dependent on the dispersion parameters E0 and Ed:
By combining the above three equations, we obtain the relation which is used to calculate the values of third-order non-linear susceptibility:
B is constant and it is equal to 1.7 × 10–10. The values of the optical parameters, no, n2, χ(1) and χ(3) are shown in Table 3. It is clear that the Ni content has a positive effect on the non-linear parameters where no, n2, χ(1) and χ(3) are increased with increasing the number of Ni ions in ZnO lattice. This is another interesting result which confirms the controlling as well as improvement of the optical properties of ZnO by Ni doping and this enables the possibility of the development of ZnO to be promising materials for the optoelectronic applications.
3.3 Photoluminescence (PL)
The characteristic of UV band edges against the optimum value of PL intensity for pure and Ni-doped ZnO is shown in Fig. 10. However, a lot of continuous peaks are recorded in which the wave number is slightly shifted by Ni to higher values (lower energy against each peak), and the intensity is increased. Interestingly, some of strong UV common emissions listed in Table 4 are recorded at about 390, 420 and 440 nm, in consistent with the reported for ultraviolet energy gap of ZnO ~ (3–3.2) eV [84]. This of course is another clear evidence for decreasing the bandgap energy of ZnO by Ni at these most intensive peaks due to their shift to higher wave lengths as indicated above. These original emissions are, respectively, due to e–h or exciton recombination below CB to VB and transition of electron from the level of shallow donor of Zn ion to the upper limit of VB at about 340 meV below CB [85,86,87]. It has been demonstrated that the free carrier has an effect on the ionization state of the oxygen vacancy, which can strongly impact the green emission intensity [88, 89]. Therefore, the increase of PL intensity by Ni is due to increasing the electron free carries in conduction band with doping of donor impurities such as Ni. This was supported by increasing (N/m*) as listed in Table 2. The alternative reason may be related to the higher rate of donor-bound excitonic recombination due to Ni doping. Therefore, the number of absorbed photons will be increased, and consequently the intensity was increased. While the other extra of emissions between (438–525 nm) and (2.6–2.35 eV) are described by radiative transitions and also acceptor–donor recombination to oxygen antisites [90,91,92], the emissions due to host ZnO phosphors above 600 nm and also below 380 nm are not observed for the present samples, which indicated that the samples are free from defects [93, 94]. Anyhow, a green emission is commonly observed in ZnO prepared in oxygen-deficient environments resulting in vacancy of oxygen. They attributed it to donor Vo–acceptor VZn recombination. Recently, photoluminescence studies on sol–gel-derived ZnO: Gd and ZnO nanorods doped by Al have been investigated. They have shown multi-peaks of emission lines in blue, green and red regions (390–650 nm) as well the present case [95]. They also found a difference in the peak position and the nature of the PL spectra between these lines. They attributed these missions to crystallization conditions and intrinsic defects such as oxygen vacancies VO, zinc vacancies VZn, oxygen interstitials Oi, zinc interstitials Zni, oxygen antisites OZn and acceptor level of VZn located above VB [86,87,88,89,90, 95].
3.4 RTFM measurements
Room temperature magnetization versus magnetic field (M–H) of the samples is shown in Fig. 11. It is apparent that the pure and 0.025 of Ni samples do not exhibit a clear ferromagnetic as the reported for ZnO diamagnetic behavior [95]. With increasing Ni above 0.025, a clear ferromagnetic behavior with evaluated magnetization is observed and gradually increased by Ni up to 0.20. The saturated magnetization Ms listed in Table 5 is increased from 0.026 (emu/g) for pure ZnO to 0.027, 0.044, 0.062 and 0.081 (emu/g) for Ni samples. Similar behavior is obtained for Mr, ∆M and M–H slope, but Hc is reduced as listed in Table 5. This is due to the effective magnetic moment of Ni (μ = 2.83 μB), when Zn2+ is replaced by Ni2+ in either tetrahedral or octahedral coordination. This is also supported by antiferromagnetic behavior recorded for NiO synthesis at higher temperatures and superparamagnetism/spin-glass-like behavior at low temperatures. The magnetic moment μ and magnetic anisotropy factor γ are calculated by \(\mu (\mu_{B} ) = \frac{{WM_{s} }}{5585}\), \(\gamma = \frac{{H_{c} M_{s} }}{0.98}\), respectively (W is the atomic weight) [96, 97]. It is clear that μ is increased by Ni from 0.0004 to 0.0012 μB and γ from 0.171 to 0.265 (emu G/g), supporting RTFM for Ni-doped samples. However, it is reported that Ni-doped ZnO within the soluble limit exhibits room temperature weak ferromagnetism due to formation of bound magnetic polarons within the localized spins of the Ni ions through interaction with the charge carriers such as oxygen vacancies. This is of course resulting in a magnetic polarization of the surrounding local magnetic moments of ZnO by Ni [98,99,100]. Therefore, the observed weak ferromagnetism at RT seems to be the combined effect of the interaction of Ni2+ spin at the tetrahedral sites with charge carriers such as oxygen vacancies in forming bound magnetic polarons [10, 101].
4 Conclusion
Optical, photoluminescence and ferromagnetic properties of pure and Ni-doped ZnO are well investigated. Although the wurtzite structure is approved for all samples, the crystallite size, grain size and number of unit cells in ZnO particle are increased by Ni. The residual stress is compressive as Ni increases up to 0.10, but it is changed to tensile at Ni = 0.20. An absorption band centered at a wavelength of 552 nm is observed for all samples and is gradually shifted towards longer wavelengths (red shift) as Ni increases. Interestingly, two bandgaps could be obtained for all samples and their values are gradually decreased as Ni increases. Furthermore, most of the obtained optical constants are increased by Ni, whereas dissipation factor is reduced. Moreover, strong UV emission peaks recorded at about 390, 420 and 440 nm for all samples. Finally, the signature of a clear weak ferromagnetism with evaluated magnetic parameters could be recorded for Ni ≥ 0.05 doped samples. The present study approved that addition of Ni to ZnO not only showed an interesting feature, but it also has a positive impact on the properties of ZnO compound that makes it highly suitable for optoelectronic applications.
References
J. Jose, M. Abdul Khaddar, Mater. Sci. Eng. A 304–306, 810 (2001)
D.C. Look, Mater. Sci. Eng. B 80, 383 (2001)
L. Gao, Q. Li, W. Luan, H. Kawaoka, T. Sekino, K. Niihara, J. Am. Ceram. Soc. 85(4), 1016 (2002)
D.R. Clarke, J. Am. Ceram. Soc. 82(3), 485 (1999)
K. Mukae, K. Tsuda, I. Nagasawa, Jpn. J. Appl. Phys. 16(8), 1361 (1977)
G.E. Pike, C.H. Seager, J. Appl. Phys. 50(5), 3414 (1979)
F. Oba, Y. Sato, T. Yamamoto, Y. Ikuhara, T. Sakuma, J. Am. Ceram. Soc. 86(9), 1 (2003)
Z. Zhen, K. Kato, T. Komaki, M. Yoshino, H. Yukawa, M. Morinaga, K. Morita, J. Eur. Ceram. Soc. 24, 139 (2004)
M. Norouzi, M. Kolahdouz, P. Ebrahimi, M. Ganjian, R. Soleimanzadeh, K. Narimani, H. Radamson, Thin Solid Films 619, 41–47 (2016)
A. Samanta, M.N. Goswami, P.K. Mahapatra, J. Alloys Compd. 730, 399 (2018)
R. Chauhan, A. Kumar, R. Chaudhary, J. Optoelectron. Biomed. Mater. 3(1), 17 (2011)
J. Jadhav, M. Patange, S. Biswas, Carbon Sci. Tech. 5(2), 269 (2013)
H.M. Ali, A.M.A. Hakeem, Eur. Phys. J. Appl. Phys. 72, 10301 (2015)
A.A. Othman, M.A. Othman, E.M.M. Ibrahim, M.A. Ali, Ceram. Int. 43, 527 (2017)
S.A. Amin, A. Sedky, Mater. Res. Express 6, 065903 (2019)
A. Sedky, A.M. Ali, M. Mohamed, Opt. Quantum Electron. 52, 42 (2020)
A. Sedky, S.A. Amin, M. Mohamed, Appl. Phys. A 125, 308 (2019)
K. Ozawa, K. Mase, Physica Status Solidi (A) 207, 277–281 (2010)
J.-C. Deinert, D. Wegkamp, M. Meyer, C. Richter, M. Wolf, J. Stahler, Phys. Rev. Lett. 113, 057602 (2014)
V.P. Zhukov, P.M. Echenique, E.V. Chulkov, Phys. Rev. B 82, 094302 (2010)
L. Jihui, H. Qiang, L. Changsheng, Y. Jinghai, L. Xue, Y. Lili, W. Dandan, Z. Hongju, G. Ming, Z. Yongjun, L. Xiaoyan, W. Maobin, Appl. Surf. Sci. 256, 3365 (2010)
J. Kaur, R.K. Kotnala, V. Gupta, K.C. Verma, Curr. Appl. Phys. 14(5), 637 (2014)
K. Eda, IEEE Electr. Insul. Mag. 5, 28 (1989)
J. Han, P.Q. Mantas, A.M.R. Senos, J. Eur. Ceram. Soc. 22, 49 (2002)
D.C. Look, J.W. Hemsky, J.R. Sizelove, Phys. Rev. Lett. 82, 2552 (1999)
W.G. Carlson, T.K. Gupta, J. Appl. Phys. 53, 5746 (1982)
A. Sedky, M. Abu-Abdeen, A.-M. Abdel-Aziz, Phys. B 388, 266 (2007)
A. Sedky, S. Ayman, Y. Amal, Phys. B 404, 3519 (2009)
A. Sedky, E. El-Suheel, Phys. Res. Int. 2010, 1 (2010)
A.B. Glot, J. Mater. Sci. 17, 755 (2006)
A.M.R. Senos, M.R. Santos, A.P. Moreira, J.M. Vieira, Surface and interfaces of ceramic materials, in NATO ASI Series. ed. by L.C. Dufour, C.C. Monty, G. Petot-Ervas (Kluwer Academic, London, 1988)
A.M.R. Senos, J.M. Vieira, Proceedings of the international Conference Third Euro-Ceramics, in Duran P. ed. by J.F. Fernandez (Faenza Edit Rice Iberica Faenza, London, 1993)
F.K. Shan, Y.S. Yu, J. Eur. Ceram. Soc. 24, 1869 (2004)
J.P. Joshi, R. Gupta, A.K. Sood, S.V. Bhat, A.R. Raju, C.N.R. Rao, Phys. Rev. B 65, 024410 (2001)
S.S. Dhar, O. Brandt, M. Ramsteiner, V.F. Sapega, K.H. Ploog, Phys. Rev. Lett. 94, 037305 (2005)
J. Hite, G.T. Thaler, R. Khanna, C.R. Abernathy, S.J. Pearton, J.H. Park, A.J. Steckl, J.M. Zavada, Appl. Phys. Lett. 89, 132119 (2006)
Y.K. Zhou, S.W. Choi, S. Kimura, S. Emura, S. Hasegawa, Supercond. Nov. Magn. 20, 429 (2007)
X.L. Wang, C.Y. Luan, Q. Shao, A. Pruna, C.W. Leung, R. Lortz, J.A. Zapien, A. Ruotolo, Appl. Phys. Lett. 102, 102112 (2013)
K.R. Kittilstved, N.S. Norberg, D.R. Gamelin, Phys. Rev. Lett. 94, 147209 (2005)
T. Dietl, Semicond. Sci. Technol. 17, 377 (2002)
P.V. Radovanovic, D.R. Gamelin, Phys. Rev. Lett. 91, 157202 (2003)
A. Sedkyand, Kh.A. Ziq, Mech. Magn. Prop. 52, 99 (2012)
S. Ramachandran, J. Narayan, J.T. Prater, Appl. Phys. Lett. 88, 242503 (2006)
L.R. Salh, H. Zhu, W.G. Wang, B. Ali, T. Zhu, X. Fan, Y.Q. Song, Q.Y. Wen, H.W. Zhang, S.L. Saleh, Physica D 43(3), 35002 (2010)
P. Sharma, A. Gupta, K.V. Rao, F.J. Owens, R. Sharma, R. Ahuja, J.M.O. Guillen, B. Johansson, G.A. Gehring, Nat. Mater. 2(10), 673 (2003)
A. Sedky, E. El-Suheel, Chin. Phys. B 21(11), 116103 (2012)
G. Pei, C. Xia, S. Cao, J. Zhang, F. Wu, J. Xu, J. Magn. Mater. 302(2), 340 (2006)
G. Pei, C. Xia, S. Cao, J. Zhang, Wu. Feng, Xu. Jun, JMMM 302(2), 340 (2006)
A. Sedky, Braz. J. Phys. 44(4), 305 (2014)
Ü. Özgür, A. Ya, I. Alivov, C. Liu, A. Teke, M.A. Reshchikov, S. Doğan, C.V. Avrutin, S.J. Cho, J. Appl. Phys. 98, 041301 (2005)
A. Sedky, S.B. Mohamed, Mater. Sci. 32(1), 16 (2014)
T. Yao, S.-K. Hong, Oxide and Nitride Semiconductors (Springer, Berlin, 2009).
T. Hanad, http://www.Springer.com/978-3-540-88846-8(2009).
S. Aksoy, Y. Caglar, S. Ilican, M. Caglar, Chem. Eng. Civ. Eng. Mech. Eng. 227, 1–8 (2010)
U. Seetawan, S. Jugsujinda, T. Seetawan, A. Ratchasin, C. Euvananont, C. Junin, C. Thanachayanont, P. Chainaronk, Mater. Sci. Appl. 2, 1302 (2011)
E. Muchuweni, T.S. Sathiaraj, H. Nyakotyo, Heliyon 3, e00285 (2017)
A. Sedky, Adv. Mater. Sci. Eng. 2, 1 (2018)
F.K. Shan, Z.F. Liu, G.X. Liu, W.J. Lee, G.H. Lee, I.S. Kim, J. Electroceram. 13, 195 (2004)
X.S. Wang, Z.C. Wu, J.F. Webb, Z.G. Liu, Appl. Phys. A 77, 561 (2003)
X. Li, X. Cao, Xu. Liang, L. Liu, Y. Wang, J. Alloys Compd 675, 90 (2016)
A. Sedky, M. Abu-Abdeen, A. Abdul-Aziz, A. Almulhem, Phys. B 388, 266 (2007)
C.M. Jay, M. Sathya, K. Pushpanathan, Acta Metall. Sin. (Engl. Lett.) 28, 394 (2015)
H.S. Wasly, J. Al-Azhar Univ. Eng. Sector 13(49), 1312 (2018)
M. Chaari, A. Matoussi, Z. Fakhfakh, Mater. Sci. Appl. 2, 765 (2011)
T.P. Rao, M.C.S. Kumar, A. Safarullaa, V. Ganesan, S.R. Barman, C. Sanjeeviraja, Phys. B 405(9), 2226 (2010)
H.C. Ong, A.X.E. Zhu, G.T. Du, Appl. Phys. Lett. 80, 941 (2002)
C. Wang, P. Zhang, J. Yue, Y. Zhang, L. Zheng, Phys. B 403, 2235 (2008)
D.I. Rusu, G.G. Rusu, D. Luca, Acta Phys. Pol. A 119(6), 850 (2011)
J.C. Wurst, J.A. Nelson, J. Am. Ceram. Soc. 55, 109 (1972)
D. Bhattacharyya, S. Chaudhuri, A.K. Pal, S.K. Bhattacharyya, Vacuum 43, 1201 (1992)
J. Tauc, R. Grigorovici, A. Vancu, Phys. Status Solidi 15, 2 (1966)
A. Sedky, A.M. Ali, M. Mohamed, Opt. Quantum Electron. 52, 62–78 (2020)
R. Kumar, F. Singh, B. Angadi, J.-W. Choi, W.-K. Choi, K. Jeong, J.-H. Song, M.W. Khan, J.P. Srivastava, A. Kumar, R.P. Tandon, J. Appl. Phys. 100, 113708 (2006)
E. Burstein, Phys. Rev. 93, 632 (1954)
V.P. Gupta, N.M. Ravindra, Phys. Status Solidi 100, 715 (1980)
A. Walsh, J.L.F. Da Silva, S.H. Wei, Phys. Rev. B 78, 1 (2008)
M.Y. Ali, M.K.R. Khan, A.M.M.T. Karim, M.M. Rahman, M. Kamruzzaman, Heliyon 6, e03588 (2020)
N.A. Subrahamanyam, A Text Book of Optics (BRJ Laboratoray, Delhi, 1977).
T.C.S. Girisun, S. Dhanuskodi, Cryst. Res. Technol. 44, 1297 (2009)
M. Mohamed, A.M. Abdelraheem, M.I. Abd-Elrahman, N.M.A. Hadia, E.R. Shaaban, Appl. Phys. A 125, 483 (2019)
S.H. Wemple, Phys. Rev. B 7, 3767 (1973)
M.A. Ordal, R.J. Bell, R.W. Alexander, L.L. Long, M.R. Querry, Appl. Opt. 24, 4493 (1985)
L. Tichý, H. Tichá, P. Nagels, R. Callaerts, R. Mertens, M. Vlček, Mater. Lett. 39, 122 (1999)
Y.S. Wang, P.J. Thomas, P. O’Brien, J. Phys. Chem. B 110(43), 21412 (2006)
K. Vanheusden, W.L. Warren, C.H. Seager, D.R. Tallant, J.A. Voigt, B.E. Gnade, J. Appl. Phys. 79, 7983–7985 (1996)
X.M. Fan, J.S. Lian, L. Zhao, Y. Liu, Appl. Surf. Sci. 252, 420–424 (2005)
T. Tatsumi, M. Fujita, N. Kawamoto, M. Sasajima, Y. Horikoshi, Jpn. J. Appl. Phys. 43, 2602–2606 (2004)
K. Vanheusden, C.H. Seager, W.L. Warren, D.R. Tallant, J.A. Voigt, Appl. Phys. Lett. 68, 403 (1996). https://doi.org/10.1063/1.116699
W. Shan, W. Walukiewicz, J.W. Ager, K.M. Yu, H.B. Yuan, H.P. Xin, G. Cantwell, J.J. Song, Appl. Phys. Lett. 86, 191911 (2005). https://doi.org/10.1063/1.1923757
Y.W. Heo, D.P. Norton, S.J. Pearton, J. Appl. Phys. 98, 073502 (2005)
T.E. Murphy, K. Moazzami, J.D. Phillips, J. Electron. Mater. 35, 543–549 (2006)
B. Lin, Z. Fu, Y. Jia, Appl. Phys. Lett. 79, 943–945 (2001)
K. Potzger, S. Zhou, F. Eichhorn, M. Helm, W. Skorupa, A. Mcklich, J. Jassbender, T. Herrmannsdorfer, A. Bianchi, Appl. Phys. Lett. 99(1–5), 063906 (2006)
S. Rani, B. Lal, S. Saxena, S. Shukla, J. Sol-Gel Sci. Tech. 81, 586–592 (2017)
G. KrishnaReddy, A. JagannathaReddy, R. HariKrishna, B.M. Nagabhushana, G. RamGopal, J. Asian Ceram. Soc. 5, 350–356 (2017)
Q. Xu, S. Zhou, H. Schmidt, J. Alloys Compd. 487, 665–667 (2009)
E.E. Ateia, L.M. Salah, A.A.H. El-Bassuony, Inorg. Organomet. Polym. Mater. 25, 1362 (2015)
A.A.H. El-Bassuony, H.K. Abdelsalam, J. Supercond. Nov. Magn. (2017). https://doi.org/10.1007/s10948-017-4340-x
G. Srinet, R. Kumar, V. Sajal, J. Appl. Phys. 114, 033912 (2013)
J.M.D. Coey, K. Wongsaprom, J. Alaria, M. Venkatesan, J. Phys. D 41, 134012 (2008)
J.M.D. Coey, S.A. Chambers, MRS Bull. 33, 1053 (2008)
Acknowledgements
This research has been funded by Research Deanship in University of Ha’il-Saudi Arabia through Project Number RG-20133. The authors thank Scientific Research Deanship at University of Ha’il-Saudi Arabia for funding this research project
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, M., Sedky, A., Alshammari, A.S. et al. Optical, photoluminescence and ferromagnetic properties of Ni-doped ZnO for optoelectronic applications. J Mater Sci: Mater Electron 32, 5186–5198 (2021). https://doi.org/10.1007/s10854-021-05250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05250-9