Abstract
In the present work, imidazolinium L-tartrate (IMLT) crystals and L-proline-doped IMLT crystals were grown by slow cooling solution growth technique. The powder X-ray diffraction technique reveals the lattice parameters and strains developed due to the doping of L-proline in 1, 3 and 5 mol concentrations. The transmittance spectrum unveils the contribution of the dopant atoms on the optical property of the grown crystals. Bandgap and cut-off wavelength are affected by the increase in the dopant concentration. The vibrational frequencies around 3132, 2925 and 1438 cm−1 are due to the O–H stretching in the carboxyl group, C–H stretching vibration of carboxyl group and aromatic ring vibrations, respectively, present in the grown crystals are observed in the FTIR spectroscopic analysis. The dielectric constant and dielectric loss of pure and L-proline-doped IMLT crystals with respect to the frequency of the applied electric field have been investigated by the dielectric measurements at room temperature. The surface morphology of the grown crystals was examined by the etching study which reveals the growth mechanism of pure and L-proline-doped IMLT crystals. Etch pits in rectangular pattern were found to appear. The percentages of carbon, hydrogen and nitrogen elements present in pure and L-proline-doped IMLT crystals were determined using CHN analysis. The third-order nonlinear susceptibility was calculated from the nonlinear absorption coefficient and nonlinear refractive index data obtained from the Z-scan analysis. The dopant, L-proline, has altered the properties of IMLT single crystal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Device-grade single crystals with extremely high transparency in the ultraviolet to near IR spectral range are in the forefront of contemporary research communities, due to their potential applications in the domain of scientific and industrial purposes. Organic compounds have unique physical and chemical properties due to their specific structural features. The weak covalent bonds and π-bond system offer more alluring platform for nonlinear optical (NLO) applications. The asymmetric electronic distribution leads to large mobility of electron density resulting in enhanced optical polarization response. The overlap of π-orbitals and the functional groups such as donor and acceptor groups influences the optical nonlinearity of the organic molecules, when compared to their inorganic counterparts. The inclusion of donor and acceptor groups in organic compounds through hydrogen bonds overcomes the tendency of dipoles to form centrosymmetric structures [1]. The strong delocalization of π electrons which are adequately packed in the organic compounds augments the molecular hyper polarizability that emphasizes the nonlinear behavior of the organic crystals. Their efficient inter-molecular charge transfer property induces large dipole moment which is an essential attribute for optimizing the second and third harmonic generation in the crystals. The most essential property expected from quadratic NLO materials is structure flexibility favoring device fabrication.
Imidazole (C3H4N2) an organic compound with amphoteric behavior is added with L-tartaric acid (C4H6O6), which is an organic resilient diprotic chiral α-hydroxyl acid. Imidazole combines with L-tartaric acid to form an imidazolinium L-tartrate (IMLT) molecular compound. The structure of the imidazolinium L-tartrate is decided by infinite chains of hydrogen L-tartrate anions connected using hydrogen bond in a head to tail fashion [2]. The properties of the IMLT single crystal are modified by adding a suitable dopant. Among the organic compounds, amino acids have phenomenal features, e.g., zwitter ionic nature, weak van der Waals force, enhanced dipole moment, that can amend the characteristic properties of the title compound. Amino acids have asymmetric carbon atom functionalized at both ends with proton donor and acceptor group that can readily promote electron charge transfer such that the nonlinear optical property can be enhanced.
L-proline (C5H9NO2) is a polar amino acid which is also called imino acid. L-proline is abundantly available in biological systems, as its amine group is a part of the pyrrolidine ring which is an exception from other amino acids. This compound has a rotationally constrained rigid ring structure by means of the three-carbon R-group of proline that is fused to the α-nitrogen group. L-proline cadmium chloride monohydrate, L-proline zinc chloride, L-prolinium picrate, L-prolinium phosphite, L-prolinium tartrate and L-proline lithium chloride monohydrate are the series of L-proline-based organic and semiorganic compounds that have shown excellent SHG efficiency [3, 4].
In this work, L-proline was incorporated into the IMLT crystals and their properties were analyzed using various characterization techniques. The inclusion of polar amino acids in noncentrosymmetric crystal overcomes its shortcomings, by which the doped crystal surpasses that of the parent crystal. Also, the addition of small ground-state dipole molecules induces a wide transparency range and lowers the absorption cut-off wavelength in the obtained crystals [5]. In this report, we determine the effects of doping L-proline in 1 mol%, 3 mol%, and 5 mol% concentration in the host IMLT crystal and its properties have been analyzed.
2 Experimental technique
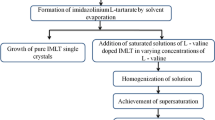
Imidazolinium L-tartrate single crystals were grown by slow cooling solution growth technique. Equimolar ratio of imidazole and L-tartaric acid was taken and dissolved in de-ionized water. The solution was stirred well for 6 h to get a homogeneous saturated solution. Later, the prepared solution was filtered using a Whatman filter paper to get rid of the impurities and dust particles. The filtered solution was kept in a perforated beaker at room temperature for slow evaporation of the solvent to obtain the seed crystals. Then the tiny transparent seed crystals were obtained in a period of 4 days. The tiny flawless seed crystals with perfect morphology were harvested and bulk crystals were grown from the seed crystals adopting slow cooling method.
The saturated mother solution was prepared at 40 °C in a temperature-controlled magnetic stirrer with an accuracy of ± 0.01 °C. Then the prepared homogeneous saturated solution placed inside in a perforated beaker was maintained at 40 °C for two days in a constant temperature bath, whose temperature can be controlled with an accuracy of ± 0.01 °C for bulk crystal growth. Then, a tiny, flawless seed crystal was introduced into the mother solution which is kept in the constant temperature bath. The temperature of the bath was reduced at the rate of 1 °C/day. 1 Transparent good quality large-size single crystals of pure IMLT were grown in a period of 20 days. L-proline was added as an additive in 1, 3 and 5 mol% concentrations to the pure IMLT solution. The above-mentioned same procedure was repeated to grow all the L-proline-doped IMLT crystals reported in this paper. The photographs of the grown crystals are shown in Fig. 1.
3 Results and discussion
3.1 Powder X-ray diffraction analysis
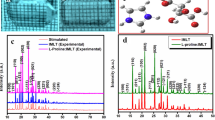
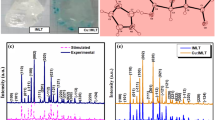
The powder X-ray diffraction (PXRD) of pure and L-proline-doped IMLT was measured using a Bruker D8 Advance powder X-ray diffractometer (45 kV, 30 mA) with CuKα radiation with the wavelength of 1.5406 Å in the angular range (2θ) 10–50° being employed to collect the data of the crystal system and lattice parameters which are tabulated in Table 1. The recorded X-ray diffraction patterns are shown in Fig. 2. The sharp intense peak indicates the good crystallinity of the grown crystals. All the grown crystals belong to the monoclinic crystal system. The obtained results in powder X-ray diffraction pattern imply that their space group P21 fits in the noncentrosymmetric crystal system which is an elementary requirement for SHG materials. The P21 space group possesses few advantages, i.e., they permit maximum contribution to the molecular nonlinearity [6]. The diffraction peak with low FWHM indicates that the crystal has a good crystalline perfection. The lattice parameters and cell volume have been found to increase, which implies that the dopant atoms have successfully incorporated into the IMLT crystals. However, in the present investigation, the diffraction curve does not contain any additional peak which indicates the absence of clustering of L-proline at macroscopic levels. In the diffraction pattern, the intensity of certain peaks is found to increase, whereas the other peaks are found to decrease due to the incorporation of the dopant atoms while increasing the concentration by forming more number of hydrogen bonds. The dopant concentration is not systematically distributed as mentioned in the UV studies, which has introduced a strain in the IMLT crystals. The strain can be tensile strain due to the vacancy type of defects [7]. The increase in the dopant concentration has produced defects and structural grain boundaries in the grown crystals. The prominent peaks with maximum intensity are (011), (102), (012), (020), (201), (121), (12 \( \overline{2} \)) and the peak with minimum intensity is (002); their 2θ values are noted and strain calculations were performed with respect to the data obtained using the Debye–Scherrer relation.
Crystallite Size, D
Strain, ε
Dislocation density, δ
where λ indicates the wavelength of the X-ray beam used, β denotes the full-width half maximum (in radians), K denotes the Scherrer constant K = 0.9, and θ indicates the Bragg’s diffraction angle. The values obtained are furnished in Table 2. From Table 2 it is explored that the minimum percentage of the dopant, i.e., 1 mol%, has large crystallite size and lower strain value compared to the pure IMLT crystal which has led to minimum number of defects and reduced scattering centers. The quality of the crystal is explored by the dislocation density. The optimization and control of defects is an ultimate issue from the application point of view. Therefore, 1 mol% L-proline-doped IMLT is the optimized concentration as per UV spectroscopic analysis and also in powder X-ray diffraction analysis. As mentioned in the UV spectroscopy analysis, the impurity concentration is lower in the case of 1 mol% LP-doped IMLT, and hence the strain has been minimized and the crystallite size has increased. The enhancement of second harmonic generation effect requires favorable alignment of molecules within the crystal to facilitate the nonlinear optical efficiency. The higher amount of dopant concentration leads to a greater number of grain boundaries, as the dopant atom tends to segregate at the boundaries of the crystal [8]. Therefore, this may inhibit the SHG efficiency of the 3 and 5 mol% LP-doped IMLT. In Fig. 3, the powder XRD patter of 1 mol% L-proline-doped IMLT crystal is presented. The intensity of the (011) peak has the maximum intensity which is due to the rapid growth of (011) plane compared to other crystallographic planes. This explains that the morphology of the crystal is not affected due to doping, but only the intensity of the peaks is affected [9].
3.2 UV spectroscopic analysis
Crystals should have excellent transparency window for NLO applications such as frequency doubling and optical switching. Its transparency must be extending from UV to near IR region, i.e., wider transparency window provides them better practical applicability. The optical spectrum of pure and L-proline-doped IMLT crystals was recorded using Varian Cary 5000 UV–Vis–NIR absorption spectrometer. Crystals of about 2 mm thickness were used to examine their optical property. The transmission spectrum of the grown crystals is shown in Fig. 4, which evidences that the dopant has contributed to an increase in the transmittance of the pure IMLT crystals due to prominent n to π transitions of electrons in –σ, –π, –n orbitals in nitrogen group of L-proline [10]. The absorption peak at 217 nm is assigned to π–π*, and 320 nm is assigned to n–π* transitions. The transition of electrons from orbitals suggests the existence of bandgap. The bandgap of the grown crystals was calculated using the following relation:
where T is the transmittance (%), t is the thickness of the sample, h is the Planck’s constant, ν is the frequency, α is the absorption coefficient ‘hν’ is the energy of the photon, A is a constant, Eg is the optical bandgap that is to be calculated for the grown crystals, and n represents the characteristic transition. The characteristic transition ‘n’ value is 2 for the pure IMLT single crystal; therefore, this implies that the grown pure crystals undergo indirect allowed transition, as mentioned in the reported data [11]. The optical bandgap of the grown crystals is indirect bandgap in nature; substituting the value of ‘n’ in Eq. (1), the following relation has been obtained:
Rearranging the above equation, we get
The optical bandgap values of the grown crystals were calculated using the above relation and Tauc’s plot \({\left(\alpha h\nu \right)}^\frac{1}{2} vs h\nu \) was constructed to obtain the optical bandgap. Tauc’s plots are shown in Fig. 5. The obtained data are presented in Table 3. The variation in the bandgap energy is connected with the molecular arrangement, i.e., atomic and electronic structures [12]. It is found that the bandgap which has been calculated is high for 1 mol% LP IMLT, whereas it is low for pure IMLT. This implies that the dopant amino acid L-proline has entered into the matrix of the host compound [13]. In the transmission spectrum, it is observed that there is a sharp narrow decrease in the spectrum at 1435–1445 cm−1 which is due to the C–H vibration modes of imidazole present in the title compound. The 1 mol% LP-doped IMLT has the maximum transparency; this is due to the reason that L-proline at 1 mol% concentration improves the structure perfection of the IMLT and also noted that the cut-off wavelength of the 1 mol% LP-doped IMLT is minimum (Table 3). At lower concentration of the dopant, the rate of absorption of L-proline by IMLT crystal is more, whereas at higher concentrations more amount of impurities may be absorbed at the surface, by which the rate of deposition of immobile impurities becomes high, which diminishes the transmittance of the crystals [13]. At higher concentration, the rate of deposition of growth entities may be low by which the transparency of 1 mol% L-proline IMLT has increased. This factor confirms that the grown 1 mol% LP-doped IMLT crystal is suitable for effective laser frequency conversion applications. The higher bandgap is because of the presence of dopant [14]. The number of hydrogen bonding has increased and their spatial location in the host matrix plays a vital role in controlling the optical properties of the crystals. The dopants are not systematically distributed in the host matrix which may lead to defects in the highly concentrated L-proline-doped IMLT. Therefore, a decrease in the transmittance upon increasing the dopant concentration is observed.
3.3 Dielectric measurements
The dielectric response of the grown crystals is obtained by exposing the material to an applied electric field, so that the negative charges align opposite to the field, whereas the positive charges align in the direction of the applied field. The degree of polarization measures the dielectric constant of the material. The grown crystals were cut and polished with about 2 mm thickness and with a surface area of about 3 mm2. The crystals were coated with silver paste on either side to make uniform electrical contact with the electrodes, like a parallel plate capacitor. The dielectric measurements of the grown crystals were made using Hioki 3532–50 LCR Hitester for different frequencies from 50 Hz to 5 MHz and are shown in Figs. 6 and 7. The dielectric constant is measured using the relation:
where C is the capacitance, ε0 is the permittivity of free space, d is the thickness of the grown crystal, and A is the surface area of the crystal.
The Pockel’s effect emphasizes that the change in the dielectric constant is a linear function of the electric field. Operation of the electro-optic devices is based on the Pockel’s effect. Dielectric materials exhibiting Pockel’s effect can be used as a transducer to transfer the surface charge density to retard the light beam transmitted. There is a need for the low dielectric constant material in microelectronic industries in multilevel interconnect structures as an interlayer dielectrics (ILD). The low dielectric constant values lower the power consumption and reduce the cross-talk between the nearby interconnects. From Fig. 6, it is observed that at lower frequencies, the charges can easily shift their alignment in phase with respect to the applied electric field; hence all types of polarization arise, but when the frequency increases, each polarization vanishes progressively except electronic polarization. Therefore, there is a decrease in the dielectric constant at higher frequencies. When the concentrations of the dopant molecules are low, a smaller number of hydrogen bonds are introduced in the crystal; therefore, there is a dipole interaction between the molecules, whereas, when the dopant concentration is increased, the additional hydrogen bonds obstruct the movement of the dipoles. [15]. Figure 6 implies that the 1 mol% LP-doped IMLT has lower dielectric constant and hence it is suited for optical switching device application enabling faster switching speeds. This also implies that the maximum limit that the dopant L-proline can be incorporated for successful device-related applications is limited to 1 mol%.
Dielectric loss (tan δ) has been calculated using the following relation:
where
D is the dissipation factor; the dielectric loss at various frequencies is shown in Fig. 7. From Fig. 7, it is observed that the low dielectric loss of 1 mol% L-proline-doped IMLT implies that, at lower concentration of the dopant, there is low power dissipation which is attributed to lesser number of defects and cracks in the grown crystals.
3.4 FTIR analysis
Table 4 lists the wavenumbers of the bands with respect to their intensities and their estimated functional groups present in the compound. They were analyzed by FTIR spectral analysis in the range of 400–4000 cm−1 by KBr pellet technique, using IR tracer-100 (Shimadzu). The hydrogen bond vibrations and their internal vibrations contribute to the bands observed and are shown in Fig. 8 [16]. At lower wavenumber in the spectrum, the lattice vibrations and internal deformation motion of the parent molecules contribute to the peaks observed. The peak at 3170–3180 cm−1 in 1, 3 and 5 mol% LP-doped IMLT is observed due to the NH3+ asymmetric stretching of L-proline. The band at 1580–1770 cm−1 is due to C=N stretching vibration of the imidazole ring. The band at 1240–1280 cm−1 is due to the breathing mode of the imidazole ring in plane C–H deformation of the parent compound. C–C stretching of imidazole vibration appears at 1120–1160 cm−1. The intense signal at 1040–1090 cm−1 is due to the CO–OH stretching vibrations of carboxylate groups.
3.5 Z-scan method
Z-scan analysis is a widely used method in material characterization due to their high sensitivity and due to the availability of well-explained and elaborated theory calculations and ease in analyzing the third-order susceptibility of the crystals. The requirement for optical limiting devices has grown rapidly owing to the pervasive usage of optical detectors and sensors for scientific and industrial purposes [17]. The optical detectors and sensors shield the photosensitive modules from intense laser optical radiation. These nonlinear materials could be used for the protection of sensitive human eye from the laser effects, as their permissible exposure is minimum up to 2.5 mWcm−2 in the visible spectrum.
The Z-scan technique can be used as a convenient tool to determine the magnitude and sign of nonlinear refractive index and nonlinear absorption coefficient of the crystals which was introduced by Sheik Bahae et al. [17, 18]. In this method, the third-order nonlinear properties of the grown crystals were analyzed using intense laser beam. The beam from a continuous-wave 532-nm diode-pumped Nd: YAG Gaussian laser beam is focused through a convex lens on the optical material to be analyzed using this method. The thickness of the sample or the optical material should be less than the Rayleigh length (ZR), ZR > L.
where ω0 represents the beam waist diameter (2ω0) and λ is the wavelength of the laser beam. The incoming laser beam alters the absorption property of the sample, i.e., the optical material; therefore, the resulting transmitted intensity is greatly minimized by which the nonlinear property such as third-order susceptibility can be calculated. This can be used to analyze the suitability of the grown crystals in optical limiting applications [19].
The optically focused Gaussian laser beam is passed on to the sample and the sample is translated along the positive and negative z-axis by the control of the stepper motor through focal point of the lens to vary the intensity of the laser beam that is incident on the sample [20]. The recorded normalized transmittance of the sample in open and closed apertures is shown in Fig. 9. The corresponding transmitted intensity from the closed and open aperture method is extracted and the data are analyzed.
3.6 Closed aperture method
While placing the sample (crystal) in the path of a laser beam, the behavior of the laser gets altered since there is a variation in the refractive index of the passage medium. The sample can have self-focusing or self-defocussing behavior depending upon the property of the sample. The self-focusing behavior of the sample is characterized by positive refractive index of the sample (\({n}_{2}>0)\) , i.e., the corresponding transmitted intensity has prefocal transmitted valley followed by post-focal transmitted peak. The self-defocussing behavior of the sample is characterized by prefocal transmitted peak followed by post-focal transmitted valley indicating the negative refractive index of the sample \({(n}_{2}<0)\). The grown crystals were subjected to closed aperture Z-scan method and the obtained data are shown in Fig. 9a. The estimated data by closed aperture method imply that the grown crystal has self-defocussing behavior as it has transmitted peak followed by valley, which is due to the local variation of the refractive index with temperature [21]. The self-defocussing nature of the sample is an essential property in the production of optical sensor devices. This induces the material to have large laser damage threshold value as it has defocussing nature.
\(\Delta \varphi \) is the on-axis phase shift at the focus which is calculated using the relation:
where \({\Delta T}_{p-v}\) is the difference between transmitted peak and transmitted valley and S represents the linear transmittance aperture.
\( S = 1 - \exp \left( {\frac{{ - 2r_{a}^{2} }}{{\omega _{a} }}} \right) \), and then the third-order nonlinear refractive index (n2) is related to the on-axis phase shift by using the following relation:
where \( k = \frac{{2\pi }}{\lambda } \), I0 is the intensity of the laser beam at the focus (z = 0) and \( L_{{{\text{eff}}}} = \frac{{1 - \exp ( - \alpha L)}}{\alpha } \) is the effective thickness of the sample. The nonlinear refractive index values for the grown crystals were calculated and are furnished in Table 5.
3.7 Open aperture method
The open aperture Z-scan data provide the nonlinear absorption coefficient (β)
The value of β can be positive or negative depending on the behavior of the sample with the laser beam. The positive value of nonlinear absorption coefficient (β) is obtained for the sample with reverse saturable absorption curve in the open aperture normalized transmittance curve and the negative value is obtained for the sample with saturable absorption behavior [22]. The obtained data are shown in Fig. 9b which confirms the saturable absorption behavior of the sample; therefore, the sample has negative nonlinear absorption coefficient (β).
The determination of nonlinear absorption coefficient (β) is less accurate in closed aperture method [23], since the transmittance is affected by the nonlinear refractive index and saturation absorption coefficient (β). Therefore, to detach the transmitted nonlinear refractive index from nonlinear absorption coefficient the corresponding transmitted values from closed aperture scan are divided by open aperture scan and the data are shown in Fig. 9c
The real and imaginary parts of the third-order nonlinear susceptibility were calculated using the following relations:
where \({\varepsilon }_{0}\) is the vacuum permittivity\((8.8518\times {10}^{-12}\left( \frac{F}{\text{m}} \right))\), C is the velocity of light (3 × 108 m/s), n0 is the linear refractive index of the sample and λ is the wavelength of the laser beam (532 nm).
The third-order nonlinear susceptibility of the grown crystals was calculated by using the following relation and is tabulated in Table 5.
1 mol% L-proline-doped IMLT has the highest nonlinear optical susceptibility, which implies enhanced hydrogen bonding by significant distribution of the dopant atoms and delocalization of \(\pi \)-electron cloud resulting in the increase in dipolar moment. The self-defocussing behavior of the pure IMLT and L-Proline-doped IMLT suggests its suitability for sensor devices and optical limiting since it has large laser damage threshold value.
3.8 Etching studies
The etching study is a widely used powerful tool to analyze the crystalline perfection and to examine the growth features of the crystals. It reveals the quality of the crystals. Structural defects and crystal perfection can also be revealed by using etching studies [24]. It is an eminent tool to analyze the quality of the crystal. The growth features of the crystals were analyzed using an optical microscope in the reflection mode by which the morphology of the etch pits was studied. Crystals with 2 mm thickness were used for analysis, with de-ionized water being used as an etchant. The grown crystals were immersed in the etchant for about 10 s and then dried by using the tissue paper to investigate the growth pattern using optical microscope. The growth patterns, i.e., the growth features of the crystal samples, were analyzed and are shown in Fig. 10. In the present work, the morphology of the etch pits was identified to be rectangular in nature, which are visible by etching the surface. The etch pits are produced due to the inclusion of the dopant at the dislocation lines. Therefore, a greater number of etch pits are produced as a result of strain [25] and due to the mismatch in the normal lattice arrangement in 3 and 5 mol% LP-doped IMLT. Hence, more number of etch pits are developed surrounding the dislocation line in highly doped crystals in order to relax the strain developed.
3.9 CHN analysis
The presence of carbon, hydrogen and nitrogen elements in the pure IMLT and L-proline-doped IMLT was analyzed using the instrument Elementar Vario EL III CHNS analyzer. The CHN analysis confirms the chemical composition of carbon, hydrogen and nitrogen elements in pure IMLT and L-proline-doped IMLT crystals [26]. The obtained results of the analysis are presented in Table 6 in which the experimental values are compared with the theoretical values. Table 6 confirms the formation of IMLT and LP-doped IMLT. The experimental and theoretical values of carbon, hydrogen and nitrogen elements agree with each other. The composition of carbon, hydrogen and nitrogen elements are increasing with the increase in the dopant concentration. This clarifies the incorporation of L-proline in the IMLT crystals [27].
3.10 TG/DTA analysis
The thermal stability of the grown crystals was analyzed using thermogravimetric and differential thermal analysis (TG–DTA). The TG–DTA curves were recorded using Perkin-Elmer Diamond thermal analyzer. The experiment was carried out in the temperature range between 30 and 400 °C in nitrogen atmosphere with the gas flow rate of 20 ml/min and is shown in Fig. 11a, b for pure IMLT and L-proline-doped IMLT. It is observed that there is no remarkable weight loss till 206 °C which implies that there is no inclusion of water molecules in the lattice of the grown crystals. The TG curve reveals that the thermal stability of the L-proline-doped IMLT is slightly higher than pure IMLT since the decomposition of L-proline-doped IMLT is at 209 °C and pure IMLT is 208 °C. The two main endothermic peaks (206 and 222 °C) of L-proline-doped IMLT are also observed at higher temperature than pure IMLT. Therefore, the early decomposition of pure IMLT implies that the pure crystal is less stable at higher temperature than L-proline-doped IMLT. Hence, L-proline-doped IMLT crystal can be utilized for NLO applications up to 208 °C.
3.11 Vicker’s microhardness analysis
Hardness measurement for the grown crystals was made using Vicker’s microhardness tester with a diamond indenter. The Vicker’s hardness number (Hv) was calculated using the relation:
where d is the length of the indentation mark on the grown crystal in millimeter. Figure 12a shows the graph of load versus hardness value (Hv). From Fig. 12 a, it was observed that the Vicker’s hardness number was found to increase with the applied load. The graph between log p versus log d provides the work hardening coefficient ‘n’ which is calculated for L-proline-doped and pure IMLT crystal by using Meyer’s relation as 3.2, 3.7, 3.6, 2.9 shown in Fig. 12b. The work hardening coefficients are greater than 1.6 and it shows that the grown crystals belong to a soft material category. Elastic stiffness, C11, explains the tightness of the bonding of an atom with the neighboring atoms, which is derived from the hardness value (HV) at different loads using Wooster’s empirical relation. L-proline-doped IMLT crystals have large value of elastic stiffness compared to that of the pure and all the other L-proline-doped KHP crystals and are shown in Fig. 12a and b. These results indicate that the L-proline-doped IMLT single crystals have moderate hardness useful for device applications.
4 Conclusions
The slow cooling method was adopted for the growth of pure and LP-doped IMLT single crystals. The incorporation of the dopant (L-proline) was confirmed from the variations in the cell parameters determined using powder X-ray analysis. The development of lattice strain due to the high concentration of the dopant has been studied using equations available in the literature. The property of the grown crystals can be controlled and optimized by limiting the dopant concentration into the host crystal. The transmittance of the grown crystals was found to decrease due to the increase in the dopant concentration. The introduction of a greater number of dopant atoms develop scattering centers by which the transmittance gets decreased. 1 mol% LP-doped IMLT was found to be the optimized concentration that enables an increase in the optical quality of the IMLT crystals. The hydrogen bond vibrations and their internal vibrations contribute to the bands observed in FTIR analysis. The dopant atoms form more number of hydrogen bonding that reduces the motion of protons and therefore the interaction of the dipoles gets inhibited. This leads to an increase in the dielectric constant value while increasing the dopant concentration. The etch pattern of the grown crystals was analyzed and rectangular etch pits were observed. The nonlinear absorption coefficient and nonlinear refractive index were analyzed and the third-order susceptibility was calculated to be maximum for 1 mol% LP-doped IMLT crystals. The crystals undergo self-defocussing behavior which is a desired property to possess large laser damage threshold. The self-defocussing behavior of the L-Proline-doped IMLT suggests its suitability for sensor devices and optical limiting since it has large laser damage threshold value. The compositions of carbon, hydrogen and nitrogen were found to increase upon increasing the dopant concentration, which implies that the dopant has got incorporated in the host compound.
References
Z. Kotler, R. Hierle, D. Josse, J. Zyss, R. Masse, JOSA B. 9(4), 534–547 (1992). https://doi.org/10.1364/JOSAB.9.000534
C.B. Aakeröy, P.B. Hitchcock, Acta Crystallogr. Sect. C. 50(5), 759–761 (1994). https://doi.org/10.1107/S0108270193014258
M. Shakir, S.K. Kushwaha, K.K. Maurya, R.C. Bhatt, M.A. Wahab, G. Bhagavannarayana, Mater. Chem. Phys. 120(2–3), 566–570 (2010). https://doi.org/10.1016/j.matchemphys.2009.12.008
C. Serbutoviez, J.F. Nicoud, J. Fischer, I. Ledoux, J. Zyss, Chem. Mater. 6(8), 1358–1368 (1994). https://doi.org/10.1021/cm00044a039
R.N. Shaikh, M. Anis, G. Rabbani, M.D. Shirsat, S.S. Hussaini, Adv. Mater. Rapid Commun. 10, 526–531 (2016)
C. Ji, T. Chen, Z. Sun, Y. Ge, W. Lin, J. Luo, Q. Shi, M. Hong, CrystEngComm 15(11), 2157–2162 (2013). https://doi.org/10.1039/C3CE26942F
M.A. Rajkumar, S.S. Xavier, S. Anbarasu, P.A. Devarajan, Opt. Mater. 55, 153–159 (2016). https://doi.org/10.1016/j.optmat.2016.03.022
I.M. Pritula, E.I. Kostenyukova, O.N. Bezkrovnaya, M.I. Kolybaeva, D.S. Sofronov, E.F. Dolzhenkova, A. Kanaev, V. Tsurikov, Opt Mater. 57, 217–224 (2016). https://doi.org/10.1016/j.optmat.2016.04.044
A. Jayarama, S.M. Dharmaprakash, Appl. Surf. Sci. 253(2), 944–949 (2006). https://doi.org/10.1016/j.apsusc.2006.01.051
A. Mohd, G.G. Muley, M.D. Shirsat, S.S. Hussaini, Mater. Res. Innov. 19(5), 338–344 (2015). https://doi.org/10.1179/1433075X15Y.0000000002
A.M. Petrosyan, R.P. Sukiasyan, H.A. Karapetyan, S.S. Terzyan, R.S. Feigelson, J. Cryst. Growth. 213(1–2), 103–111 (2000). https://doi.org/10.1016/S0022-0248(00)00011-7
K. Thukral, N. Vijayan, D. Haranath, K.K. Maurya, J. Philip, V. Jayaramakrishnan, J. Chem. (2015). https://doi.org/10.1016/j.arabjc.2015.08.022
G. Bhagavannarayana, S. Parthiban, S. Meenakshisundaram, Cryst. Growth Des. 8(2), 446–451 (2007). https://doi.org/10.1021/cg0702129
P. Dhivya, R.A. Kumar, T. Theivasanthi, G. Vinitha, M.D. Kannan, J. Electron. Mater. 48, 1–11 (2019). https://doi.org/10.1007/s11664-019-07218-2
M. Meena, C.K. Mahadevan, Cryst. Res. Technol. 43(2), 166–172 (2008). https://doi.org/10.1002/crat.200711064
J. Baran, A.J. Barnes, H. Ratajczak, J. Mol. Struct. 1009, 55–68 (2012). https://doi.org/10.1016/j.molstruc.2011.09.016
T. Thilak, M. Basheer Ahamed, G. Vinitha, Optik-Int. J. Light Electron Opt. 124(21), 4716–4720 (2013). https://doi.org/10.1016/j.ijleo.2013.01.111
M. Sheik-Bahae, D.C. Hutchings, D.J. Hagan, E.W. Van Stryland, Opt. Lett. 14(17), 955–957 (1989). https://doi.org/10.1364/OL.14.000955
M. Sheikbahae, A.A. Said, T. Wei, D.J. Hagan, IEEE J. Quantum Elect. 26(4), 760–769 (1990). https://doi.org/10.1109/3.53394
T.S. Girisun, S. Dhanuskodi, G. Vinitha, Mater. Chem. Phys. 129(1–2), 9–14 (2011). https://doi.org/10.1016/j.matchemphys.2011.04.013
K. Senthil, S. Kalainathan, A.R. Kumar, P.G. Aravindan, RSC Adv. 4(99), 56112–56127 (2014). https://doi.org/10.1039/C4RA09112D
M. Saravanan, T.S. Girisun, G. Vinitha, S.V. Rao, RSC Adv. 6(94), 91083–91092 (2016). https://doi.org/10.1039/C6RA21428B
P.V. Dhanaraj, N.P. Rajesh, J. Kalyana Sundar, S. Natarajan, G. Vinitha, Mater. Chem. Phys. 129(1–2), 457–463 (2011). https://doi.org/10.1016/j.matchemphys.2011.04.041
R.P. Jebin, T. Suthan, N.P. Rajesh, G. Vinitha, S.A. Britto Dhas, Opt. Mater. 57, 163–168 (2016). https://doi.org/10.1016/j.optmat.2016.04.030
S. Redrothu Hanumantharao, G. Kalainathan, U.M. Bhagavannarayana, Spectrochim. Acta A 103, 388–399 (2013). https://doi.org/10.1016/j.saa.2012.10.044
T. Wang, L. Cao, D. Zhong, J. Liu, F. Teng, S. Ji, S. Sun, J. Tang, B.G. Teng, CrystEngComm 21(17), 2754–2761 (2019). https://doi.org/10.1039/C9CE00150F
S. Vasuki, R.T. Karunakaran, G. Shanmugam, J. Mater. Sci. 28(17), 12916–12928 (2017). https://doi.org/10.1007/s10854-017-7122-0
Acknowledgements
One of the authors (P. Dhivya) is thankful to the TEQIP-III, PSG College of Technology (TEQIP/No A/17 dated 25.11.2017) for the financial assistance to carry out this work. The corresponding author (Dr. R. Arun Kumar) is thankful to the University Grants Commission—South Eastern Regional Office, Hyderabad, India, for awarding the Minor research project (No.F. MRP-6721/16 (SERO UGC) Link 6721, dated June 30, 2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhivya, P., Kumar, R.A. & Vinitha, G. Investigations on the properties of L-proline doped imidazolinium L-tartrate (IMLT) single crystals. J Mater Sci: Mater Electron 32, 3673–3687 (2021). https://doi.org/10.1007/s10854-020-05113-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-05113-9