Abstract
The organic material 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline was synthesised and the single crystal has been grown by slow evaporation technique. Single-crystal X-ray diffraction studies show that the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal belongs to the monoclinic system with the centrosymmetric space group P21/n. The FTIR and FT-Raman spectra were recorded to identify the functional groups present in the grown crystal. The optical property of the grown crystal was analyzed by UV–Vis–NIR studies and determines the optical band gap, the refractive index, the optical conductivity and the Urbach energy. The photoluminescence (PL) studies show that the grown crystal has green colour emission. The thermal property of the grown crystal was analysed by thermogravimetric (TG) and differential thermal analyses (DTA). The kinetic parameters such as the activation energy, frequency factor, entropy, enthalpy and Gibbs-free energy are computed from TGA data using Coats–Redfern method. The dielectric studies were analyzed by using the parallel plate capacitor method. The activation energy is calculated from Arrhenius plot. The electronic properties such as the plasma energy, the Penn gap, the Fermi energy and the electronic polarizability are calculated. The third order nonlinear optical properties of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline was measured by the Z-scan technique using 532 nm diode pumped continuous wave (CW) Nd:YAG laser.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the recent years, many innovative research reports have been focused on NLO materials due to its potential applications in modern optical data storage, frequency mixing, holographic imaging, telecommunication, integrated optics etc. Nonlinear optical materials have the ability to generate new electromagnetic fields with changed frequencies, phases and other physical properties. Nonlinear optics has come under the limelight of scientific research since the late 1960s with the onset of laser followed by the confirmation of harmonic generation in quartz. Nonlinear optics is the study of the interaction of light with matter when the response of a material system to an applied optical field depends in a nonlinear manner on the strength of the optical field. The synthesis of new and efficient frequency conversion materials has resulted in the development of novel organic materials.
Organic NLO materials offer a much larger versatility in assembling different crystal structures, enabling the fine tuning of the linear and nonlinear optical properties of the materials compared to inorganic materials. The advanced development in the crystal growth technology has exposed the attractive nonlinear optical properties of the crystals. This has enabled the commercial development of single crystals with promising nonlinear optical (NLO) properties [1,2,3,4]. Most of the optical fields researchers are also require a clear understanding of the third order nonlinear optical properties, which is an important part of these investigations. A vital goal for designing the molecules with large third-order nonlinearities has a feature devices used in the optical signal processing [5, 6]. To evaluate a material for the above application, its nonlinear refractive index and nonlinear absorption coefficient must be characterized. Several techniques are available for the measurement of nonlinear refraction in materials such as nonlinear interferometry, degenerate four-wave mixing, nearly degenerate three-wave mixing, ellipse rotation and beam-distortion measurements. Z-scan is a simple and popular experimental technique for the accurate measurements of intensity dependent nonlinear susceptibilities, nonlinear refractive index and nonlinear absorption coefficient [7,8,9].
The 3,4-dimethoxybenzaldehyde is a non-hygroscopic organic compound and its molecular formula is C9H10O3. It can be used as an intermediate in the synthesis of some pharmaceutical drugs. The 2,4-dinitroaniline is a non-hygroscopic organic compound and its molecular formula is C6H5N3O4. The dinitroanilines are intermediates in the preparation of various industrially important chemicals including dyes and pesticides. The organic material 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal was successfully grown by slow evaporation technique using acetone as the solvent. The grown crystals were characterized by the single crystal X-ray diffraction, powder XRD, FTIR, FT-Raman, optical (UV–Vis–NIR), photoluminescence (PL), thermal (TG/DTA), dielectric measurements and the third-order nonlinear optical studies.
2 Experimental
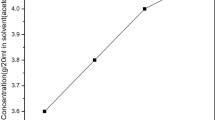
The organic material 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline was successfully synthesized using commercially available AR grade 3,4-dimethoxybenzaldehyde and 2,4-dinitroaniline chemicals in the equimolar ratio (1:1). The synthesized material was dissolved in acetone solvent. The solubility curve of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline at the various temperature ranges from 32 to 40 °C is shown in Fig. 1. The result shows that the solubility increases with the increase in temperature. The prepared solutions were then filtered and the purity of the materials was achieved by repetitive recrystallization process. For the crystal growth process the solvent was allowed to evaporate slowly at room temperature. The good optical quality crystals were harvested within few days. The photograph of the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal is shown in Fig. 2.
3 Results and discussion
3.1 Single-crystal XRD analysis
The Enraf Nonius CAD4MV31 single crystal X-ray diffractometer with the MoKα (λ = 0.717073 Å) is used to estimate the structure and the crystallographic parameters of the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal. This technique is directly connected to a single crystal refinement module, where the data generated from the X-ray analysis is interpreted and refined to obtain the crystal structure. The structure was solved by direct methods using the 2772 reflections and the refinements were carried out by the full-matrix least-squares technique using SHELXL97 package. It is observed that the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal belongs to monoclinic crystal system with the centrosymmetric space group P21/n. The structure and the lattice parameter values of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal are deposited in the Cambridge Crystallographic Data Centre (CCDC) [10]. The obtained cell parameters and the technical details of data acquisition and selected refinement results are listed in Table 1. The molecular structure and the crystallographic numbering scheme of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal is shown in Fig. 3 and the Packing diagram of the grown crystal is shown in Fig. 4.
3.2 Powder XRD studies
Powder X-ray diffraction pattern was recorded using a XPERT-PRO diffractometer with CuKα1 (λ = 1.54056 Å) radiation. The diffraction was recorded over a range of 10 to 70° at the rate of 1° min−1. The recorded X-ray pattern of the 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline is shown in Fig. 5. All the reflections are observed at room temperature. The obtained hkl planes are determined by using the two theta software. The determined sharp diffraction Bragg’s peaks at the specific 2θ angle shows the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystals have the good crystalline quality.
3.3 FTIR and FT-Raman spectral analyses
The functional groups of the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal has been identified by FTIR and FT-Raman analyses. The FTIR spectrum was recorded using the Perkin–Elmer FTIR spectrum RIX spectrometer by the KBr pellet technique, with a limit of 4000–400 cm−1. The FT-Raman spectrum was recorded using the BRUKER-RFS27 FT-Raman spectrometer in the limit of 4000–50 cm−1. The FTIR spectrum of the grown crystal 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline is shown in the Fig. 6 and the FT-Raman spectrum is shown in the Fig. 7. The absorption peaks observed in the IR and the Raman spectrum at 2845 cm−1 and 2843 cm−1 may be due to H–C=O stretching and it confirms the presence of aldehyde. The peaks in the IR spectrum at 1627 cm−1 and in the Raman spectrum at 1620 cm−1 is due to N–H bending. The absorption peak in the FTIR spectrum at 1675 cm−1 and the peak in the Raman spectrum at 1674 cm−1 were assigned as –C=C– stretching. The peaks observed almost nearly 1327 cm−1 in FTIR and around 1328 cm−1 in Raman have been assigned as the C–N stretching. The absorption peaks at 1497 cm−1 and 1518 cm−1 in FTIR and Raman spectra have been assigned as the N–O asymmetric stretching and it shows the presence of nitro compounds. The absorption peak at 922 cm−1 in FTIR spectrum and the absorption peak at 924 cm−1 in Raman spectrum is due to O–H bending.
3.4 UV–Vis–NIR studies
The optical transmittance plays a vital role in identifying a potential NLO material because a material can have applicability only if it has a wide optical transparent window without absorption [11]. Hence it is important to study the transmission of electromagnetic waves of the UV–Vis–NIR range through the NLO material. The transmission spectrum was recorded using a Perkin-Elmer Lambda 35 UV–Vis–spectrophotometer in the range 190–1100 nm. The transparent single crystal of thickness 2 mm was used for the analysis and the recorded transmittance spectrum is shown in Fig. 8a. The obtained result shows that the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal has nearly 80% transparency. The electronic transition σ → σ* fall into the far-ultraviolet region; π → π* and n → σ* transitions appear near the border line of the near-ultraviolet and far-ultraviolet regions; and n → π* transition fall into the near-ultraviolet and visible regions [12]. The cut-off wave length of the crystal is observed around 315 nm which is due to n → π* transition in the compound. The crystal has good transmittance in the entire visible and the near infrared regions. The wide transmittance range and the lower cut-off region will be helpful for these crystals for NLO applications.
a UV–Vis–NIR spectrum of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline. b Tauc plot of (αhν)2 versus energy for grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline. c Plot of extinction coefficient versus wavelength for 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal. d Plot of refractive index versus wavelength for 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal. e Optical conductivity as a function of photon energy for grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline. f Plot of ln(α) versus photon energy for grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline
The measured transmittance (T) was used to calculate the absorption coefficient (α) using the formula
where t is the thickness of the sample. The optical band gap (Eg) was calculated using the Tauc relation
where A is a constant, Eg is the optical band gap, h is the Planck’s constant, ν is the frequency of the incident photons and m is the index, which can have different values (2, 3, 1/2 and 3/2) corresponding to indirect allowed, indirect forbidden, direct allowed and direct forbidden transitions respectively [13, 14]. The band gap of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal was estimated by plotting (αhν)2 versus hν as shown in Fig. 8b and extrapolating the linear portion near the onset of absorption edge to the X-axis. From the figure, the value of optical band gap is found to be 3.9 eV.
The optical behaviour of materials is important to determine their usage in optoelectronic devices. The study of optical constants of a material such as refractive index and extinction coefficient is quite essential to examine the material’s potential optoelectronic applications. The extinction coefficient (K) can be obtained from the equation
Figure 8c shows the variation of extinction coefficient (K) with wavelength. The transmittance (T) is given by
The reflectance (R) in terms of the absorption coefficient can be obtained from the above equation
The refractive index (n) can be determined from reflectance data using the equation
The variation of refractive index with wavelength for 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal is shown in Fig. 8d. The optical conductivity (σop) is a measure of the frequency response of the material when it is irradiated with light.
where c is the velocity of light. The energy dependence of optical conductivity of the grown crystal is shown in Fig. 8e. It can be seen clearly from the figure that the optical conductivity increases abruptly after 2.75 eV for the grown crystal.
Urbach energy (EU) characterizes the amount of the absorption edge smearing due to the lattice disorder and non-stoichiometry showing the imperfections in crystalline nature. An exponential part called Urbach tail is found along the absorption coefficient curve near the optical band edge [15]. This exponential tail appears to be more for poor crystalline materials and amorphous materials. If this tail appears to be a minimum, this indicates the good crystalline nature and the perfection in lattice sites. The relationship between absorption coefficient α and energy of photons (hν) near the optical band gap edge is known as Urbach empirical rule, which is given by the following exponential equation
where α0 is a constant and EU denotes the Urbach energy. Taking logarithm on both sides of the above equation, one can get an equation of straight line and it is given as follows
The Urbach energy (EU) can be obtained from the reciprocal of the slope of the straight line obtained by plotting ln(α) against the incident photon energy (hν) which is shown in Fig. 8f. The value of EU is found to be 0.3 eV which indicates the good crystallinity of the grown crystal and less disorder near the optical band edge. Another relation correlating absorption coefficient α and the optical band gap energy Eg suggested by Urbach is given below.
where β is the pre-exponential constant, σ is the steepness parameter and E0 is the transition energy which is equal to Eg for direct allowed transition. The above equation can be rewritten in the following form:
Comparing the equations of ln(α), we get
where KB is the Boltzmann constant in eV/K and T is the absolute temperature. The steepness parameter σ can be calculated using the relation
The steepness parameter σ is found to be 0.078 which is used for evaluating the strength of the electron–phonon interaction by the relation
The various linear optical parameters of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal are listed in Table 2.
3.5 Photoluminescence studies
In recent years, the efficient organic luminescent nonlinear optical (NLO) materials have attracted worldwide tremendous research interests in the field of opto-electric devices. The organic benzaldehyde derivatives single crystals have obtained good photoluminescence properties [16,17,18,19]. The photoluminescence (PL) spectrum of the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal has been recorded by using the spectrofluorometer with the 450 W as high pressure Xenon lamp as the excitation source. The photoluminescence emission spectrum for the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal was shown in Fig. 9. The emission band was observed in the wave length range from 300 to 700 nm. The high intensity sharp peak was observed at 530 nm. The obtained results indicate that the grown, 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal may have green emission.
3.6 Thermal analyses
The TG and DTA analyses are of massive importance as far as fabrication technology is concerned, as they provide the thermal stability of the material for fabrication, where a considerable amount of heat is generated during the cutting process. Thermal analysis was performed on the grown crystals to study the thermal stability and the melting point. The thermal behavior of the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal was carried out by the thermogravimetric (TG) and the differential thermal analyses (DTA) using SDT Q600 V20.9 Build20 instrument, and the temperature ranges from 35 to 500 °C in the nitrogen atmosphere at a heating rate of 10 °C/min is being noted. The results obtained from the TG and the DTA are shown in Fig. 10a. The thermogravimetric analysis shows that the material is stable up to 137 °C. The first sharp endothermic curve in DTA is observed around 90 °C which indicates the melting point of the grown crystal. The second sharp endothermic peak is observed around 279 °C which is assigned as the decomposition point. From the TG/DTA analyses, it is observed that the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal have no phase transition or decomposition up to the melting point. The sharpness of the endothermic peaks observed in DTA indicates good degree of crystallinity of the sample.
TGA data can be used to study the kinetic parameters of thermal decomposition reaction such as activation energy, frequency factor and order of reaction. Several methods have been reported for calculating the kinetic parameters of solid state reactions. In the present investigation, Coats–Redfern method is used for the evaluation of kinetic parameters from non-isothermal TG data at a constant heating rate [20,21,22,23]. The following equations are used for the kinetic analysis.
Since the term (2RT/Ea) ≪ 1, the above equations can be rewritten as
where α is the fraction of conversion; n is the order of the reaction; A is the frequency factor; Ea is the activation energy of the reaction; R is the gas constant; β is the heating rate and T is the absolute temperature. The value of fraction of conversion, α was estimated by the relation
where W0 is the initial weight; W is the instantaneous weight depending on time and temperature and Wf is the final weight of the sample. The plot of \({\text{ln}}\left[\frac{g\left(\alpha \right)}{{T}^{2}}\right]\) versus \(\frac{1000}{T}\) is drawn for different values of n. Figure 10b shows the best linear fitted plot for n = 0.5 which has been chosen as the order of reaction for the determination of the kinetic parameters. The slope of the straight line is used to calculate the activation energy, Ea and the intercept is used to calculate the frequency factor, A.
The thermodynamic parameters of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal such as entropy of activation (ΔS*), enthalpy of activation (ΔH*) and Gibbs-free energy (ΔG*) were calculated using the equations:
where K is the Boltzmann constant; h is the Planck’s constant and Tm is the maximum decomposition temperature. The calculated activation energy, frequency factor and other thermodynamic parameters are listed in Table 3. The negative value of the entropy of activation indicates that the activated complex is more ordered than the reactant and the decomposition reaction is slow. The change in Gibbs free energy ΔG* is the criterion that is used to indicate whether a chemical reaction is spontaneous or not. The positive value of ΔG* indicates that the decomposition of the grown crystal is non-spontaneous. The positive value of ΔH* indicates that the reaction is endothermic in nature and enhanced by the increase in temperature [24].
3.7 Dielectric properties
The dielectric analysis is an important tool that provides valuable information on the electrical properties of the material medium as a function of temperature and frequency. Dielectric properties are correlated with electro-optic properties of the crystals. The dielectric analysis for the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal was carried out by the Agilent 4284A LCR Meter using the conventional parallel plate capacitor method [25, 26]. The capacitance and dielectric loss \((\mathrm{tan}\delta )\) were measured at various temperatures ranging from 308 to 373 K for five different frequencies 800 Hz, 1 kHz, 10 kHz, 100 kHz and 200 kHz. The dielectric constant of the crystal was calculated using the relation
where Acrys is the area of crystal touching the electrode and Aair is the area of electrode. The ac electrical conductivity \({\sigma }_{\mathrm{ac}}\) was calculated using the relation
where \({\varepsilon }_{0}\) is the permittivity of free space, \(\omega\) is the angular frequency (\(\omega =2\pi f,\) where f is the ac frequency considered) and \(\mathrm{tan}\delta\) is the dielectric loss factor. Figure 11a–c shows the dielectric constant (εr), dielectric loss (tanδ) and ac conductivity (σac) evaluated for the grown single crystal at different temperatures and frequencies. It was observed that the dielectric constant is gradually increased with increase in temperature. This indicates the presence of space charge effects in addition to the electronic and the atomic conduction in the samples. The values of the dielectric constant and the dielectric loss decrease with the increase in frequency while the ac conductivity value increases with the increase in frequency. The high value of dielectric constant at low frequency is due to the contribution of all the polarization mechanisms namely electronic, ionic, orientation and space charge polarization. At high frequencies, the dielectric constant value decreases due to the absence of space charge polarization near the grain boundary interface. The low dielectric loss with high frequency implies that the grown 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal possess enhanced optical quality with minimum defects and this parameter play a vital role for the construction of devices from nonlinear optical materials [27,28,29].
The activation energy in terms of electrical performance for 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal was evaluated from Arrhenius plot using the relation [30]
where σac is the electrical conductivity at temperature T, σ0 is the pre-exponential factor, Ea is the activation energy, T is the absolute temperature and k is the Boltzmann constant. The activation energy can be calculated by using the slope of the Arrhenius plot of ln (σac) verus \(\frac{1}{T}\). (− Ea/k) is the slope and the above equation can be written as
The value of slope can be computed from the linear graph of ln (σac) versus \(\frac{1}{T}\) and the activation energy is now calculated as
The variation of lnσac with 1000/T for the frequency of 1 kHz is shown in Fig. 12 and the value of Ea is found to be 0.139 eV.
The dielectric permittivity and dielectric loss are dependent on the Penn gap, plasma energy, Fermi energy and the valence electron of compound [31]. The valence electron plasma energy (\(\hbar\omega_{{\text{P}}}\)) is calculated using the relation
where \(Z = (15 \times Z_{{\text{C}}} ) + (15 \times Z_{{\text{H}}} ) + (7 \times Z_{{\text{O}}} ) + (3 \times Z_{{\text{N}}} ) = 132\) is the total number of valence electrons. The density \(\left( \rho \right)\) and molecular weight (M) of 3,4-dimethoxybenzaldehyde-2,4-dinitro aniline are 1.476 g/cm3 and 349.3 g/mol, respectively. The Penn gap is calculated by fitting the dielectric constant with the Plasmon energy. The Penn gap, Ep and the Fermi energy, EF in eV are given by the following relations
where \(\varepsilon_{{\text{r}}}\) is the dielectric permittivity of the material at high frequency (1 kHz in the present case).
The electronic polarizability \(\left( \alpha \right)\) of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal is calculated,
-
(a)
Using the valence electron plasma energy relation
$$\alpha = \left( {\frac{{\left( {\hbar \omega_{{\text{P}}} } \right)^{2} S_{0} }}{{\left( {\hbar \omega_{{\text{P}}} } \right)^{2} S_{0} + 3E_{{\text{P}}}^{2} }}} \right) \times \frac{M}{\rho } \times 0.396 \times 10^{ - 24} {\text{cm}}^{3}$$where \(\left( {S_{0} } \right)\) is a constant which given as
$$S_{0} = 1 - \left( {\frac{{E_{{\text{P}}} }}{{4E_{{\text{F}}} }}} \right) + \frac{1}{3}\left( {\frac{{E_{{\text{P}}} }}{{4E_{{\text{F}}} }}} \right)^{2}$$ -
(b)
(b) Using the Clausius–Mossotti relation
$$\alpha = \left( {\frac{3M}{{4\pi N_{{\text{a}}} \rho }}} \right)\left( {\frac{{\varepsilon_{{\text{r}}} - 1}}{{\varepsilon_{{\text{r}}} + 2}}} \right)$$where Na is Avogadro number (Na = 6.023 × 1023). The calculated electronic properties like plasma energy, Penn gap, Fermi energy and electronic polarizability obtained using valence electron plasma energy and Clausius–Mossotti relation for the 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline crystal is listed in Table 4.
Table 4 Electronic parameters of 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal
3.8 Nonlinear optical studies
Z-scan technique is a well known technique for the measurement of optical nonlinearities of the materials, especially nonlinear refraction and nonlinear absorption. This method facilitates the simultaneous measurement of magnitude as well as sign of the nonlinear refractive index and nonlinear absorption coefficient of the material using open, closed and ratio of the closed-to open configurations of Z-scan. The position vector z of the sample is varied with respect to the focal plane of the lens and hence this technique is called Z-scan. In the present investigation, Z-scan experiment was performed using a 50 mW 532 nm diode pumped CW Nd:YAG Laser which was focused by a lens of focal length 3.5 cm. The intensity of laser output was 4.38 kW/cm2.
The laser beam waist (ω0) at the focus is measured to be 15.84 µm. The corresponding Rayleigh length z0 is calculated using the relation
where λ is the laser wavelength. The value of z0 is calculated to be 1.48 mm. The optical cell of 1 mm width containing 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline solution satisfied the thin sample condition L ≪ z0 is translated across the focal region along the direction of the propagation laser beam. The transmission of the laser beam through the aperture placed in the far field was measured by using photo detector which is fed to the digital power meter. In the open aperture Z-scan mode, the aperture is replaced by a lens to collect the laser beam transmitted through the sample.
Figure 13a–c shows the closed, the open and the ratio of the closed to open normalized Z-scan of the 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline sample in acetone at 60% transmittance. The peak followed by a valley normalized transmittance obtained from the closed aperture Z-scan data indicate that the signs of the refraction nonlinearity is negative, i.e., self-defocusing. The self-defocusing effect is due to the local variation in the refractive index with the temperature [32,33,34].
The difference between the normalized peak and valley transmittances, ΔTp–v can be evaluated from the closed aperture normalized transmittance data by the relation
where Δϕ0 is the on-axis phase shift at the focus and S is the aperture linear transmittance which is given by the equation
where ra denotes the aperture radius and ωa denotes the radius of the laser spot before the aperture. The on-axis phase shift is related to the third order nonlinear refractive index (n2) by,
where Leff = (1 − e−αL)/α, with L the sample length, α the linear absorption coefficient, I0 the intensity of the laser beam at focus z = 0 and k the wave number (k = 2π/λ). The nonlinear absorption coefficient β is estimated from the open aperture Z-scan mode data using the relation
where ΔT is the maximum value of the open aperture normalized transmittance obtained from the Z-scan plot. Experimentally determined nonlinear refractive index n2 and nonlinear absorption coefficient β can be used to find the real and imaginary parts of the third-order nonlinear optical susceptibility [\(\chi^{3}\)] according to the following relations
where ε0 is the vacuum permittivity, and c is the light velocity in vacuum. The absolute value of the third-order nonlinear optical susceptibility is given by the relation
The nonlinear parameters are calculated and the obtained values are tabulated in Table 5.
4 Conclusion
The organic material 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal was successfully grown by slow evaporation technique using acetone as solvent. The single crystal XRD studies show that the grown crystal belongs to the monoclinic crystal system with the centro-symmetric space group P21/n. The powder XRD reveals that the grown crystal has good crystalline nature. The FTIR and the FT-Raman spectral analyses confirmed the presence of various functional groups in the 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal. The optical (UV–Vis–NIR) analysis reveals that the crystal have good transmission in the entire visible and near infrared regions. The optical band gap of the grown crystal was found to be 3.9 eV using Tauc plot. The linear optical parameters such as refractive index, extinction coefficient and optical conductivity were calculated for the grown crystal. The Urbach energy was calculated as 0.3 eV which indicates the crystalline perfection of the grown crystal. The photoluminescence analysis shows that the high intensity peak was obtained around 530 nm, and it shows green colour emission. Thermal properties indicate that the obtained single crystal melting point is almost nearly 90 °C and the decomposition point is around 279 °C. The kinetic parameters for the thermal decomposition of the grown crystal were calculated using Coats–Redfern method. The dielectric analyses reveal that the dielectric constant, the dielectric loss and the AC conductivity were gradually increases with the increase of temperature and it may be realized as due to the temperature variations of the polarizability. The value of activation energy is found to be 0.139 eV. The electronic properties such as the plasma energy, the Penn gap, the Fermi energy and the electronic polarizability are calculated. The third-order nonlinear analysis reveals that the organic 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal exhibits negative optical nonlinearity.
References
P.A. Franken, A. Hill, C.W. Peters, G. Weinrich, Phys. Rev. Lett. 7, 118–119 (1961)
S.R. Marder, J.E. Sohn, G.D. Stucky, Materials for Nonlinear Optics (American Chemical Society, Washington, 1991).
S. Suresh, A. Ramanand, D. Jayaraman, P. Mani, Rev. Adv. Mater. Sci. 30, 175–183 (2012)
D. JanardhanaRao, B. Hari Babu, A.V.V.S. Swami, S. Sumithra, Univ. J. Environ. Res. Technol. 3, 225–232 (2013)
M. Somac, A. Somac, B.L. Davies, M.G. Humphery, M.S. Wong, Opt. Mater. 21, 485–488 (2002)
J.G. Breitzar, D.D. Diott, L.K. Iwaki, S.M. Kirkpatrick, T.B. Rauchturs, J. Phys. Chem. A 103, 6930–6937 (1999)
R.K. Choubey, R. Trivedi, M. Das, P.K. Sen, P. Sen, S. Kar, K.S. Bartwal, R.A. Ganeev, J. Cryst. Growth 311, 2597–2601 (2009)
R.K. Choubey, S. Medhekar, R. Kumar, S. Mukherjee, S. Kumar, J. Mater. Sci. Mater. Electron. 25, 1410–1415 (2014)
B.K. Dadhich, I. Kumar, R.K. Choubey, B. Bhushan, A. Priyam, Photochem. Photobiol. Sci. 16, 1556–1562 (2017)
R.P. Jebin, T. Suthan, CCDC 1569865: Experimental Crystal Structure Determination (2017). https://doi.org/10.5517/ccdc.csd.cc1ppktr
J. Ramajothi, S. Dhaanuskodi, Spectrochim. Acta Part A 68, 1213–1219 (2007)
C.N. Banwell, E.M. McCash, Fundamentals of Molecular Spectroscopy, 4th edn. (Tata McGraw-Hill, New Delhi, 1994).
F. Moser, F. Urbach, Phys. Rev. 102, 1519–1523 (1956)
B.K. Periyasamy, R.S. Jebas, N. Gopalakrishnan, T. Balasubramanian, Mater. Lett. 61, 4246–4249 (2007)
P. Karuppasamy, M.S. Pandian, P. Ramasamy, S. Verma, Opt. Mater. 79, 152–171 (2018)
T. Suthan, N.P. Rajesh, J. Cryst. Growth 312, 3156–3160 (2010)
T. Suthan, P.V. Dhanaraj, N.P. Rajesh, Spectrochim. Acta Part A 87, 194–198 (2012)
R.P. Jebin, T. Suthan, N.P. Rajesh, G. Vinitha, U. Madhusoodhanan, Spectrochim. Acta Part A 135, 959–964 (2015)
M. Saravanan, S.A. Rajasekar, Opt. Mater. 54, 217–228 (2016)
M.A. Ashok, B.N. Achar, Bull. Mater. Sci. 31, 29–35 (2008)
S. Rama, C.S. Dilip, R.N. Perumal, J. Cryst. Growth 409, 32–38 (2015)
V. Gupta, K.K. Bamzai, P.N. Kotru, B.M. Wanklyn, Mater. Sci. Eng. B 130, 163–172 (2006)
V. Hangloo, K.K. Bamzai, P.N. Kotru, M.L. Koul, Bull. Mater. Sci. 27, 395–401 (2004)
K. Gayathri, P. Krishnan, P.R. Raj Kumar, G. Anbalagan, Bull. Mater. Sci. 37, 1589–1595 (2014)
T. Suthan, N.P. Rajesh, P.V. Dhanaraj, C.K. Mahadevan, Spectrochim. Acta Part A 75, 69–73 (2010)
T. Suthan, P.V. Dhanaraj, N.P. Rajesh, C.K. Mahadevan, G. Bhagavannarayana, Cryst. Eng. Comm. 13, 4018–4024 (2011)
T. Akutagawa, S. Takeda, T. Hasegawa, T. Nakamura, J. Am. Chem. Soc. 126, 291–294 (2004)
T. Arumanayagam, P. Murugakoothan, J. Miner. Met. Mater. Eng. 10, 1225–1231 (2011)
T. Suthan, N.P. Rajesh, C.K. Mahadevan, G. Bhagavannarayana, Mater. Chem. Phys. 129, 433–438 (2011)
S. Karuppusamy, K. Dinesh Babu, V. Nirmal Kumar, R. Gopalakrishnan, Appl. Phys. A 122, 498 (2016)
S. Siva Bala Solanki, N.P. Rajesh, T. Suthan, Opt. Laser Technol. 103, 163–169 (2018)
P.B. Chapple, J. Staromlynska, J.A. Hermann, T.J. Mckay, J. Nonlinear Opt. Phys. Mater. 6, 251–293 (1997)
S. Suresh, M.N. Mohideen, G. Vinitha, R.M. Kumar, Mod. Electron. Mater. 4, 103–111 (2018)
T. Cassano, R. Tommasi, M. Ferrara, F. Babudri, G.M. Farinola, F. Naso, Chem. Phys. 272, 111–118 (2001)
Acknowledgements
This work was supported by the University Grants Commission (UGC), South Eastern Regional Office (SERO), Government of India, under the grant of Minor Research Project UGC Reference No: F. MRP-7005/16 (SERO/UGC) Link No: 7005, is hereby gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jebin, R.P., Suthan, T., Anitha, T.R. et al. Growth and characterization of organic material 3,4-dimethoxybenzaldehyde-2,4-dinitroaniline single crystal. J Mater Sci: Mater Electron 32, 3232–3246 (2021). https://doi.org/10.1007/s10854-020-05072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-05072-1