Abstract
The raspberry-like hollow SnO2-based (bare SnO2 and Pd-doped SnO2) nanostructures with different dominant crystal facets were prepared facilely using carbon nanospheres as templates via solvothermal method. Volatile organic compounds (VOCs) and ammonia (NH3) gas sensing performances of the hollow SnO2-based structures were studied systematically. The gas sensing performances were investigated in a temperature range of 150–315 °C. It was found that 285 °C was the optimum operating temperature for both the sensors. The SnO2 sensor showed excellent VOCs (1–100 ppm) sensing performances, with a fast response/recovery behavior (around 4 s/30 s) at 285 °C. While the Pd-SnO2 sensor displayed selective NH3 sensing characteristics at low concentrations of 1.5–12 ppm, interestingly, with a response/recovery time of about 4 s/80 s at 285 °C. Both the SnO2 and Pd-SnO2 sensors showed great repeatability for 8 response/recovery cycles, and very slight response recession for a long period. It was found that not only the morphology, the synergistic effect from the heterojunctions of doped Pd and SnO2, and the Pd catalysis, but also the crystal facets could modulate the sensing performance of metal oxides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Volatile organic compounds (VOCs) are important precursors of urban haze and photochemical smog. Certain VOCs are identified as hazardous gases and could cause severe diseases (e.g., lung cancer and allergy) [1]. They are encountered in various activities, such as oil refining, driving, cooking, painting, and so on. In order to evaluate the air quality, environmental monitoring is essential. Ammonia is a colorless and toxic gas with a pungent odor, and it could be noticed at a low level of 5 ppm in real environment [2]. Additionally, ammonia is immediately dangerous to life and health at a concentration of 300 ppm, causing damage to the skin, eyes, and respiratory systems of human beings [3, 4]. To ensure human safety, durable NH3 sensors are indispensable.
Chemi-resistive gas sensors based on tin dioxide (SnO2) has attracted widespread attention, and is considered as the most promising gas sensing material due to its low cost, high chemical durability and simple preparation [5,6,7,8,9,10]. As elucidated, response of resistive gas sensors is closely related to the amounts of active sites on the sensing material surface [11]. Various porous nanostructures (such as hollow spheres [7, 12], porous films [13, 14], et al.) have been designed to circumvent superior gas sensing performance, providing lots of surface active sites and well surface permeability. Among of them, SnO2 hollow nanostructures exhibit notably interesting gas sensing performance, due to their additional exterior openings and inner voids inducing large surface area [7, 12, 15, 16].
Moreover, sensors based on undoped SnO2 usually exist significant shortages, especially the lack of selectivity and low response at low analyte concentration [17, 18]. Noble metals (e.g., Pd, Ag, Pt) doping has proved to be the most suitable approach to overcome these weaknesses of pure SnO2, due to the catalytic properties and the special synergistic effects in heterojunctions between the noble metal and SnO2 [17, 18]. According to the previous reports, Pd doping could improve the selective sensing of metal oxides to NH3 [19, 20]. Additionally, selectively exposing facets of the sensing material has been elucidated previously to improve the gas sensing properties of metal oxides [17, 21]. In the literature [21], (221) facet with higher surface energy of SnO2 could improve the sensitivity to ethanol than (110) facet with lower surface energy. Crystallographic facets effect has been extensively investigated in catalyst field, nevertheless, this effect on metal oxides sensors is still limited and quite elusive.
Herein, raspberry-like hollow SnO2 and Pd-SnO2 nanostructures with different dominant crystal facets are implemented facilely using carbon nanospheres as templates via solvothermal method. The VOCs and NH3 sensing performances of the synthesized products are investigated, by integrating the products on micro-hotplates. Due to the special hollow structure with additional exterior openings and inner voids, different crystal facets, and the Pd doping, greatly superior VOCs sensor devices based on SnO2 and NH3 sensor devices based on Pd-SnO2 are prepared. The corresponding sensing mechanism is proposed together with the first-principles calculation.
2 Experiments
2.1 Materials and synthesis
The carbon spheres were hydrothermally prepared according to the reported method [22]. In order to prepare SnO2-based hollow structures, 3.6 g synthesized carbon spheres were dispersed into 90 mL ethanol with ultrasonic agitation, and a homogeneous supernatant solution (labeled A) was obtained. Meanwhile, 15 mmol SnCl2·2H2O was dispersed in 15 mL DI water to form a solution as labeled B. Solution B was added into solution A drop by drop with ultrasonic agitation. Afterwards, 0 or 0.15 mmol PdCl2 was added into the mixed solution, and then kept stirring for 16 h to ensure a sufficient diffusion of Sn2+ onto the surface of carbon spheres because of the hydrogen bond interactions. After that, precipitates were collected by centrifugation, washed with DI water and ethanol 3 times successively, and dried at 80 °C in air. Finally, the product was calcinated in air at 500 °C for 2 h with the heating rate of 2 °C/min. The raspberry-like hollow SnO2 and Pd-SnO2 nanostructures were obtained.
2.2 Characterization
The morphology of the SnO2 and Pd-SnO2 products was characterized by the scanning electron microscopy (SEM, FEI Nanosem 430) and transmission electron microscopy (TEM, JEOL JEM-2100). Powder X-ray diffraction (XRD) analyses were performed on a Bruker D8 Advance diffractometer with Cu Kα radiation (λ ≈ 1.54 Å). X-ray photoelectron spectra (XPS) were performed on an RBD upgraded PHI-5000C ESCA system (PerkinElmer). BET surface areas were determined by (Quantachrome Inc., USA) nitrogen adsorption and desorption method.
2.3 Sensor fabrication
The gas sensor was prepared by integrating SnO2-based products onto the center of a commercial aluminum oxide micro-hotplate. On the front of the micro-hotplate is interdigitated electrodes for integrated sensing material, while the back is the heating electrode for providing needed temperatures. Figure S1 shows the voltage input to the micro-hotplate versus temperature of the micro-hotplate. The SnO2-based products were sonicated into suspension in an aqueous solution of isopropyl alcohol (0.5 mg/mL). A 1 µL drop was placed onto the micro-hotplate, while the micro-hotplate was heated to 100 °C and maintained there for 2 h to promote solvent evaporation and material deposition at the center of the hotplate. Figure 1a displays the schematic structure of the sensor device, with interdigitated electrodes for sensing layer resistance readout and heater circuitry for sensing temperature controlling.
2.4 Sensor testing
The gas sensing properties were investigated by a homemade measuring system with an 8 L glass test chamber as shown in Figure S2. Before measurements, the chamber is filled with ambient air. When an analyte gas was injected into the chamber, sensor resistance began to change. Concentration of an analyte was controlled by amount of the injected pure gas. Relative humidity (RH) is adjusted by air conditioner and humidifier. Both the sensor signal collection and the micro-hotplate temperature are controlled using a Keithley 2602B source-meter. In order to form a measurement circuit, a reference resistor (Rref.) was put in series. The circuit diagram is illustrated in Fig. 1b. By monitoring the voltage (Voutput) across Rref., the sensor response (Rsensor) could be calculated by Ohm’s law according to the Eq. (1):
Response is defined by the percentile resistance change when the sensor is exposed to an analyte gas as follows:
where R0 and Rg are the resistances of the sensor before and after exposed to the test gas, respectively.
3 Results and discussion
3.1 Characterization
SEM image in Fig. 2a shows that carbon spheres have well-defined spherical shape and roughly 70 nm in sphere diameters. SEM image in Fig. 2b exhibits the raspberry-like hollow nanostructures of Pd-SnO2 inherited from the structure of carbon spheres. During the synthesis process, the carbon spheres derived from hydrothermal conditions were hydrophilic with plenty of − OH and C=O groups on the surface. These functional groups enabled the carbon spheres to bind Sn2+. The carbon cores could be sacrificed in the form of CO2 during calcination. Hence, the hollow structures were induced by the carbon spheres loaded with Sn2+ after calcination [5, 22]. The hollow structures possess small openings like raspberries, leading to great surface accessibility and effect gas diffusion and contributing to the gas sensing behavior. The Brunauer–Emmett–Teller (BET) specific area of Pd-SnO2 is 25 m2/g according to the N2 adsorption–desorption measurement (shown in Fig. S3), which is slightly higher than that of the reported Pt-SnO2 hollow nanospheres exhibiting extreme ethanol sensitivity [8].
The XRD patterns for SnO2 and Pd-SnO2 products are shown in Fig. 2c. All the peaks could be assigned to the tetragonal rutile SnO2 (JCPDS No. 41-1445). The obvious characteristic peaks for SnO2 at 2θ = 26.8°, 33.9°, 38.1° and 51.9° correspond, respectively, to the (110), (101), (200) and (211) planes of the pure SnO2. And the strongest two peaks are (101) and (200) in SnO2. For Pd-SnO2, however, there is no obvious Pd or PdOx peaks, which is possibly because of the small amount of Pd. Compared to pure SnO2, (110) peak of SnO2 strengthens dramatically while (200) peak almost disappears in Pd-SnO2. The broaden width and shift of the SnO2 diffraction peaks with Pd additive could be explained in two aspects: (1) the crystallite size of SnO2 became smaller after Pd addition; (2) the poor crystallinity of SnO2 due to strong chemical interactions with Pd additive [23]. The additive Pd phase will be analyzed using XPS in the following. In addition, the EDX analysis for Pd-SnO2 demonstrates coexistence of Sn, O, and Pd (Fig. 2d). And the distribution of every color confirms these three elements distribute uniformly over the whole outline.
More morphological and crystal structure characterizations for SnO2 and Pd-SnO2 were investigated using TEM analysis. The low-resolution TEM images (Fig. 3a, c) further demonstrate the spherical shape and rough surface of SnO2 and Pd-SnO2, similar to previous report [5, 6], as well as an obvious void space in the interior confirming the hollow feature. The high-resolution TEM image (Fig. 3b, d) displays SnO2 is high crystallinity, with the lattice fringe of 3.3, 2.6 and 2.36 Å, respectively, well matched with the (110), (101) and (200) plane of SnO2 (JCPDS No. 41-1445). The diffraction rings in the SAED patterns (Figure S4) confirm the polycrystalline structure of SnO2 and Pd-SnO2 products, which are agree with the XRD patterns (Fig. 2c).
In order to investigate the electronic state and surface chemical composition of elements of SnO2 and Pd-SnO2 products, XPS measurement was taken. The binding energy values of Sn 3d, Pd 3d and O 1s, and their atomic percent are listed in Table S1. Obviously, both SnO2 and Pd-SnO2 are nonstoichiometric. The survey spectrums of SnO2 and Pd-SnO2 are shown in Fig. 4a. For Pd-SnO2, the relative intensity of Pd 3d binding energy peak is extremely weak because of its low content. In the spectrum of Pd 3d (Fig. 4b), the fitted peaks at 341.87 and 336.59 eV are indexed to Pd2+ 3d3/2 and Pd2+ 3d5/2, respectively, indicating the PdO phase in Pd-SnO2 due to the oxidation of Pd during calcination process, which is agree with the Ref [24]. Figure 4c demonstrates that the two peaks around at 495.2 and 486.7 eV assigned to Sn4+ of Pd-SnO2 move to low banding energy, compared with those of the pure SnO2. The Sn 3d peak movement is attributed to the electrons flow from SnO2 to PdO [15], due to their different Fermi levels (see Sect. 3.3). The obvious deviation further confirms the strong interaction and illustrates heterogeneous formation between SnO2 and PdO. Figure 4d shows the high-resolution O 1 s core level XPS spectrum and its fitting curve over a small binding energy window for SnO2 and Pd-SnO2. It could be resolved into 3 fitted peaks corresponding to O1, O2 and O3. O1 is ascribed to the lattice oxygen species, having no influence on gas sensing behavior. O2 and O3 refers to the oxygen vacancies and chemisorbed oxygen species, respectively, dedicating to the gas sensing properties [25,26,27].
3.2 Gas sensing performance
For reliable gas sensing measurements, a series of sensors were made from each product. Resistance values of these sensor samples are shown in Fig. S5, indicating resistance of Pd-SnO2 is much higher than pure SnO2. Each sensor was measured several times to obtain reliable testing data. According to the I–V curves shown in Fig. S6, nearly linear current vs. voltage behaviors illustrate an Ohmic contact between the sensing materials and sensor electrodes, further demonstrate conductivity of the Pd-SnO2 is lower than that of pure SnO2. The higher resistance of Pd-SnO2 could be explained as follows: according to the XPS analysis, Pd dopant is PdO phase in the Pd-SnO2 hybrid. The work functions of PdO (5.5 eV) and SnO2 (4.5 eV) are different. Electrons flow from the conduction band of SnO2 to PdO, leading to a depletion layer and upward band bending on the surface of SnO2. Hence, the resistance of Pd-SnO2 is much higher than that of pure SnO2.
Prior to investigating the gas sensing performances, selectivity to various gases of the SnO2 and Pd-SnO2 sensors was measured. Obviously, the SnO2 sensor shows excellent selectivity to various VOC gases (ethanol, methanol, acetone, isopropyl alcohol (IPA), formaldehyde), while the Pd-SnO2 sensor shows great selectivity to NH3 (shown in Fig. 5 a, b). Due to the adsorption/desorption behaviors of oxygen and analyte molecules on the surface of sensing materials are closely related to temperature, the response and response/recovery time of the sensors at different temperatures were studied (Table S2). The response/recovery time of both SnO2 and Pd-SnO2 sensors decrease with the working temperature increasing (Fig. 5c, d). While the response values of the sensors increase at the beginning and then decrease with further increasing in the working temperature, resulting in a peak value at 285 °C for SnO2 sensor to ethanol gas and 240 °C for Pd-SnO2 sensor to ammonia gas. Considering response and recovery performances and power consumption of the devices, 285 °C is taken as the optimum operating temperature. Further tests for both SnO2 and Pd-SnO2 samples are taken at 285 °C under the relative humidity of 40%.
The SnO2 sensor responses to various VOCs (ethanol, methanol, acetone, IPA and formaldehyde) from 1 to 20 ppm at 285 °C are shown in Fig. 6a. Resistance of the SnO2 sensor decreases upon exposure to VOCs, consistent with a n-type semiconductor behavior [28]. Full and rapid recovery of the sensor resistance (e.g., returning back to the baseline), when VOCs is replaced by air, indicates the excellent reversibility of the SnO2 sensor. The response increases with the VOCs gas concentration increasing, while tending to saturate above 20 ppm (Fig. 6b). The typical power-law fitting of acetone response as a function of acetone concentration is shown in Fig. 6b. The similar fitting of the responses vs. concentration for the other VOC gases is shown in Fig. S7.
The SnO2 sensor: a resistance versus time for various VOCs gas (1–20 ppm) and b the corresponding response values with fitting of the typical acetone response as a function of acetone concentration. The Pd-SnO2 sensor: c resistance versus time for NH3 gas (1–12 ppm) and d the corresponding response values with fitting of the response as a function of NH3 concentration. The error bars represent the standard deviation of response of the 5 exposures to a given target gas concentration
Figure 6c shows dynamic response-recovery curves of Pd-SnO2 sensor at various NH3 concentrations at 285 °C. The sensor resistance decreases exposed to NH3, indicating the n-type behavior of Pd-SnO2. After the atmosphere returns to air, resistance of the Pd-SnO2 sensor reaches to the initial state in air quickly, exhibiting excellent recovery performance to NH3 exposure. The response to a minimum concentration of 1.5 ppm is very obvious and the power-law fitting can be obtained between the responses and NH3 concentrations, as shown in Fig. 6d. Furthermore, Table 1 summarizes the comparison of NH3 sensing performance between our Pd-SnO2 sensor and previously similar NH3 sensor based on Pd-functionalized metal oxides. Obviously, the raspberry-like hollow Pd-SnO2 demonstrates more sensitive to NH3 gas at low concentrations.
Repeated response/recovery measurements of the SnO2 sensor to 3 ppm VOCs and that of the Pd-SnO2 sensor to 3 ppm NH3 for 8 cycles are shown in Fig. 7a, b, respectively, indicating well repeatable sensing behaviors. Excellent long-term stability of gas sensors is another essential issue for reliable application. Responses of the SnO2 sensor to 3 ppm ethanol and that of the Pd-SnO2 sensor to 3 ppm NH3 were measured over a period of 30 days. As shown in Fig. 7c, the response average variation of around 15% and 8% for SnO2 and Pd-SnO2 sensor, respectively. Moreover, repeat response of the Pd-SnO2 sensor to 1.5 ppm NH3 under different relative humid conditions (Fig. S8) indicates the stability of the sensor at high working temperature of 285 °C under wide humid conditions (RH% 40–70%).
a Repeatability of the SnO2 sensor to 3 ppm VOCs; b repeatability of the Pd-SnO2 sensor to 3 ppm NH3; c long-term stability of the SnO2 sensor to 3 ppm ethanol and that of the Pd-SnO2 sensor to 3 ppm NH3. The error bars represent the standard deviation of response of the 5 exposures to a given ethanol (or ammonia) gas concentration
3.3 Gas sensing mechanism
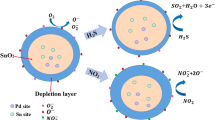
Response of the SnO2-based sensor refers to the surface-related redox reaction, depending on the composite and structure of the SnO2, analyte species and the operating temperature. Among of various nanostructures, the hollow structure here maximizes the gas accessibility of internal surfaces, giving rise to excellent sensing behavior, due to its additional exterior openings and inner voids (Figs. 2b and 3a, c) [6].
The response first step is the surface reaction on SnO2: the adsorbed oxygen molecules capture electrons from the conduction band of SnO2, inducing an electron depletion layer on the surface of SnO2, i.e., a high resistance state. Oxygen chemisorbs on SnO2 at T ≤ 150 °C in the molecular form \({O}_{2}^{-}\), notably, and at higher temperatures it dissociates to atomic \({O}^{-}\) [36]. For the present work working at 285 °C, upon exposure to VOCs such as ethanol, the analyte vapor reacts with chemisorbed oxygen species \({O}^{-}\) releasing electrons back to sensing material as the following reaction:
Hence, upon exposed to VOCs, the SnO2 resistance decreases.
As for the Pd-SnO2 sensor, there are two sensitization mechanism to explain the improved NH3 selectivity. For one thing, Pd dopant as PdO phase, observed based on XPS results, introduces a depletion layer on the surface of SnO2 and an upward band bending. It could be explained that PdO traps electrons from the conduction band of SnO2 to equilibrate Fermi level in the hybrid, due to the different work function of PdO (5.5 eV) and SnO2 (4.5 eV). The increased resistance of Pd-SnO2, observed based on I–V behaviors (Figure S3), further probes the initial upward band bending.
For another thing, when the Pd-SnO2 exposed to NH3 vapor, PdO reacts with NH3 and back to \({Pd}^{0}\) as the following reaction [18, 29]:
With that, more oxygen chemisorbed on the Pd-SnO2 surface due to the “spillover” effect of \({Pd}^{0}\) as catalyst. It’s probably due to that the \({Pd}^{0}\) atoms provide oxygen adsorption sites via available free valences, as well as lower the energy barrier of oxygen adsorption/dissociation [37]. NH3 molecules react with chemisorbed oxygen \({O}^{-}\) releasing electrons back to SnO2 and reducing its resistance, according to the following reaction:
Both the electrical sensitization, upward band bending contribution created by PdO, and the catalytic activation of PdO maximize the NH3 sensing ability of the Pd-SnO2 sensor.
A comparative first-principles calculation was conducted to further clarify the gas sensing mechanism of the SnO2-based materials with different dominant crystal facets. Theorical simulation for gas sensing of Pd-ZnO [38], Pt-SnO2 [39], and CO sensing of Pd-SnO2 [40] have been reported, as well as the NH3 sensing improvement of Pd additive [18, 29, 37]. Moreover, the simulation of VOCs and NH3 adsorption on Pd-SnO2 is quite elusive. Compared the XRD patterns of SnO2 and Pd-SnO2, the main discrepancy is that (110) peak strengthens dramatically while (200) peak tends to disappear after Pd doping. We calculated the adsorption ability of both ethanol (as an example of VOCs) and NH3 on Pd-SnO2 (110) surface, respectively, using Monte Carlo simulation. More details are described minutely in Supporting Information (Fig. S9). The adsorption fields in Fig. 8a, b display the fields of adsorption sites (in white dotted circle), higher density of points showing more likely locations. What’s more, the more likely adsorption areas are shown in green and less likely sites appear red. Obviously, compared with ethanol molecule, NH3 is easier to adsorb on the Pd-SnO2 surface. In addition, adsorption energy of NH3 molecule (727 kcal/mol) is much higher than that of ethanol molecule (51 kcal/mol) on Pd-SnO2 (110) facet, further verifying the great effect of the Pd additive on the NH3 selectivity of the Pd-SnO2.
4 Conclusions
The raspberry-like hollow SnO2 and Pd-SnO2 nanostructures with different dominated crystal facets were designed and synthesized solvothermally via carbon spheres as templates. Both them possessed additional exterior openings and inner voids, which could contribute to the enhanced gas sensing performances. The pure SnO2 showed great VOCs gas sensing performances, while Pd dopants modulated the NH3 sensing selectivity of the SnO2. The optimum working temperature for both sensors was found to be 285 °C, with low power, appropriate response, and fast response/recovery behavior. Both the SnO2 and Pd-SnO2 sensors show great repeatability for 8 response/recovery cycles. But the slight response recession for a long period need to be improved in the future. The corresponding sensing mechanism is explained in terms of morphology, crystallographic facets effect, as well as doping and heterojunctions. The facile approach opens up a prospect for commercialization of the relative micro sensors.
References
T.T. Lin, X. Lv, Z.N. Hu, A.S. Xu, C.H. Feng, Sensors (2019). https://doi.org/10.3390/s19020233
I.P. Liu, C.H. Chang, T.C. Chou, K.W. Lin, Sens. Actuator B 291, 148 (2019). https://doi.org/10.1016/j.snb.2019.04.046
V.B. Raj, A.T. Nimal, Y. Parmar, M.U. Sharma, K. Sreenivas, V. Gupta, Sens. Actuator B 147, 517 (2010). https://doi.org/10.1016/j.snb.2010.03.079
D.D. Nguyen, D.V. Dang, D.C. Nguyen, Adv. Nat. Sci. Nanosci Nanotechnol 6, 035006 (2015). https://doi.org/10.1088/2043-6262/6/3/035006
J. Zhang, S.R. Wang, Y.M. Wang et al., Sens. Actuator B 135, 610 (2009). https://doi.org/10.1016/j.snb.2008.09.026
P.M. Bulemo, H.J. Cho, D.H. Kim, I.D. Kim, ACS Appl Mater Interfaces 10, 18183 (2018). https://doi.org/10.1021/acsami.8b00901
J.Y. Liu, T.S. Wang, B.Q. Wang et al., Sens. Actuator B 245, 551 (2017). https://doi.org/10.1016/j.snb.2017.01.148
B.-Y. Kim, J.S. Cho, J.-W. Yoon et al., Sens. Actuators B 234, 353 (2016). https://doi.org/10.1016/j.snb.2016.05.002
Z. Li, J.X. Yi, Sens. Actuator B 243, 96 (2017). https://doi.org/10.1016/j.snb.2016.11.136
S.L. Bai, W.G. Tong, Y. Tian et al., J. Mater. Sci. 54, 2025 (2019). https://doi.org/10.1007/s10853-017-1588-2
K. Suematsu, H. Uchino, T. Mizukami, K. Watanabe, K. Shimanoe, J. Mater. Sci. 54, 3135 (2019). https://doi.org/10.1007/s10853-018-3020-y
L. Xiao, S.M. Shu, S.T. Liu, Sens. Actuator B 221, 120 (2015). https://doi.org/10.1016/j.snb.2015.06.099
J. Rebholz, K. Grossmann, D. Pham et al., Sensors 16, 1437 (2016)
K. Großmann, K.E. Kovács, D.K. Pham, L. Mädler, N. Barsan, U. Weimar, Sens. Actuators B 158, 388 (2011)
L.P. Yang, X.Y. Zhou, L.F. Song et al., ACS Appl. Nano Mater. 1, 6327 (2018). https://doi.org/10.1021/acsanm.8b01529
F. Gyger, A. Sackmann, M. Hübner et al., Part. Part. Syst. Charact. 31, 591 (2014). https://doi.org/10.1002/ppsc.201300241
J.M. Walker, S.A. Akbar, P.A. Morris, Sens. Actuator B 286, 624 (2019). https://doi.org/10.1016/j.snb.2019.01.049
D. Degler, U. Weimar, N. Barsan, ACS Sens. 4, 2228 (2019). https://doi.org/10.1021/acssensors.9b00975
F. Shao, M.W.G. Hoffmann, J.D. Prades, J.R. Morante, N. Lopez, F. Hernandez-Ramirez, J. Phys. Chem. C 117, 3520 (2013). https://doi.org/10.1021/jp3085342
B. Cho, J. Yoon, M.G. Hahm et al., J. Mater. Chem. C 2, 5280 (2014). https://doi.org/10.1039/c4tc00510d
X. Han, M. Jin, S. Xie, Q. Kuang, L. Zheng, Angew Chem. Int. Ed. Engl. 48, 9180 (2009)
X. Sun, Y. Li, Angew Chem. Int. Ed. Engl. 43, 597 (2004). https://doi.org/10.1002/anie.200352386
T. Takeguchi, O. Takeoh, S. Aoyama, J. Ueda, R. Kikuchi, K. Eguchi, Appl. Catal. A 252, 205 (2003). https://doi.org/10.1016/S0926-860x(03)00418-6
Y. Masayoshi, T. Masaki et al., Sens. Actuators B 136, 99 (2009)
T.S. Wang, S.F. Zhang, Q. Yu et al., Sens. Actuator B 276, 262 (2018). https://doi.org/10.1016/j.snb.2018.07.020
B.Y. Huang, Z.X. Zhang, C.H. Zhao et al., Sens. Actuator B 255, 2248 (2018). https://doi.org/10.1016/j.snb.2017.09.022
L.L. Guo, F. Chen, N. Xie et al., Sens. Actuator B 272, 185 (2018). https://doi.org/10.1016/j.snb.2018.05.161
P. Sun, W. Wang, Y. Liu, Y. Sun, J. Ma, G. Lu, Sens. Actuators B 173, 52 (2012)
G.H. Mhlongo, D.E. Motaung, F.R. Cummings, H.C. Swart, S.S. Ray, Sci. Rep. 9, 9881 (2019). https://doi.org/10.1038/s41598-019-46247-z
P.G. Su, L.Y. Yang, Sens. Actuator B 223, 202 (2016). https://doi.org/10.1016/j.snb.2015.09.091
M. Shahabuddin, A. Sharma, J. Kumar, M. Tomar, A. Umar, V. Gupta, Sens. Actuator B 194, 410 (2014). https://doi.org/10.1016/j.snb.2013.12.097
N. Van Toan, C.M. Hung, N. Van Duy, N.D. Hoa, D.T.T. Le, N. Van Hieu, Mater. Sci. Eng. B 224, 163 (2017). https://doi.org/10.1016/j.mseb.2017.08.004
Y. Li, H.T. Ban, M.J. Yang, Sens. Actuator B 224, 449 (2016). https://doi.org/10.1016/j.snb.2015.10.078
S.G. Leonardi, W. Wlodarski, Y.X. Li, N. Donato, A. Bonavita, G. Neri, J. Alloy Compd. 781, 440 (2019). https://doi.org/10.1016/j.jallcom.2018.12.110
A.M. Al-Enizi, M. Naushad, A.H. Al-Muhtaseb et al., Chem. Eng. J. 345, 58 (2018). https://doi.org/10.1016/j.cej.2018.03.138
A. Rothschild, Y. Komem, Sens. Actuator B 93, 362 (2003). https://doi.org/10.1016/S0925-4005(03)00212-0
D. Koziej, M. Hubner, N. Barsan, U. Weimar, M. Sikora, J.D. Grunwaldt, Phys. Chem. Chem. Phys. 11, 8620 (2009). https://doi.org/10.1039/b906829e
Y.H. Zhang, Y.L. Li, F.L. Gong, K.F. Xie, H.L. Zhang, S.M. Fang, Phys. Chem. Chem. Phys. 21, 22039 (2019). https://doi.org/10.1039/c9cp04242c
D.P. Xue, P.T. Wang, Z.Y. Zhang, Y. Wang, Sens. Actuator B 296, 126710 (2019). https://doi.org/10.1016/j.snb.2019.126710
P. Bechthold, M.E. Pronsato, C. Pistonesi, Appl. Surf. Sci. 347, 291 (2015). https://doi.org/10.1016/j.apsusc.2015.03.149
Acknowledgements
The authors acknowledge M. L. for help with the TEM, XRD, and XPS characterizations. The authors W. Y., H. Z, and C. G. acknowledge the 2011 Zhejiang Regional Collaborative Innovation Center for Smart City.
Funding
This research was supported by the Zhejiang Science and Technology Foundation (LQ20F040006), 2011 Zhejiang Regional Collaborative Innovation Center for Smart City, and Research Foundation of Hangzhou Dianzi University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, W., Zeng, X., Wu, G. et al. Raspberry-like hollow SnO2-based nanostructures for sensing VOCs and ammonia. J Mater Sci: Mater Electron 31, 14165–14173 (2020). https://doi.org/10.1007/s10854-020-03971-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03971-x