Abstract
The normal and superconducting properties of Bi1.7Pb0.30Sr2Ca2−xLaxCu2Oy (Bi, Pb):2223 system with various x values (0.00 ≤ x ≤ 0.30) have been reported. It is found that the replacement of Ca2+ by La3+ up to 0.30 does not influence the phase purity of the pure system, while the orthorhombic distortion, excess oxygen, effective Cu valance and hole carriers/Cu ions are clearly affected. Further, the doping distance and crystal geometry factor are decreased, but the distance between neighboring Cu-atoms and density of excess doping are increased. Furthermore, the DTA diagrams show strong endothermic peaks Tm at 844.1, 848, 850.1 and 857.4 °C for pure and La samples, respectively. The mass loss determined by TGA displayed a sharp mass loss started at about 800 °C and extended to 1000 °C for all samples. Although the critical temperatures Tc of the samples are decreased by La insertion from 119 to 104, 71, and 53 K, the critical concentration for quenching superconductivity may be extended above 0.30. On the other hand, the Vickers hardness Hv is increased by La up to 0.30, but it is decreased by the applied load F. Additionally, a negative linear relation between Tm or HV and Tc is obtained as due to cooperative interactions, (Tc ∝ –Tm) i.e. there are an extended forces acting along such a wide temperature range. The Hv–F characters are divided into two linear parts; the first at lower loads (0.00–1.96 N) and the second at higher loads (2.94–4.90 N). The variation of surface energy ϒ against La is different according to the range of applied loads, while the elastic indentation de is decreased, and the resistance pressure Fo is increased. But, the values of Fo and de at higher loads are generally more than that of lower loads, while the vice is versa for the ϒ. Our results are discussed in terms of the balance between the hole carriers lost by La3+ with that introduced by excess oxygen in the Cu–O2 planes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is known that superconductivity, SC, phenomenon is cooperative in nature. Normally, cooperative phenomenon is that when there are several entities that are taking part to produce the physical phenomena in question. According to Matthias [1] superconductivity is one of the cooperative phenomenon that may correlate normal to the superconducting states properties. That is to say, the macroscopic properties such as melting temperature and mechanical properties are correlated to the critical SC temperature.
In the present study, we will consider Bi(Pb):2223 superconducting system with a critical temperature TC of 110 K, where it has three CuO2 planes and the superconductivity occurs at these planes, while the other layers such as Sr–O, CaO and Bi–O are non-superconductors [2, 3]. Due to large number of CuO2 planes and also the difficulty to mix of Bi(Pb):2212 minority phase with Bi:2223 majority phase, it is not easy to obtain a single-phase free from intergrowths in such a system, and consequently TC vary between 106 and 110 K [4, 5]. However, heat treatment during Bi(Pb):2223 synthesis may supports increasing the content of the Bi-2223 phase relative to the amount of the Bi-2212 phase [6, 7]. On the other hand, the partial substitution of Bi by Pb has been used to stabilize and improve the formation of its original phase, as well as the superconducting critical parameters [8]. For example, the highest values of the critical temperature, Tc, critical fields, Hc, and critical current, Ic, are obtained when Pb = 0.30 [9].
Generally, the unit cell of Bi (Pb):2223 compound with a ~ b = 0.544 nm and c = 3.600 nm consists of Bi1.7Pb0.30Sr2Ca2Cu3O10+δ unit and about 19 layers and planes. It is approved that the chemical formula of 10-oxygen atoms (10 O2−) needs at least 20 electrons provided by 1.7Bi3+ + 0.30Pb2+ + 2Sr2+ + 2Ca2+ + 3Cu2+ ions. To be superconductor, this compound should be over doped with excess oxygen atoms by transforming 3Cu2+ ions into 3Cu3+ ions, and therefore, the electronic arrangement becomes 1.7Bi3+ + 0.30Pb2+ + 2Sr2+ + 2Ca2+ + 3Cu3+ + 11O2− [2].
The melt process is considered as the highly essential method for optimization of thermal behavior of superconductors through solid–liquid phase diagram. Furthermore, the transition points such as melting, sublimation and solidification can be obtained. Differential thermal analysis (DTA) and thermo-gravimetric analysis (TGA) are considered for identifying the above points [10]. TGA is a powerful technique to explore the thermal stability of a material through accurately monitoring the weight of a sample and heating at a constant rate. The change in the sample weight during thermal treatments is attributed to a specific material response due to thermal stress. This is the perfect way for exploring, in detail, decomposition temperatures and ensuring a material performs adequately in a given temperature range. DTA is used for measuring the temperature difference between the sample and the standard reference for obtaining solid–liquid phase diagram. However, DTA up to 1000 °C with 10 °C/min heating rate was considered earlier to investigate the amount of Bi (Pb):2223 phase relative to the amounts of other phases such as Bi (Pb):2212 phase. The DTA show that the structure of Bi (Pb):2223 phase is more stable and can be easily prepared as stable pure phase rather than Bi (Pb):2212 phase [11].
Generally, chemical substitution plays a crucial role in high Tc superconductors, and consequently the substitution into (Bi, Pb):2223 system have been carried out to improve their normal and superconducting properties [12,13,14,15,16,17,18,19,20,21,22,23,24,25]. However, it is approved that the superconducting properties depend on the density of holes carriers in the CuO2 planes as well as the effective Cu-valance [26,27,28]. Therefore, the substitution of Ca2+ ions by RE3+ elements (rare earth in the lanthanum series) leads to a decrease of the formal Cu-valence state, and ultimately Tc decreases [29, 30]. The excess of positive charge for trivalent RE3+ cation, as compared to divalent Ca2+ cation, causes repletion between CuO2 planes, thereby the separation between these planes may be increased. Furthermore, the increase of RE content induces excess oxygen incorporated between the Bi2O3 double layers [31]. In this respect, (Bi, Pb):2223 system holds some advantage in that the oxygen stoichiometry is relatively invariant with respect to RE3+ substitution when the samples are prepared in an identical environment [32,33,34].
Most of the studies have concluded that even though RE3+ improves the stability of Bi: 2223 structure, the critical parameters show a degrading tendency as the doping concentration increases [19, 35,36,37,38,39]. On the other hand, very few studies show an improvement of Tc in Bi(Pb):2223 systems [40,41,42,43,44]. Regardless of the RE3+ elements which already show superconductivity in the RE: 123 phases such as Y, Gd, Sm and Nd in lanthanide series while only few studies were based on La. Additionally, the ionic size of La3+ (1.18 Ẳ) is higher than that of Ca2+ (1.12 Ẳ), if the substitution occurs at the same eightfold coordination sites. Although, the substitution of La at Ca position in BSCCO systems show a decrease of the superconducting properties, a question mark should be considered as due to Tc variations by such substitution. Anyhow, the answer is still unclear and needs to pay more attentions, especially for higher La3+ content that is up to 0.30 as due to the non-superconducting phases of Bi2Sr2CaLaCu3Oy compound [45].
On the other hand, high Tc superconductors, HTS, have relatively poor mechanical properties, which may limit their practical applications. In this aspect, Vickers microhardness testing is considered as a convenient method for investigating the mechanical properties of bulk specimens. Where the elastic deformation is observed when the deformation is due to very small-applied loads. While above a critical loads a plastic (irreversible) deformation is produced. Generally, indentation size effect (ISE) occurs when the microhardness decreases as the applied load increases [46, 47]. While, the reverse indentation size effect occurs when the microhardness increases with the applied load [45,46,47]. However, the improved mechanical properties of Bi:2223 system may be achieved by substitution of RE3+ ions at Ca2+ site [48, 49]. It is found that RE3+ substitution improve the connection between grains, and consequently the mechanical resistance is increased [10, 50,51,52].
As an extension to the above works, a systematic study on the normal and superconducting properties of Bi1.7Pb0.30Sr2Ca2−xLaxCu2Oy (Bi, Pb):2223) with various, x, values (0.00 ≤ x ≤ 0.30) is reported. On the light of the present work we are able to increase the Tc of pure sample to 119 K. Further, it is shown that although the superconducting critical temperatures are decreased by La3+ substitution, the critical concentration for quenching superconductivity is increased to about 0.50. Furthermore, the relation between the normal state properties, such as melting temperature, Tm, hardness, HV, Young’s modulus, E, and yield strength, Y, and the superconducting critical temperature state property, Tc, are studied and correlated to shade some light on their association. Moreover, the distance between neighboring Cu-atoms, density of excess doping, decomposition temperature, hole carries/Cu ions. However, these findings are discussed in terms of the resulting hole carriers due La3+ and excess oxygen.
2 Experimental
The required amounts of Bi2O3, SrO, La2O3, CaCO3 and CuO oxides and carbonates of 4 N purity Bi1.7Pb0.30Sr2Ca2−xLaxCu2Oy (Bi, Pb):2223) are thoroughly mixed and calcinated at 825 °C in air for 24 h. The calcination process is repeated three times with intermediate grinding at each stage. The resulting powder regrounded, and pressed into pellets at a force up to 10 tons, then sintered in air at 850 °C for 150 h and cooled down to room temperature at the rate of (5°/min). For optimizing the excess oxygen in the samples, the pellets are finally annealed in air at 850 °C for 20 h and left in the furnace for slow cooling to room temperature. The phase purity of the samples is examined by using X-ray diffraction pattern (PhilipsPW-1700) with Cu–Kα radiation of ave length λ = 1.5418 Ẳ at 40 kV and 30 mA settings, and the data diffraction angle range (20°–70°) with a step of 0.06 in order to evaluate crystalline phase and crystallite orientation. The DTA and TGA thermal analysis are performed from room temperature up to 1000 °C with heating rate of 10 °C/min. The melting behaviors of the samples are analyzed by Schimadzu differential thermal analyzer-50 (DTA). The electrical resistivity of the samples is obtained by using the standard four-probe technique in closed cycle cryogenic refrigerator within the range of (18–300) K (Displex) employing helium gas. A 1.0 mA dc current, I, is supplied by a digital Keithley 6221 constant current source and the dc voltage drop, V, in the normal and reverse directions across the sample is measured by dc Keithley 181 digital nanovoltmeter. High-quality silver paint is used for electrical ohmic contacts. A calibrated chromel versus gold with 0.07 at.% iron (Kp-Au 0.07 at.% Fe) thermocouple is used for recording the sample temperature. The electrical resistivity of the sample is obtained using the relation,, where w, L and t are the sample width, length and thickness, respectively. Finally, the Vickers microhardness of the samples at room temperature are determined using manual microhardness tester model IN-412A with an applied loads of (0.49–10 N).
3 Results and discussion
3.1 Structural analysis
The theoretical and measured densities ρth and ρexp of the samples are listed in Table 1. ρth is calculated from XRD analysis using the relation, \(\rho_{{{\text{th}}}} = \frac{M}{{2N_{{\text{A}}} a^{3} }}\), where NA is the Avogadro's number, M is the molecular weight of the sample and a is the unit cell lattice parameter. While, ρexp is obtained for the samples using Archimedes principle in terms of the buoyant force FB and volume using the relation, \(\rho_{\exp } = \frac{{F_{B} }}{gV}\). It is clear that both values increased by increasing La content, however the values of ρth are higher than that of ρexp. The porosity of the samples, PS = [1—(ρexp/ρth)], listed in Table 1 is decreased by La content due to decreasing the vacancies [53, 54].
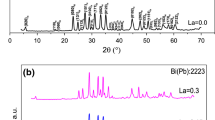
It is evident from XRD pattern shown in Fig. 1 that all samples are single phase and are free from any impurity phases. The peaks of high intensity indicated by H (hkl) belong to the Bi(Pb):2223 phase, while the peaks of very low intensity indicated by L (hkl) intensities such as (131), (115), (019) (240), (172), (310) and (3110) belong to Bi(Pb):2212 phase. However, the composition of low 2212 superconducting phase (Tc = 89 K) is normally formed as a minority phases in 2223 high phase, and cannot be consider as an impurity phase [22, 23, 51]. Furthermore, these peaks have very lower intensities as indicated above.
The volume fractions of the 2223 phase (V2223) in the samples are determined by using the relation [55, 56]:
where I2223 is the peak intensity of 2223 phase, I2212 is the peak intensity of 2212 phase and Iother is the peak intensity of any other phases. As listed in Table 1, the V2223 fraction of the samples are 78.58%, 70.08%, 74.75% and 80.73%, respectively.
The average crystallite size Dhkl is evaluated in terms of Scherer's equation [57,58,59]:
where λXRD = 15.418 nm, ∆θ is the half maximum line width, θ is the Bragg angle and k = 0.9. By using the Lorentz square method, the Dhkl values are listed in Table 1. On the other hand, the dislocation density is given as, β = 1/D2 and are listed in Table 1 as well, indicating that the samples have very few lattice defects and are of good crystalline quality [60].
The lattice parameters a, b and c are listed in Table 1. The lattice parameters a and c are gradually decreased by increasing La content, while "b" increases. Similar behavior has been reported previously during substitution of rare-earth elements at Ca sites in Bi (Pb):2223 system such as Y, Er, Dy and/or Nd [20,21,22,23]. However, the decrease in the "c" parameter may be related to the amount of excess oxygen arising from the replacement of 2CaO by La2O3. The BiO double layers take up this excess oxygen and causing a tighter binding in these layers [38, 42, 61]. Consequently, the length of the in-plane Cu–O bond is decreased and helpful for reducing the "a" parameter. Otherwise, the increase in the "b" parameter is related to the change of hole carrier concentration per Cu ion within these planes [53,54,55,56]. This together with the ratio (c/a) is generally used to characterize the Jahn–Teller distortion of the oxygen octahedron around Cu2+ [62, 63]. Alternatively, the orthorhombic distortion, OD = (b − a)/a, given in Table 1 is increased by increasing La content, although c/a is decreased. The change of the unit cell parameters indicates that La release the John–Teller distortion of the CuO6 octahedron markedly [64].
Furthermore, the oxygen content, y, is obtained by titration using the following formula [64]:
where V1, V2 are the volumes of Na2S2O3 solution and m1, m2 are the masses of the sample during the first and second titration. The oxygen content listed in Table 2, indicating that La assist more excess oxygen to the system (y is higher than 10 for all samples). On the other hand, the effective Cueff valance can also be obtained with the help of the valence state of the compound elements (Bi+3, Pb+2, Sr+2, Ca+2, La+3 and O−2) using the relation:
where x is the La content. Increasing La content as well as y increases the values of Cueff, as listed in Table 2.
The formation of 2223 high Tc phase generally requires the existence of Cu3+ ions in the superconducting planes as well as excess oxygen in/or near to CuO2 planes [65,66,67,68]. It is supposed that the Coulomb potential caused by excess oxygen atoms pins and distributes the doped holes in the CuO2 planes to minimize their total energy as possible [3]. The density of excess doping, \(\sum^{ - 1}\), due to excess oxygen is given by; \(\sum^{ - 1} = (1 - 10y^{ - 1} )\) [69, 70]. The excess oxygen can be also converted into a doping distance, d, given by; \(d = a{\sqrt{\sum}} \), where \(\sum\) is the hole doping density. Furthermore, the distance, z, between two neighboring Cu-atoms can be calculated by:
Moreover, the crystal geometry factor (CGF) of the area of superconducting planes can be obtained by [3, 69, 70]:
where n is the number of CuO2 planes (n = 3 for the 2223 phase) and meff is the effective mass of the hole doped cup rates (meff = 2me = 18.210–31 kg). We basically used the above mathematical calculations to check the response of La cations in pure 2223 system. The calculated values of d, \(\sum^{ - 1}\), z and CGF for La free and La containing samples are listed in Table 2. It is clear that both d, z and CGF are gradually decreased by increasing La content, while \(\sum^{ - 1}\) is increased. Decreasing the doping distance d and also increasing the density of excess hole doping \(\sum^{ - 1}\) by La are considered as the clear evidence for the increase of the hole carriers concentration, and consequently the distance between Cu-atoms z is decreased as obtained.
3.2 DTA, TGA analysis
Figure 2a shows the DTA patterns of the samples. It is noted that by increasing the temperature above RT, some exothermic and endothermic peaks with lower intensity are observed. These peaks are shifted to higher temperature as La content increases. They are shifted from the temperature range of (30–200 °C) to (200–450 °C), (450–700 °C) and (650–800 °C) for La pure and substituted samples. This is corresponding to crystallizations of 2201, 2212 and (Sr,Ca)2CuO3 phases, and also transformation of 2212 to 2223 phases. While with increasing temperature to about 800 °C, the tendencies of 2212 crystallization is completely disappear. Interestingly, the strong endothermic peaks listed in Table 3 occurred at 848.55, 855.24, 857.93 and 874.05 °C as La content increases, while no other strong peaks could be recorded, which reinforces the purity of the samples. This may be due to the decomposition reaction of 2223 superconducting phase. The increase of melting temperature by La may be attributed to increasing the optimum temperature of the mixture due to La, which facilitates the growth of the 2223 phase. Such observed increase in Tm emphasizes the role of La addition on the internal structure of the examined samples. This behavior will be correlated in terms of the SC, Tc, values in the following section. On the other hand, the activation energy related to the endothermic heat are listed in Table 3, which is increased from 0.651 J for La-free sample to 2.91, 2.07 and 1.49 J as La content increases, in agreement with previously reported results [71, 72]. These results indicated that La addition increases the melting temperature of 2223 phase without producing any other additional compound as indicated from the above XRD results.
The TGA graph on the other hand shown in Fig. 2b indicates that the La containing samples with La = 0.00, 0.05 and 0.30, respectively, displaying a gradual mass loss starting from 40 °C to about 600 °C, followed by a significant mass gain at about 800 °C, followed by a sharp mass loss at about 1000 °C. However, the sample with x = 0.15 shows a mass gain starting from 40 °C up to about 400 °C, followed by a mass loss up to about 1000 °C. Furthermore, the temperature region of mass gain above 600 °C is completely disappeared. It is obvious that a gradual mass loss is due to de-oxidization of the samples, where the mass gain is due oxidation. However, a sharp mass gain close to 800 °C may be due to the decomposition temperature as indicated by the DTA analysis. Nevertheless, the increase of mass loss/mass gain is due to more excess of oxygen introduced by La doping [10, 11, 73]. Additionally, the oxygen content of the samples is calculated in terms of TGA mass loss for YBCO123 (Y:123) using the relation [74];
where α is a parameter (α = 3.5) for Y:123 system, x is the doping content, m is the mass of the sample, l is the average mass loss and M is the atomic weight. Substituting by the values of l and m deduced from TGA and also y obtained from iodometric titration, we found that (α = 8.08) for the 2223 phase.
3.3 Resistivity measurements
Figure 3 depicts the electrical resistivity versus temperature for the samples. From this Figure it can be observed that the resistivity decreases almost linearly from room temperature and beyond which it turns to the superconducting state at the critical temperature Tc. It is also noted that all samples exhibit a clear metallicity which starts to decrease slightly by La addition. The linear part of the ρ(T) curves has a positive slope dρ/dT and its extrapolation to T = 0 K provides the residual resistivity ρo. Usually, ρo is connected to the impurity scattering and dρ/dT is connected with carrier–carrier scattering [21, 75]. However, the values of normal state resistivity ρ300 at 300 K, residual resistivity ρo at 0 K, Tc and width of transition ΔTc for all samples are presented in Table 4. It is noted that the values of both ρo,ρ300 and ΔTc are increased by La, while, Tc and dρ/dT is decreased. Interestingly, the Tc values are 119 K for pure sample and decreased to 104, 71 and 53 as La content increases. Therefore, we could improve the Tc of pure sample up to 119 K and also 104 K for La = 0.05. (The highest reported value is 110 K for the pure Bi-2223 sample [2,3,4]). This increase may be due to the final annealing period which is done during sample synthesis.
Next it is useful to consider the perspective reported in refs [39, 61] concerning the substitution of RE3+ in exchange of Ca2+ in Bi(Pb):2223 system. The reported data of reduced critical temperature [Tc(x)/Tc(0)] against RE3+ content are drawn and shown in Fig. 4a. For comparison, our present results are included within the same Figure. It is clear that the critical concentration xc of La for quenching superconductivity can be extended above 0.30, which is more than that reported earlier (0.35) for Gd, Sm and Nd elements [20, 22, 43, 45]. This behavior indicates that La has a higher solubility in Bi(Pb):2223 system and less detrimental to the superconductivity. However, different solubility’s of rare-earth elements RE3+ in Bi(Pb):2223 system can be understood by a comparison of their ionic radii at eightfold coordination with respect to Ca2+ [76]. The reported data have indicated that the solubility of RE3+ in Bi(Pb):2223 system decreased as one move towards the rest of the lanthanide series, which are not given in the present case.
As reported above by Matthias [1] superconductivity is related to one of the cooperative phenomenon in nature and which may be correlated, to some normal state properties. This is examined here as shown in Fig. 4b which shows the relation between Tc as obtained from electrical conductivity measurements and Tm as obtained from DTA. As can be seen from the Figure, an inverse linear relation between Tm and Tc is obtained and is given by; Tc (K) = 2200 − 2.462 Tm, as estimated. This behavior is completely consistent with the reported data for the inverse linear relation between Tc and Tm for Y:123, Bi:2223 and TI:2223 superconducting systems [77]. Although the Tc values are, respectively, 92, 110 and 125 K for the systems, the Tm are decreased to 1283, 1140 and 1080 K. These suggest that to get higher Tc, a eutectic compound of lower melting point should be looked for.
On the other hand, the phase diagram for undoped and doped high Tc systems is described by the following parabolic formula [78]:
Tcx and Tcm are the critical and maximum critical temperatures, respectively; p is the hole-carrier concentration per Cu cation. Therefore, p, is established from the values of Tc and Tcm listed in Table 4. It is clear that, p, is increased from 0.16 for pure sample to 0.20, 0.23 and 0.24 for La containing samples. However, this behavior occurs as a result of more positive charges transferred to the CuO2 planes. This of course disagrees with the general role of substitution due to replacing Ca2+ by La+3, and therefore, p, should be expected to decrease. However, according to the mechanism of excess oxygen in BSCCO system, the electrons are transferred from Cu to BiO layer leading to the formation of holes on Cu and electrons on Bi as follows; Bi3+ + Cu2+ → Bi3−x + Cu2+x [79]. These changes in the valence state of Bi are owing to excess oxygen and is reflected as the change of hole carriers of the system. To clarify this point according to the role of substitution, we supposed that La decreases the hole carrier concentration, and at the same time the excess oxygen introduces some excess holes in the Cu–O2 planes, and consequently the effective (net) number of holes will be increased. This can be supported also by decreasing the doping distance and increasing the density of excess doping and excess oxygen as discussed in the above section.
3.4 Microhardness measurements
The apparent Vickers microhardness Hv is calculated by the following relation [80]:
where Hv is given by GPa, dp is the diagonal length of the indentation in µm and F is the applied load in N (F = 0.49, 0.981, 1.962, 2.942 and 4.904 N). It is obvious from Hv versus F curves shown in Fig. 5 that Hv decreases as the F increases up to 4.904 N. This behavior is nonlinear and usually due to increase in the mechanical penetration depth where the inner layers effect becomes more prominent as well as the well-known indentation size effect [80, 81]. Interestingly, at fixed F, the Hv is increased as La content increases [Hv α (1/Tc)] shown in Fig. 5b. Anyhow, the behavior of Hv against rare earth element RE3+ is normally controlled by the formation of impurity non-superconducting phases and irregularities. These phases and irregularities are mainly distributed at the grain boundaries, causes distortion of the bonding strength, and consequently the Hv decreases [45]. However, as indicated in the XRD analysis, we could not report these phases and also no significant change in the structure distortion c/a listed in Table 1 is nearly the same for all samples. Therefore, the increase of Hv values by La is probably due to the intercalation of La between superconducting grains in compositions which may be provide a plastic-flow region that allows relaxation of undesirable stresses resulting from the grain anisotropy of superconductors. This behavior is consistent with the reported elsewhere and indicated that Hv could be achieved by replacement of Ca by Y and Cd in BSCCO superconducting systems [40, 49, 51].
To test the original suggestion made by Matthias [1] for cooperative phenomenon. which link a normal state property to a superconducting property. Following Mathias [1] argument the relation between Vickers hardness and SC critical temperature, TC, is tested as shown in Fig. 5b at two different applied loads. The obtained relation is a negatively sloped liner curves. This result indicates that both properties are strongly correlated and demonstrate a cooperative phenomenon as suggested earlier [1]. The linearity is tested and the following relations were produced to describe this phenomenon;
In addition, the elastic Young’s modulus, E, and yield strength, Y, on the other hand are related to the Hv as follows [81, 82]
However, a similar behavior of both, E, and, Y, against, F, and La content is obtained and shown in Fig. 6a and b as well as Hv behavior. This is probably due improving the roughness and interlayer bonding between grains when Ca is replaced by La.
Otherwise, the diagonal length dp is strongly dependent on the applied load F according to [58, 83,84,85]:
where Ht is the true hardness and γ is the surface energy. The plots of (F/dp) against dp for lower and higher loads are shown in Fig. 7a and b, in which the slope represents Ht and the intercept represents γ. It is noted that the slope of the linear plot is positive for all samples at lower loads (0–1.962 N), while it is changed to negative at higher loads (2.94–4.90 N). The different values of Ht and γ are listed in Table 4. It is clear that Ht has a similar behavior of Hv against La concentration which indicating a decrease of crack faces and supporting hardness improvements. But the values of γ are decreased at lower loads from 0.01 N/μm for pure sample to 0.009, 0.008, 0.007 (N/μm) for La substituted samples, and from 0.019 N/μm to 0.021, 0.023, 0.027 N/μm at higher loads. From atomistic point of view, the more compacted surface the higher hardness and thus having lower surface energy as obtained. This is right for lower loads, but it is unclear at higher loads at present.
The indentation size effect observed in the present case can be explained by two different methods [86, 87]. The first method assumes that the indentation contains an elastic portion of the deformation which is supposed to relax upon loading beside plastic deformation. This can be accounted by adding an elastic component de to the measured in elastic dp, supposing they are in the same direction, as follows [37, 88, 89];
The plot of dp versus F1/2 is shown in Fig. 8a and b, in which the slope may be represented by [(1854.4)1/2(Ht)−1/2], and the vertical intercept represents the de. It is clear that de values listed in Table 5 are decreased as La content increased, but their values at higher loads are more than that of lower loads. This of course emphasizes the hardness suppression as discussed above. It is decreased at lower loads from 26.76 μm for pure sample to 21.57, 15.73, 13.28 μm for La substituted samples, and from 421.66 to 406.91, 382.64, 379.55 μm at higher loads.
The second method considers energy dissipative processes and Ht can be defined by subtracting a dissipative part F0 from the applied load F as follows [90, 91]:
The slope of linear plot of F against dp2 shown in Fig. 9 (a, b) represented by (Ht/1854.4) while the intercept represents the resistance pressure Fo. It is clear that Fo values listed in Table 5 are slightly increased by La concentration, but their values at higher loads are more than that of lower loads. It is increased at lower loads from 0.201 N for pure sample to 0.302, 0.261 and 0.216 N for La containing samples, and from 2.28 to 2.31, 2.32, 2.39 N at higher loads.
Summing up the following points as obtained for the La substitution in place of Ca in the Bi (Pb):2223 ceramic superconducting system: (i) A clear 2223 majority single phase. (ii) increasing the carriers per Cu ion, effective Cu valence, critical concentration for quenching superconductivity, hardness and resistance pressure. (iii) decreasing the doping distance, crystal geometry factor, critical temperature, surface energy and elastic indentation. (vi) shifting endothermic peaks towered higher temperatures; (v) a sharp mass loss starting at about 800 °C.
There are several possible reasons for Tc suppression by La substituted at Ca site in Bi (Pb):2223 system as obtained in the present work. The first indicating that Tc depression is not related to the phase purity or bad microstructure in the form of secondary phases rich in Sr [92, 93]. The second reason indicated that the excess oxygen causing a tighter binding in the CuO2 planes, and therefore, c-parameter orthorhombic distortion, excess oxygen, distance between Cu-atoms, density of excess doping, hole is reduced [60]. The third indicates that although La decreased the hole carriers, the excess oxygen is more than enough to compensate the variation of charge in the system, and consequently the number of holes in the CuO2 planes increases. The fourth reason that may be put forward, as due to the verified correlations between the melting temperature, hardness and SC critical temperature. These properties all have an origin related to some sort of electron–phonon interaction as all phenomenon are related to such a physical source; i.e. it may be said that there are an extended forces acting along such a wide temperature range.
Finally, the consistency of these points gives a fair degree of certainty to the suggestion of La substitution in place of Ca in Bi (Pb):2223 system.
4 Conclusion
Structural and different properties of Bi1.7Pb0.30Sr2Ca1−xLaxCu2Oy superconductor with various x values are investigated. We have shown that the replacement of Ca2+ by La3+ increased the excess oxygen, effective Cu valance and hole carrier per Cu ion. Further, the doping distance and crystal geometry factor are decreased, while the distance between Cu-atoms and density of excess doping are increased. Furthermore, a strong shift of endothermic peaks to higher temperatures and a sharp mass loss are obtained. Although the critical temperatures Tc are decreased by La, the critical concentration for quenching superconductivity may be extended above 0.30. Moreover, an inverse linear relation between Tm and Tc given by; Tc (K) = 2200 − 2.462 Tm could be obtained. On the other hand, Vickers hardness Hv is decreased by the applied loads F, but it is increased by increasing La content, i.e. Hv ∝ 1/Tc. Although the variation of surface energy against La is found to be load dependent, the elastic indentation de are decreased and the resistance against pressure Fo is increased. But the values of Fo and de at higher loads are generally more than that of lower loads, while the vice is versa for the ϒ. The balance between the hole carriers lost by La3+ with that introduced by excess oxygen resulting for increasing the effective (net) of hole carriers. This of course helps for altering the quenching of superconductivity by La3+, which highlights the present work.
References
B.T. Matthias, The Empirical Approach to Superconductivity, Chapter 7, in Applied Solid State Physics, ed. by W. Low, M. Schieber (Plenum Press, New York, 1970)
H. Maeda, Y. Tanaka, M. Fukutomi, T. Asano, Jpn. J. Appl. Phys. 27(2), L209 (1988)
H.P. Roesera, F. Hetfleischa, F.M. Huberb, M.F. Von Schoenermarka, M. Steppera, A. Moritza, A.S. Nikoghosyanc, Acta Astronaut. 63, 1372 (2008)
H. Eisaki, N. Kaneko, D.L. Feng, A. Damascelli, P.K. Mang, K.M. Shen, Z.X. Shen, M. Greven, Phys. Rev. B 69, 064512 (2004)
P. Bordet, J.J. Capponi, C. Chaillout, J. Chenavas, A.W. Hewat, E.A. Hewat, J.L. Hodeau, M. Marezio, A. Narliker (Ed.), vol. 2, Nova Science Publisher, p. 171 (1991).
A. Amirabadizadeh, S. Memarzadeh, N. Tajabor, H. Arabi, World J. Condens. Matter Phys. 2, 148 (2012)
I.H. Gul, M.A. Rehman, M. Ali, A. Maqsood, Physica C 432(1–2), 71 (2005)
A. Ehmann et al., J. Less Common Met. 151, 55 (1959)
A. Jeremie, K.A. Yadri, J.C. Grivel, R. Fiñkiger, Supercond. Sci. Technol. 6, 730 (1993)
M.A. Aksan, M.E. Yakinci, J. Alloy Compd. 433, 22 (2007)
C.H. Hwang, G. Kim, Supercond. Sci. Technol. 5, 586 (1992)
H.A. Qureshi, M. Arshad, K.S. Durrani, Waqas H 94, 175 (2008)
S.B.M. Dogruer, G.Y.A. Varilci, C.T.Y. Zalaoglu, J. Supercond. Novel Magn. 25, 847 (2012)
A.V. Pop, G. Ilonca, D. Ciurchea, V. Pop, I.G. Deac, Phys. B 284–288, 1101 (2000)
M.A.M.C. Kaya, B. Ozcelik, B. Ozkurt, A. Sotelo, J. Mater. Sci. Mater. Electron. 24, 1580 (2013)
S. Vinu, P.M. Sarun, A. Biju, R. Shabna, P. Guruswamy, Supercond. Sci. Technol. 21, 3 (2008)
M.A.S. Aksan, Y. Balci, M.E. Yakinci, Mater. Chem. Phys. 106, 428 (2007)
C.A.M. dos Santos, S. Moehlecke, Y. Kopelevich, A.J.S. Machado, Physica C 390, 21 (2003)
A.Y. Ilyushchkin, T. Yamashita, L. Boskovic, I.D.R. Mackinnon, Supercond. Sci. Technol. 17, 1201 (2004)
A. Sedky, Physica C 468, 1041 (2008)
S. Singh, Physica C 294, 249 (1998)
K. Nanda Kishore, S. Satyavathi, M. Muralidhar, O. Pena, V. Hari Babu, Physica C 252, 49 (1995)
A. Sedky, J. Phys. Chem. Solids 70, 483 (2009)
A. Sedky, J. Alloys Compd. 499, 238 (2010)
D. Marconi, G. Stiufiuc, A.V. Pop, J. Phys. Conf. Ser. 153, 012022 (2009)
B. Chevalier et al., Mater. Sci. Eng. B 2, 277 (1989)
M.A. Aksan, S. Altin, M.E. Yakinci, A. Guldeste, Y. Balci, J. Mater. Sci. Technol. 27(1), 314 (2011)
S. Altin, M.A. Aksan, M.E. Yakinci, Mater. Chem. Phys. 133(2–3), 706 (2012)
B. Jayaram, P.C. Lanchester, M.T. Weller, Physica C 160, 17 (1989)
A. Coskun, A. Ekicibil, B. Ozcelik, K. Kiymac, Chin. Phys. Lett. 21, 2041 (2004)
P. SumanaPrabhu, M.S. RamachandraRao, U.V. Varadaraju, G.V. SubbaRao, Phys. Rev. B 50, 6929 (1994)
V.P.S. Awana, S.K. Agarwal, R. Ray, S. Gupta, A.V. Narlikar, Physica C 43, 191 (1992)
J.M. Tarason, P. Barboux, G.W. Hull, R. Ramesh, L.H. Greene, M. Giroud, M.S. Hegde, W.R. Mckinnon, Phys. Rev. B 39, 4316 (1989)
K. Koyama, S. Kanno, S. Noguchi, Jpn. J. Appl. Phys. 29, L53 (1990)
H. Jin, J. Kotzler, Physica C 325, 153 (1999)
N. Hudakova, Physica C 406, 58 (2004)
A.D.M. dos Santos, G.S. Pinto, B. Ferreira, A.J.S. Machado, Physica C 354, 388 (2001)
S. Kobayashi, N. Suzuki, Physica C 185–189, 869 (1991)
Q. Cao, K.Q. Ruan, S.Y. Li, X.H. Chen, G.G. Qian, L.Z. Cao, Physica C 334, 237 (2000)
S.M. Khalil, J. Phys. Chem. Solids 64, 855 (2003)
X. Sun, X. Zhao, W. Wu, X. Fan, X.-G. Li, H.C. Ku, Physica C 307, 7 (1998)
X. Zhao, X. Sun, X. Fan, W. Wu, X.G. Li, S. Guo, Z. Zhao, Physica C 307, 265 (1998)
M. Yilmazlar, H.A. Cetinkara, M. Nursoy, O. Ozturk, C. Terzioglu, Physica C 442, 101 (2006)
K.A. Jassim, T.J. Alwan, J. Supercond. Nov. Magn. 22, 861 (2009)
H. Aydin, O. Cakiroglu, M. Nursoy, C. Terzioglu, Chin. J. Phys. 47(2), 192 (2009)
S.M. Khalil, J. Phys. Chem. Solids 62(3), 457 (2001)
A.A. Elmustafa, D.S. Stone, J. Mech. Phys. Solid 51, 357 (2003)
K. Sangwal, Mater. Chem. Phys. 63, 145 (2000)
M.M. Abbas, Int. J. Curr. Eng. Technol. 5(3), 1908 (2015)
S.M. Khalil, Smart Mater. Struct. 14, 804 (2005)
S.M. Khalil, J. Low. Temp. Phys. 143(112), 31 (2006)
I.K. Schuller, J.D. Jorgfnsen, MRS Bull. 14, 27 (1989)
S.M. Khalil, A. Sedky, Phys. B 357, 299 (2005)
A. Simon, P.S. Mukherjee, M.S. Sarma, A.D. Damodaran, J. Mater. Sci. 29, 5059 (1994)
S.A. Halim, S.B. Mohamed, H. Azhan, S.A. Khawaldeh, H.A.A. Sidek, Physica C 312, 78 (1999)
M.S. Lee, K.Y. Song, Supercond. Sci. Technol. 15, 851 (2002)
A.I. Malik, S. Celebi, S.A. Halim, Physica C 377, 421 (2002)
J. Gong, J. Wu, Z. Guan, J. Eur. Ceram. Soc. 19(15), 2625 (1999)
R. Singth, A. Gupta, S.K. Agarwal, D.P. Singh, A.V. Narlikar, Supercond. Sci. Technol. 10, 1 (1997)
F. Munakata, T. Kawano, H. Yamauchi, Y. Inoue, Physica C 190, 471 (1992)
S. Sedky, A. Gupta, V.P.S. Awana, A.V. Narlikar, Phys. Rev. B 58(18), 12495 (1998)
P. Mandel, A. Poddar, B. Ghosh, P. Choudhury, Phys. Rev. B 43(16), 13102 (1991)
A. Biju, P.M. Sarun, R.P. Aloysius, Physica C 433, 68 (2007)
W.M. Chen, X.S. Wu, J.F. Geng, J. Chen, D.B. Chen, X. Jin, S.S. Jiang, J. Superconduct 10(1), 41 (1997)
J. Hwang, T. Timusk, G.D. Gu, Nature 427, 714 (2004)
F. Jean, G. Collin, M. Andrieux, N. Blanchard, J.F. Marucco, Physics C 339, 269 (2000)
M.R. Norman, H. Ding, M. Randeria, J.C. Campuzano, T. Yokoya, T. Takeuchi, T. Takahashi, T. Mochiku, K. Kadowaki, P. Guptasarma, D.H. Hinks, Nature 392, 157 (1998)
J.W. Alldredge, J. Lee, K. McElroy, M. Wang, K. Fujita, Y. Kohsaka, C. Taylor, H. Eisaki, S. Uchida, P.J. Hirschfeld, J.C. Davis, Nat. Phys. 4, 319 (2008)
H.P. Roesera, F.M. Huberb, M.F. von Schoenermarka, A.S. Nikoghosyanc, Acta Astronaut. 65, 489–494 (2009)
S. Bernik, M. Hrovat, D. Kolar, Supercond. Sci. Technol. 7, 920 (1994)
T. Honda, T. Wada, M. Sakai, M. Miyanaga, N. Nishikawa, S. Uchida, K. Uchinokawa, S. Tanaka, Jpn. J. Appl. Phys. 27, L545 (1988)
A. Broido, J. Polym. Sci. A 7, 1761 (1969)
R. Venkataramani, S.P. Garg, S. Mazumder, Bull. Mater. Sci. 14(3), 665 (1991)
S. Ravi, V. SeshuBai, Phys. Rev. B 49, 13082 (1994)
R.K. Nkum, W.R. Datars, Supercond. Sci. Technol. 8, 822 (1995)
M.R. Presland, J.L. Tallon, R.G. Buckley, R.S. Liu, N.E. Floer, Physica C 176, 95 (1991)
A.A. Bahgat, High TC Updata (USA) Technical Report 6(19), 5 (1992). https://doi.org/10.13140/RG.2.2.13407.25761
P. Konsin, B. Sorkin, Supercond. Sci. Technol. 13, 301 (2000)
A. Leenders, M. Ullrich, H.C. Freyhardt, Physica C 279, 173 (1997)
C. Veerender, V.R. Dumke, M. Nagabhooshanam, Phys. Status Solid A 144, 199 (1994)
D. Tabor, The Hardness of Metals (Clarendon, Oxford, 1951)
B.R. Lawn, T.R. Wilshaw, J. Mater. Sci. 10, 1049 (1975)
H.A. Cetinkara, M. Nursoy, O. Ozturk, C. Terzioglu, Physica C 442, 101 (2006)
J.B. Quinn, G.D. Quinn, J. Mater. Sci. 32, 4331 (1997)
A.K. Dutta, N. Narasaiah, A.B. Chattopadhyaya, K.K. Ray, Ceram. Int. 27, 407 (2001)
B.Y. Farber et al., Superconductivity 4, 2296 (1991)
F. Frohlinch, P. Grau, W. Grellmann, Phys. Status Solidi 42, 79 (1997)
H. Li, R.C. Bradt, J. Mater. Sci. 22, 917 (1993)
C. Hays, E.G. Kendall, Metallography 6(4), 275 (1973)
J. Gong, J. Wu, Z. Guan, J. Eur. Ceram. Soc. 19, 2625 (1999)
Z. Li, A. Ghosh, A.S. Kobayashi, J. Ami Soc. 72, 904 (1989)
V.G. Prabitha, A. Biju, R.G. Abhilash Kumar, P.M. Sarun, R.P. Aloysius, U. Syamaprasad, Physica C 433, 28 (2005)
A. Biju, R.P. Aloysius, U. Syamaprasad, Physica C 440, 52 (2006)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sedky, A., Salah, A., Bahgat, A.A. et al. Cooperative effects due to Ca substitution by La on the normal and superconducting states of (Bi, Pb):2223 system. J Mater Sci: Mater Electron 31, 12502–12513 (2020). https://doi.org/10.1007/s10854-020-03798-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03798-6