Abstract
Nano Ba1-xSrxFe2O4 samples were prepared by sol-gel autocombustion procedure. The phases developed due to different annealing temperatures and Sr-substitution amounts were determined by X'Pert HighScore Plus software. The effect of composition and annealing temperature on the lattice parameters and the crystallite size was investigated using X-ray diffraction technique. Rietveld analysis has been performed to determine the cation distribution between different crystallographic sites upon the Sr-substituted BaFe2O4 sample. Sr ion was found to prefer residing at 4a(II) site and the coordinates of this site dramatically changed with the Sr content (x). Fourier transform infrared spectrometry (FTIR) confirmed the orthorhombic structure of BaFe2O4 and the presence of the hexagonal structure ascribed to Ba0.5Sr0.5Fe2O4 for Sr-substituted samples. BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples prepared at 700 °C revealed hysteresis loop with wasp-waist nature. The saturation magnetization and coercivity changed irregularly upon varying the annealing temperatures. For Ba1-xSrxFe2O4 samples annealed at 900 °C, the saturation magnetization changed depending on the amount of Sr doping in the sample. Ba0.5Sr0.5Fe2O4 has the lowest coercively (424 Oe) at 700 °C annealing temperature, while its highest saturation magnetization (3.83 emu/g) is obtained at 1050°C. Many factors were considered to explain the irregular behavior of coercivity in the Ba1-xSrxFe2O4 nano system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ferrites materials are technologically important magnetic ceramic oxides that can be classified into spinels, garnets, hexaferrites, and orthoferrites depending on their crystal structure [1]. Spinel ferrites with the general formula of MFe2O4 (M=Mn, Fe, Co, Ni, Cu or Zn, etc.) can exhibit different crystal structures depending on the difference between the ionic radii of M and Fe3+ cations; less symmetric structures are established when the difference is large [2]. The diverse properties of the different ferrites nominated them to be used in several applications such as batteries, photodegradation, photocatalytic effect and microwave device [3,4,5,6,7,8,9,10]. As illustrative examples, CoFe2O4, SrFe2O4 and BaFe2O4 have cubic [11], hexagonal [12] and orthorhombic [13] structures, respectively. Numerous studies have been done on the phase relations in Ba–Fe–O ternary system [14]; three stable phases were reported, namely, Ba2Fe2O5, BaFe2O4 and BaFe12O19 [15]. Barium monoferrite, BaFe2O4 is a member of spinel ferrites family that combines interesting soft magnetic properties with rather high electrical resistivities, low band gap and advantageous dielectric behavior attributed to barium-ion existence [16]. The magnetic properties of BaFe2O4 could be controlled via the substitution of Ba2+ and/or Fe3+ cations by other ions in the same manner as other ceramics materials [13, 17]. Peymanfar et al. [18] investigated the effect of annealing temperature and organic capping agent on the physical properties of nanosized BaFe2O4. They found that the organic capping agent reduced the crystallite size and subsequently improved the isotropic magnetic exchange and the microwave absorption bandwidth. The nanocomposite of BaFe2O4, annealed at 650 °C, and silicone rubber revealed substantial microwave absorption properties created from the geometrical influence of nano materials [18]. On the other hand, Dilip et al. [19] revealed that the level of barium relative to iron in BaFe2O4 dramatically affects the formed phases, particle size, magnetic and dielectric properties of the system. Furthermore, BaFe2O4 indirectly prepared in the oxygen atmosphere exhibited an excellent photocatalysis effect [20].
On the other hand, SrFe2O4 is a hard ferrite with a wide range of applications in magnetic recording media and memory storage [21]. SrFe2O4 is characterized by its hexagonal structure, large magnetocrystalline anisotropy, high Curie temperature and a relatively large magnetization [22]. The effect of substitution of Sr2+ in different spinel ferrites was studied before by several authors [23,24,25,26]. Ateia et al. [23] reported that elastic parameters and the magnetic properties of magnesium nanoferrite were enhanced as being doped with Sr. Lima et al. [22] recorded other phases CoO, SrCO3, and SrFeO2.96 for Sr2+-doped cobalt nano ferrite, attributed to the low solubility of strontium in CoFe2O4 matrix. Ahmad et al. [25] found that all magnetic parameters in single-phase Ba1 − xSrxFe2O4 prepared by sol gel auto-combustion method were enhanced upon increasing the degree of Sr-substitution. Mulyawan et al. [26] have used X-ray diffraction and Raman spectroscopy techniques to reveal that the crystal structure of BaFe2O4 prepared by solid state reaction followed by high-energy milling at 900 °C has changed from orthorhombic to a trigonal phase when doped with Sr (Ba0.5Sr0.5Fe2O4). Furthermore, the sample of Ba0.9Sr0.1Fe2O4 has exhibited the widest frequency range (11.16 GHz) for reflection loss of −38.25 dB, nominating it to be used as radar absorbing materials (RAM) [26]. The aim of the present work is to explore the structural and magnetic properties of nano Ba1−xSrxFe2O4 (x = 0.0, 0.1, 0.3, 0.4, 0.5) samples prepared by sol-gel auto combustion procedure at different annealing temperatures (700, 900 and 1050 °C); new Sr content is tested at different annealing temperatures. The phases development upon annealing temperatures and Sr doping and hence their influence on the magnetic properties were inspected using Rietveld analysis for X-ray diffraction (XRD), transmission electron microscope (TEM), Fourier transform infrared spectrometry (FTIR) and vibrating sample magnetometry (VSM) techniques.
2 Experimental
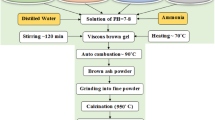
Nanocrystalline Sr-doped barium Ba1−xSrxFe2O4 (x = 0.0, 0.1, 0.3, 0.4, 0.5) monoferrite samples were prepared using sol-gel auto combustion procedure. Stoichiometric amounts of barium, strontium and iron nitrates were dissolved in small amounts of distilled water, and then mixed with anhydrous citric acid under stirring for 1 h. Meanwhile, the ammonia solution was added drop by drop to adjust the pH number from ∼1 (for the acidic solution) to 7, after which the stirring process was continued for an extra 1 h. The above solution was heated on a hot plate at 100°C until the water evaporated when a dark vicious gel was obtained. Upon continuing the heating process, an ash-like powder was formed. The formed powder was grounded in an agate mortar. For samples with x = 0.0 and 0.5, the fine powder was divided into three parts and heated at 700, 900 and 1050 °C for 2 h, separately. For samples with x = 0.1, 0.3, and 0.4, the powders were sintered at 900 °C. The mechanism of the auto-combustion method used in this study can be described as following: Mixture of metal nitrates (Ba, Sr and Fe) and the organic compounds behave in a similar manner as conventional oxidants and fuels. The reaction is carried out by dissolving metal nitrates and fuel in a minimum amount of water and heating the mixture to evaporate excess water. The effect of the citric acid is to chelate the nitrate group from the metal nitrates before forming the metal oxidants Fe2O3, BaO, and SrO. Upon continuing the heating process, the resulting viscous liquid foams, ignites, and undergoes a self-sustained combustion, producing ashes containing the oxide product. The exothermic reaction is controlled by the fuel type and the oxidizer-to-fuel ratio. Barium or strontium carbonate could be formed as a transition phase due to the presence of C═O in the citric acid, but sintering process eliminates the carbonate phase. The phase percentage, structural and microstructural parameters were analyzed with the aid of Rietveld profile method [27] and applying MAUD [28] program for the XRD patterns recorded by Philips diffractometer (X’pert MPD). The XRD patterns were recorded using Cu-Kα radiation, 2θ range of 20 –80°, and step-scan operation mode. The instrumental broadening was corrected using LaB6 standard sample. A Bruker Tensor 27 FTIR spectrometer with the range of 200–1000 cm−1 to investigate the IR spectra. The particle size and sample morphology were examined through images of HRTEM microscope (HR-TEM, Tecnai G20, FEI, Netherland) with an accelerating voltage of 200 kV. The room temperature hysteresis curves were traced from LDJ vibrating sample magnetometer (VSM, model 9600).
3 Results and discussions
3.1 Phase analysis, structural and microstructure analysis
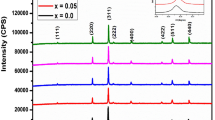
The phases characterizing the prepared samples Ba1−xSrxFe2O4 have been identified by applying the search-match program X’Pert HighScore Plus for XRD patterns of samples sintered at 900 °C shown in Fig. 1a. For x = 0.0 and 0.1, both samples exhibited a single orthorhombic phase with the space group \(Bb{2}_{1}m\). For samples with x = 0.3 and 0.4, two phases have been identified: orthorhombic \(Bb{2}_{1}m\) and hexagonal \(P\stackrel{-}{6}m2\). Single hexagonal phase \(P\stackrel{-}{6}m2\) has been identified for sample x = 0.5 (see Fig. 1a). Quantitative phase analysis has been performed applying Rietveld analysis; Table 1 gives the resulting structural and microstructural parameters and the percentages of phases developed, obtained from the refinement using MAUD program. Figures 1d-f illustrate the XRD patterns fitting produced from Rietveld analysis that shows explicitly the crystallographic phases of the samples. In the orthorhombic phase \(B b{2}_{1}m\), Sr incorporated into the lattice of BaFe2O4 replacing Ba ions at the site 4a, whereas in the hexagonal \(P \stackrel{-}{6}m2\) phase Sr ions have its own site 1b (0 0 ½). Inspecting Table 1, interesting structural changes could be noticed. The orthorhombic phase is manifested in the samples up to x = 0.4 with a decreasing percentage from 100% for x = 0.0 and 0.1 to 3.4% for x = 0.4. For low doping x = 0.1, Sr resides in a substitutional manner for Ba keeping the orthorhombic phase \(B b{2}_{1}m\). For high doping x = 0.3 and 0.4, part of Sr substitutes Ba forming orthorhombic phase while the remaining part segregates with Ba forming the hexagonal BaSrFe4O8 phase, as illustrated schematically in Fig. 2. For x = 0.5, equivalent amounts of Sr and Ba tend to form the hexagonal BaSrFe4O8 phase. The orthorhombic lattice constants (a and b) decreased with the Sr-content (x) while the parameter (c) decreased slightly, with a pronounced decrease in the unit cell volume (Table 1). This could be attributed to the smaller ionic radius of Sr relative to that of Ba and confirms the substitutional replacement of Ba by Sr. The crystallite size of the orthorhombic phase is almost isotropic and increases slightly for x = 0.1 but clearly decreases for x = 0.3 and 0.4 which can be probably assigned to the defects introduced by Sr substitution. For the hexagonal phase, the crystallite size is anisotropic, as expected, with larger value along the c-axis.
The unit cell of orthorhombic BaFe2O4\(\left(B b{2}_{1}m\right)\) contains 16 Fe ions, 8 Ba ions and 32 O ions. The 16 Fe ions reside at the same crystallographic site 8b but with different coordinates displaced from the averaged structure: 8 Fe(I) at \( \frac{1}{{24}} + \Delta x_{1} ,\frac{3}{4} + \Delta y_{1} ,\frac{1}{4}\Delta z_{1} \) and 8 Fe(II) at \( \frac{5}{{24}} + \Delta x_{2} ,\frac{3}{4} + \Delta y_{2} ,\frac{1}{4}\Delta z_{2} \). Each Fe atom is located at the center of a distorted tetrahedron of four O ions with the 16 tetrahedra forming a coordinated framework leaving cavities where tile barium atoms are accommodated. The structure is known as the ‘stuffed tridymite’ type [29] with a six-membered tetrahedral, forming a ring, have the conformation five-up and one-down, Fig. 2. The 8 Ba ions reside at the same crystallographic site 4a as two different kinds with different coordinates displaced from the averaged structure: 4 Ba(I) at \( \frac{1}{8} + \Delta x_{1} ,\frac{1}{4} + \Delta y_{1} ,0 \) and 4 Ba(II) at \( \frac{3}{8} + \Delta x_{2} ,\frac{3}{4} + \Delta y_{2} ,0 \). Ba(I) is surrounded by seven oxygen atoms while Ba(II) is surrounded by eleven oxygen atoms; such arrangement of atoms is not a close packed one. Very careful Rietveld analysis has been performed to determine the cation distribution between the crystallographic sites, specifically, the distribution of Ba ions and their replacement Sr ones. Table 2 gives the cation occupancies of Ba and Sr deduced from Rietveld analysis for x = 0.1, 0.3 and 0.4. It is obvious that Sr preferred to reside at the site 4a(II) and the coordinates of this site dramatically changed with the value of x. Figures 1b and c reveal that the crystal structure of BaFe2O4 and Ba0.5Sr0.5FeO4 did not change with increasing the annealing temperatures. The orthorhombic and hexagonal lattice constants for BaFe2O4 and Ba0.5Sr0.5FeO4 samples respectively are listed in Table 3, which illustrates their irregular change with the increase in the annealing temperatures. As expected, the crystallite size for both phases increases with increasing the annealing temperatures (Table 3). The structural morphology of the BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples, both annealed at 900 °C, is investigated by the HRTEM images (Fig. 3a, b), respectively, along with their selected-area electron diffraction (SAED) patterns. HRTEM images affirm the nanosized character of both samples, and their tendency to agglomerate. The crystallite sizes demonstrated by TEM images agree quite well with those got from XRD analysis. The corresponding SAED patterns with spotty circular rings revealed a reasonable degree of crystallization of the annealed samples.
3.2 FTIR analysis
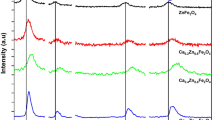
FTIR spectra of Ba1−xSrxFe2O4 annealed at 900 °C are revealed in Fig. 4. The spectrum for BaFe2O4 sample has bands at 306, 378, 499, 624 and 773 cm− 1 which are assigned for BaFe2O4 with orthorhombic structure [30]. As 10% of Ba is substituted with Sr, the bands shifted to higher wavenumber and the intensity of the band at 624 cm−1 decreased, became broader, and divided into two sub-bands 615 and 648 cm−1. These bands changed dramatically upon increasing Sr content for x > 0.1. For x = 0.3, an irregular shift in bands is observed, beside a significant reduction in their intensities, which is quite compatible with its low-intensity XRD pattern (Fig. 1a). Finally, the additional band appeared at 266−269 cm−1 for samples with x = 0.4 and 0.5, which could be correlated with the hexagonal structure of SrFe2O4. The bands around 605–628 cm− 1 correspond to the intrinsic stretching vibration of the metal ion at the tetrahedral site [31, 32], while the bands at 499–512 cm−1 corresponded to the stretching vibration of Fe3+-O2− [33]. The vibration bands due to Ba–O occurred at 773–815 cm− 1 [34]. The band at 873 cm−1 is assigned to the presence of Fe4+ cations [35]. The bands appearing at 919–926 cm−1 and 1078–1102 cm−1 are attributed to Ba–O stretching vibrations of BaO4 units in tri-, tetra- and/or penta-borate groups [36].
3.3 Magnetic properties analysis
Figure 5b , c show the magnetic measurements at different annealing temperatures for BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples, respectively. It can be noticed that there is no saturation in M-H loops for either sample at any annealing temperature, which infers the probable coexistence of a paramagnetic component with the dominating ferromagnetic phase [37]. The Wasp-Waist formation is also revealed in Fig. 5a [38] displaying the hysteresis plots for BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples prepared at 700 °C. This type of magnetic anisotropy is called exchange anisotropy, which associated to the magnetic manifestations of an exchange coupling of magnons at the interface between two different magnetically ordered systems (ferromagnetic and non-ferromagnetic systems) [37]. Dimri et al. [39] recorded a similar behavior in BaFe2O4 synthesized by citrate combustion method at different annealing temperatures (1100–1300 °C) but the Wasp-Waist formation shape appeared at a higher temperature (1300 °C). They argued this shape into the pinning of domains and magnetocrystalline anisotropy [40].
As the sintered temperature increased a typical hysteresis loop is shown with a large coercivity for both samples (Fig. 5b , c). It is noticed from Table 4 that the coercivity (Hc) of both samples has increased from 2880 to 5832 Oe for BaFe2O4 sample and from 424.1 Oe to 6145 Oe for Ba0.5Sr0.5Fe2O4 sample when the annealing temperature increased from 700 to 900 °C. Upon further increase in the annealing temperature 1050 °C (Fig. 6), coercivity decreased to 5260 and 3438 Oe for BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples, respectively. All coercivity values of BaFe2O4 samples annealed at different temperatures are much higher than those reported values (Hc=1205 Oe) for orthorhombic BaFe2O4 sample [41]. The obtained result is consistent with the result obtained by Dimri et al. [39]. The increase of the coercivity as the annealing temperature changed is due to the existence of the particles within the range of a single domain [42]. On the other hand, the decrease of coercivity with further increasing of the annealing temperature to 1050 °C, is due to the existence of the particles within multi domain range [42]. Furthermore, the saturation magnetization (Ms) was deduced by plotting M versus 1/H and taking the limit as H approaches ∞ [43]. With the increase of the annealing temperature from 700 to 900 °C, the values of Ms first decreased from 2.458 to 2.1967 emu/g to 2.0037 and 1.0536 emu/g for BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples, respectively. With further increasing annealing temperature to 1050 °C, Ms increased again to 2.2347 and 3.8346 emu/g for BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples, respectively. These changes could be mainly due to the increases in the crystallite size, and the contribution of Fe3+ ion site distribution. Maximum magnetization was lower as compared to the reported values for single crystals (Ms = 5.7 emu/g) of orthorhombic BaFe2O4 [41]. The recent low value can be partially attributed to the relative sublattice population [44], and the presence of small particles [45].
Figure 7 shows the VSM measurements of Ba1−xSrxFe2O4 samples carried out at room temperature. When Ba2+ in BaFe2O4 is substituted by Sr2+ ions, there is a drastic change in the magnetic properties including saturation magnetization (Ms), remanent magnetization (Mr) and coercivity (Hc). The variation in the magnetic property of the samples is shown in Fig. 6c. As shown in the figure, Ms values of samples varied according to the Sr-doping amount in BaFe2O4 host material. The change in the value of Ms is most probably due to the difference in the cation sharing at different sites, as shown in Table 3, the data of which achieved from XRD analysis. Mulyawan et al. [26] revealed that Ba1 − xSrxFe2O4 prepared by high energy milling method exhibited irregular behavior of Ms,Mr and Hc with increased amount of Sr in the system. This indicates that the Sr2+ cation, which replacing the Ba2+ site in the orthorhombic structure, play an important role to originate the magnetic anisotropy and influences the domain growth and finally improved the coercivity value [26]. Upon Ba-Sr substitution of BaFe2O4 structure with small levels (x = 0.1), Sr2+ entered the BaFe2O4 system with orthorhombic structure and changed its Hc value. With further substitution, a new phase has been formed beside the original one, as indicated by XRD analysis. These two factors (the involvement of Sr2+ into the orthorhombic BaFe2O4 matrix and the formation of the second phase with hexagonal structure) compete with the crystallite size variation upon Sr-doping (Table 1 , 2), and control the irregular behavior of Hc.
4 Conclusion
Samples with x = 0.0 and 0.1 in Ba1−xSrxFe2O4 resemble a single orthorhombic phase and samples with x = 0.3 and 0.4 exhibit orthorhombic and hexagonal phases. A single hexagonal phase is exhibited by a sample with x = 0.5. The orthorhombic lattice constants (a and b) decreased with increasing Sr-content while the parameter (c) decreased slightly. The crystallite size of the orthorhombic phase is almost isotropic and increased slightly for x = 0.1 but clearly decreased for x = 0.3 and 0.4. For the hexagonal phase at x = 0.5, the crystallite size is anisotropic, with larger growth along the c-axis. Sr ion was found to prefer residing at 4a(II) site and the coordinates of this site dramatically varied with the value of x. The crystal structure of BaFe2O4 and Ba0.5Sr0.5FeO4 samples revealed no change with increasing the annealing temperature. HRTEM images affirmed the nanosized character for both samples. FTIR spectra confirmed the presence of the orthorhombic phase only in the samples with x ≤ 0.1. BaFe2O4 and Ba0.5Sr0.5Fe2O4 samples annealed at 700 °C revealed hysteresis loops with Wasp-Waist behavior due to the exchange coupling of magnons at the interface. As the sintering temperature increased, a typical hysteresis loop for both samples are exhibited and their coercivity values first increased then decreased with further raising of annealing temperatures, being dependent on the sample crystallite size. The behavior of the saturation magnetization of the annealed samples depended on the crystallite size and Fe3+ distribution. Finally, the change in saturation magnetization and coercivity in Ba1−xSrxFe2O4 was correlated with the presence of one or two phases in the matrix, crystallite size and the phase in which Sr replaces Ba (orthorhombic or/and hexagonal).
References
Modern Ferrite Technology, A. Goldman, Springer; 2nd edition (November 30, 2005)
E.C. Snelling, Soft Ferrites, Butterworths, London, 2nd Edition (1988)
S.M. Peymani-Motlagh, A. Sobhani-Nasab, M. Rostami, H. Sobati, M. Eghbali-Arani, M. Fasihi-Ramandi, M.R. Ganjali, M. Rahimi-Nasrabadi, Assessing the magnetic, cytotoxic and photocatalytic influence of incorporating Yb3+ or Pr3+ ions in cobalt–nickel ferrite. J. Mater. Sci.: Mater. Electron. 30, 6902 (2019). https://doi.org/10.1007/s10854-019-01005-9
S.M. P-Motlagh, N. Moeinian, M. Rostami et al., “Effect of Gd3+-, Pr3+- or Sm3+-substituted cobalt–zinc ferrite on photodegradation of methyl orange and cytotoxicity tests”. J. Rare Earths 37, 1288 (2019). https://doi.org/10.1016/j.jre.2019.04.010
S.M. F.Gandomi, M.Rostami Peymani-Motlagh et al., ,“Simple synthesis and characterization of Li0.5Fe2.5O4, LiMg0.5Fe2O4 and LiNi0.5Fe2O4, and investigation of their photocatalytic and anticancer properties on hela cells line”. J. Mater. Sci.: Mater. Electron. 30(22), 19691 (2019). https://doi.org/10.1007/s10854-019-02320-x
Z.K. Heiba, M.M.S. Sanad, M.B. Mohamed, Influence of Mg-deficiency on the functional properties of magnesium ferrite anode material. Solid State Ionics 341, 115042 (2019). https://doi.org/10.1016/j.ssi.2019.115042
Z.K. Heiba, M.B. Mohamed, “Effect of magnesium deficiency on magnetic properties tuning and cation redistributions of magnesium ferrite nanoparticles”. J. Mater. Sci.: Mater. Electron. 30(1), 786 (2019). https://doi.org/10.1007/s10854-018-0348-7
MB Mohamed, AM Wahba, ZK Heiba, “Effect of Zn Substitution on Structural, Magnetic, and Electric Properties of Ni1 – xZnxFe1.78Al0.2Gd0.02O4 Nanoparticles”, Journal of Superconductivity and Novel Magnetism 28 (12) (2015)3675, https://doi.org/10.1007/s10948-015-3208-1.
S.M. Peymani-Motlagh, A. Sobhani-Nasab, M. Rostami et al., “Assessing the magnetic, cytotoxic and photocatalytic influence of incorporating Yb3+ or Pr3+ ions in cobalt–nickel ferrite”. J. Mater. Sci.: Mater. Electron. 30, 6902 (2019). https://doi.org/10.1007/s10854-019-01005-9
M. Rahimi-Nasrabadi, M. Rostami, F. Ahmadi et al., “Synthesis and characterization of ZnFe2 – xYbxO4–graphene nanocomposites by sol–gel method”. J. Mater. Sci.: Mater. Electron. 27, 11940 (2016). https://doi.org/10.1007/s10854-016-5340-5
M. Rajendran, R. Pullar, A. Bhattacharya, D. Das, S. Chintalapudi, C. Majumdar, Magnetic properties of nanocrystalline CoFe2O4 powders prepared at room temperature: variation with crystallite size. J. Magn. Magn. Mater. 232, 71 (2001). https://doi.org/10.1016/S0304-8853(01)00151-2
A. Mulyawan, W.A. Adi, S. Mustofa, A. Fisli, The phase transformation and crystal structure studies of strontium substituted barium monoferrite. In IOP Conference Series: Earth and Environmental Science (2017) 012070 (1–9), https://doi.org/10.1088/1755-1315/58/1/012070
R. Candeia, M. Souza, M. Bernardi, S. Maestrelli, I. Santos, A. Souza, E. Longo, Monoferrite BaFe2O4 applied as ceramic pigment. Ceram. Int. 33, 521 (2007). https://doi.org/10.1016/j.ceramint.2005.10.018
S. Castro, M. Gayoso, A.C. Rodriguez, Study of the combustion method to prepare fine ferrite particles. J. Solid State Chem. 134, 227 (1997). https://doi.org/10.1006/jssc.1997.7523
S.K. Rakshit, S.C. Parida, Z. Singh, R. Prasad, V. Venugopal, Thermodynamic properties of ternary oxides in the system Ba–Fe–O using solid-state electrochemical cells with oxide and fluoride ion conducting electrolytes. J. Solid State Chem. 177, 1146 (2004). https://doi.org/10.1016/j.jssc.2003.10.021
G. Yang, B. Han, Z. Sun, L. Yan, X. Wang, Preparation and characterization of brown nanometer pigment with spinel structure. Dyes Pigments 55, 9 (2002). https://doi.org/10.1016/S0143-7208(02)00056-6
Z.K. Heiba, M.B. Mohamed, A.M. Wahba, M.I. Almalowi, Effect of vanadium doping on structural and magnetic properties of defective nano-nickel ferrite. Appl. Phys. A-Mater 124(4), 290 (2018). https://doi.org/10.1007/s00339-018-1721-3
R. Peymanfar, M. Rahmanisaghieh, Preparation of neat and capped BaFe2O4 nanoparticles and investigation of morphology, magnetic, and polarization effects on its microwave and optical performance. Mater. Res. Express. 5(1–14), 105012 (2018)
R. Dilip, R. Jayaprakash, Effect of barium on morphological transition and magnetic and dielectric properties in ferrite nanoparticles. J. Supercond. Nov. Magn. 31, 1741 (2018). https://doi.org/10.1007/s10948-017-4379-8
Y. Yang, Y. Jiang, Y. Wang, Y. Sun, L. Liu, J. Zhang, Influences of sintering atmosphere on the formation and photocatalytic property of BaFe2O4. Mater. Chem. Phys. 105, 154 (2007). https://doi.org/10.1016/j.matchemphys.2007.04.050
R. Valenzuela, Magnetic Ceramics (Cambridge University Press, New York, 1994)
H.AllahB.Hamdollah Zohre Javdani, P. Salehi, Amiri, First principles calculations of optical and magnetic properties of SrFe2O4 compound under pressure. Phys. Lett. A 378, 2644 (2014). https://doi.org/10.1016/j.physleta.2014.06.046
E.E. Ateia, E. Takla, A.T. Mohamed, Physical and magnetic properties of (Ba/Sr) substituted magnesium nano ferrites. Appl. Phys. A 123, 631 (1–10 (2017). https://doi.org/10.1007/s00339-017-1246-1
A.C. Lima, A.P.S. Peres, J.H. Araújo, M.A. Morales, S.N. Medeiros, J.M. Soares, D.M.A. Melo, A.S. Carriço, The effect of Sr2+ on the structure and magnetic properties of nanocrystalline cobalt ferrite. Mater. Lett. 145, 56 (2015). https://doi.org/10.1016/j.matlet.2015.01.066
M. Ahmad, J. Shaheen, W.A. Hashmi, M.N. Akhtar, M. Asif, Structural, magnetic and high frequency (1–6 GHz) parameters of Sr-substituted BaFe2O4 monoferrites synthesized by sol–gel method. Mod. Phys. Lett. B 33(19), 1950219 (2019). https://doi.org/10.1142/S0217984919502191
A. Mulyawan, W.A. Adi, Yunasfi, Raman spectroscopy study, magnetic and microwave absorbing properties of modified barium strontium monoferrite Ba(1–x)Sr(x)Fe2O4. Mal. J. Fund. 14(1), 73 (2018). https://doi.org/10.11113/mjfas.v14n1.750
J. Rodriguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 192, 55 (1993). https://doi.org/10.1016/0921-4526(93)90108-I
L. Lutterotti, Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods, Phys. Res. B. 268, 334, (2010). https://doi.org/10.1016/j.nimb.2009.09.053
F.P. Glasser, L.S. Dent, Glasser, Crystal Chemistry of Some AB2O4 Compounds. J. Am. Ceram. Soc. 46, 377 (1963). https://doi.org/10.1111/j.1151-2916.1963.tb11755.x
S. Mandizadeh, M. Salavati-Niasari, M. Sadri, Hydrothermal synthesis, characterization and magnetic properties of BaFe2O4 nanostructure as a photocatalytic oxidative desulfurization of dibenzothiophene. Sep. Purif. Technol. 175, 399 (2017). https://doi.org/10.1016/j.seppur.2016.11.071
S.F. Wang, X.T. Zu, G.Z. Sun, D.M. Li, C.D. He, X. Xiang, W. Liu, S.B. Han, S. Li, Highly dispersed spinel (Mg, Ca, Ba)-ferrite nanoparticles: Tuning the particle size and magnetic properties through a modified polyacrylamide gel route. Ceram. Int. 42, 19133 (2016). https://doi.org/10.1016/j.ceramint.2016.09.075
H. Saravani, M.R. Esmaeilzaei, M.T. Ghahfarokhi, Synthesis and characterization of ferromagnetic BaFe2O4 nanocrystals using novel ionic precursor complex [Fe(opd)3]2[Ba(CN)8]. J. Inorg. Organomet. Polym. 26 (, 353 (2016). https://doi.org/10.1007/s10904-016-0327-5, “,” )
Z.K. Heiba, A.M. Wahba, M.B. Mohamed, Structural analysis and magnetic properties of biphasic chromium-substituted copper ferrites. J. Mol. Struct. 1147 (668 (2017). https://doi.org/10.1016/j.molstruc.2017.07.003
T. Vijayaraghavan, S.P. Suriyaraj, R. Selvakumar, R. Venkateswaran, A. Ashok, Rapid and efficient visible light photocatalytic dye degradation using AFe2O4 (A = Ba, Ca and Sr) complex oxides. Mater. Sci. Eng., B 210 (43 (2016). https://doi.org/10.1016/j.mseb.2016.04.005
B.P. Barbero, J.A. Gamboa, and L.E. Cadús, Synthesis and characterisation of La1 – xCaxFeO3 perovskite-type oxide catalysts for total oxidation of volatile organic compounds. Appl. Catal. B-Environ. 65, 21 (2006). https://doi.org/10.1016/j.apcatb.2005.11.018
A. Saleem, Hussain, “Investigation of the Structure and Magnetic Properties of Co+ 2 –Ti+ 4 Substituted Barium Ferrite Powder Prepared by Sol – Gel Method. J. Phys.: Conf. Ser. 1234, 012052 (2019). https://doi.org/10.1088/1742-6596/1234/1/012052
W.H. Meiklejohn, C.P. Bean, New Magnetic Anisotropy. Phys. Rev. 102, 1413 (1956). https://doi.org/10.1103/PhysRev.102.1413
M.L. Tibério Alves et al., “Wasp-waisted behavior in magnetic hysteresis curves of CoFe2O4 nanopowder at a low temperature: experimental evidence and theoretical approach,” RSC Adv. (2017) https://doi.org/10.1039/C6RA28727A
C. Mukesh, H. Dimri, P. Khanduri, J. Agarwal, R. Pahapill, Stern, Structural, magnetic, microwave permittivity and permeability studies of barium monoferrite (BaFe2O4). J. Magn. Magn. Mater. 486(1–6), 165278 (2019). https://doi.org/10.1016/j.jmmm.2019.165278
R.E. El Shater, E.H. El-Ghazzawy, M.K. El-Nimr, Study of the sintering temperature and the sintering time period effects on the structural and magnetic properties of M-type hexaferrite BaFe12O19. J. Alloys Compd. 73, 327 (2018). https://doi.org/10.1016/j.jallcom.2017.12.228
A. Jaafar, S. Arekat, A. Al-Saie, M. Bououdina, Structure and magnetic properties of nanosized BaFe2O4 material. Int. J. Nanosci. 9, 575 (2010). https://doi.org/10.1142/S0219581X10007289
K. El-Sayed, M.B. Mohamed, S. Hamdy, S.S. Ata-Allah, Effect of synthesis methods with different annealing temperatures on micro structure, cations distribution and magnetic properties of nano-nickel ferrite. J. Magn. Magn. Mater. 423, 291 (2017). https://doi.org/10.1016/j.jmmm.2016.09.100
Z.K. Heiba, M.B. Mohamed, Structural phase analysis, optical and magnetic properties of nano Mn-doped LiFe5O8. Appl. Phys. A 124, 818 (1–10 (2019). https://doi.org/10.1007/s00339-018-2241-x
V.K. Sankaranarayanan, Q.A. Pankhurst, D.P.E. Dickson, C.E. Johnson, An investigation of particle size effects in ultrafine barium ferrite. J. Magn. Magn. Mater. 125, 199 (1993). https://doi.org/10.1016/0304-8853(93)90838-S
M.V. Cabanas, J.M. Gonzalez-Calbet, M.J. Vallet-Regi, Synthesis of barium hexaferrite by pyrolysis of an aerosol. J. Mater. Res. 9, 712 (1994). https://doi.org/10.1557/JMR.1994.0712
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heiba, Z.K., Wahba, A.M. & Mohamed, M.B. Phase analysis and cation distribution correlated with magnetic properties of spinel Ba1−xSrxFe2O4 ferrites prepared at different annealing temperatures. J Mater Sci: Mater Electron 31, 12482–12492 (2020). https://doi.org/10.1007/s10854-020-03795-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03795-9