Abstract
NiO was synthesized simply via thermal decomposition of nickel ascorbate, which resulted from semi-solid reaction of nickel acetate and ascorbic acid. The prepared precursor was characterized by elemental, thermal and spectral analyses. The precursor was decomposed at 700 °C producing cubic NiO with average size 50 nm as indicated from XRD and TEM. FESEM indicated the formation of macroporous NiO. Magnetic parameters (saturation magnetization (Ms), remanence (Mr) and coercivity (Hc)) of NiO nanoparticles and its precursor were determined and compared with the bulk nickel. Zeta potential measurements at different pH values indicated that NiO is negatively charged. Adsorption of ponceau xylidine dye onto NiO nanoparticles at different parameters as pH, amount of adsorbent and time of adsorption was studied. It was found that the adsorption behavior obeys Langmuir isotherm and intra-particle diffusion model. NiO can remove 97.2% of ponceau xylidine from aqueous media. NiO showed higher adsorption than some other reported adsorbents. In additional to that, the antimicrobial effect of NiO-NPs on Staphylococcus aureus, Candida albicans and Escherichia coli was investigated. NiO nanoparticles exhibited good effects against the tested species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal oxide nanoparticles have received research interest, because of their nanosize-dependent properties and their different technological applications [1,2,3,4,5,6,7,8]. The particle shape will affect the chemical and physical properties of the synthesized nanoparticles [9,10,11,12]. Use of molecular precursors in preparing metal oxide nanoparticles is a very efficient method that gives nanoparticles in a high yield and quality [13,14,15]. Among the advantages of this method is the separation of different shapes of nanoparticles through changing the preparation conditions. NiO-NPs are a p-type semiconductor with wide bandgap in the range of 3.6 to 4.0 eV [16, 17]. In addition, they are used in batteries, smart windows, capacitors and as a gas sensor [18, 19]. NiO nanoparticles are desirable absorbents due to its high surface area, which enables it to be used as a carrier and catalyst. The effect of calcination temperature on the morphology NiO was studied. It was found that there is a direct relation between the particle size and the calcination temperature [20].
On the other hand, dyeing industries are the major source of polluting water due to their discharge in rivers, canals and other water sources. Such contaminated water will negatively affect the aquatic ecosystem. To remove organic pollutants from water, different physicochemical and biological processes such as reverse osmosis, adsorption, ozonation, precipitation, biodegradation, enzymatic decomposition and others can be used [21].
Ponceau xylidine is an azo dye that can reach to human and animal by irrigation and from surface water. It is used as coloring agent in the manufacturing process such as inks, textiles, leathers, food, pharmaceuticals and in histology for staining. It is harmful as it is metabolized releasing carcinogenic amines [22].
Nickel oxide is considered as good adsorbent due to its chemical and magnetic properties [23]. In comparison with other inorganic adsorbents such as Al, Ti and Fe, NiNPs are advantageous as Al NPs are thermodynamically unstable as it is affected by air or water. Also, in comparison with FeNPs, iron oxides exist in various oxidation states and can undergo further oxidation changing from one form to another, while NiO has only oxidation state [24].
To the best of our knowledge, no work has described the adsorption of ponceau xylidine dye. In this study, nickel oxide was synthesized by an environmental safe method and used as adsorbent for ponceau xylidine. NiO nanoparticles exhibited 97% of removal capacity of the dye; besides that, it showed antimicrobial effect against some pathogens which can exist in water such as Escherichia coli, Candida albicans and Staphylococcus aureus [25,26,27].
2 Materials and methods
2.1 Materials
Nickel acetate (99%) and ascorbic acid (99%) were brought from ADWIC, while ponceau xylidine dye was from Sigma.
2.2 Technique
CHN were determined at the Organic Microanalytical Section in National Research Centre, Egypt on Vario El Elementar Analyzer. Nickel content was determined spectrophotometrically [28]. IR spectra were recorded in the form of KBr discs by a Mattson 5000 FTIR spectrometer. UV–Visible was studied on UV2 Unicam UV–Vis spectrometer by 1 cm stoppard silica cell. TGA was run on a Schimadzu model 50 instrument using 20 mg samples. XRD was recorded by Philips X’PERT-PRO diffractometer, (λ = 1.5406 Ǻ for Cu Kα radiation). TEM images were taken with a JEOL JEM-1200EXII microscope. The magnetic properties of the samples were recorded at room temperature on a vibrating sample magnetometer (VSM) with a maximum magnetic field of 10 k Oe. FESEM images were taken by NRC QUANTA (250 FEG) microscope. Zeta potential results were determined on Malvern zeta potential and particle size analyzer.
2.3 Synthesis of precursor
Nickel acetate 0.1 mol (1.7 gm) was mixed with 0.2 mol (3.4 gm) of ascorbic acid, and about 5 ml distilled water was added dropwise. The mixture was ground for 15 min in a mortar until a semi-solid homogenous paste was formed. The paste was dried at 100 °C for 4 h. A light green powder was formed. Anal. Calc. for [Ni(Ascorbat)2 2H2O]H2O: C, 31.13; H, 4.35; Ni, 12.68. Found: C, 31.08; H, 4.16; Ni, 13.00.
2.4 Synthesis of NiO nanoparticles
Ni-ascorbate precursor was ignited in a muffle furnace at 700 °C. The heating rate was 50 °C/min in air. A dark green residue of NiO was formed (Scheme 1).
2.5 Adsorption of ponceau xylidine
Ponceau xylidine was adsorbed as batch form by changing some factors as dose of NiO, pH, the dye concentration and the contact time. To study the adsorption isotherm and the reaction kinetics, the dye solution with known concentrations was prepared and the required amount of NiO-NPs was added and stirred (JARTEST–STIRER) for a defined period of time. The time at which equilibrium conditions were achieved was estimated by kinetic measurements. Then, the mixture was centrifuged at 4000 rpm. The concentration of ponceau xylidine dye was followed at 507 nm using UV–Visible spectrophotometer. Solutions (0.1 N HCl and 0.1 N NaOH) were used to adjust the pH of the dye. The adsorption kinetic was determined as adsorption capacity at various times. The adsorption isotherms of ponceau xylidine at different concentrations were agitated with NiO until the equilibrium is achieved.
2.6 Antimicrobial activity
Escherichia coli, Staphylococcus aureus and Candida albicans were isolated from clinical and laboratory culture of Suez Canal University Center for Environmental Studies and Consultation. Two concentrations (100 and 200 ppm) of NiO were incubated in 0.7 mm hole using "disc diffusion method" against the pathogens at 37 °C for 48 h [29].
3 Results and discussion
3.1 Characterization of the precursor
IR spectrum of ascorbic acid (Fig. 1) shows bands at 3536, 3412, 3317 and 3221 cm−1 assigned, respectively, to the stretching vibrations of ν(1OH), ν( 2OH), ν(3OH) and ν (4OH) [30].
The in-plane bending of these groups appears at 1113, 1070, 1033 and 983 cm−1. In the precursor, the bands due to stretching vibrations and bending of (4OH) have disappeared as a result of coordination of this group to the Ni(II) ion with liberation of hydrogen. The band at 1760 assigned to the starching vibration of the free acid carboxyl group [31, 32] has been shifted to 1717 cm−1 as a result of coordination to Ni(II). From these observations, it is clear that ascorbic acid chelates Ni(II) as a mononegative bidentate via carbonyl oxygen and phenolic OH after deprotonation (Fig. 2). This mode of chelation has been supported also, by the following observations: the shift of the band at 1654 cm−1 of (C=COH) in the free acid to 1623 cm−1 in Ni(II) precursor. The band at 1080 cm−1 of the ring oxygen (C–O–C) remains more or less unaltered in the spectrum of the precursor. The appearances of two new bands at 575 and 671 cm−1 in the spectrum of the precursor were attributed to M–O of the formed chelate [33].
The electronic spectrum of Ni(II) precursor in DMSO (Fig. S2) shows two bands at 25,188 and 14,641 cm−1. These bands were assigned to 3A2g → 3T1g(P) (ν3) and 3A2g → 3T1g (ν2) transitions, respectively. These bands suggest an octahedral stereochemistry [34].
Thermal analysis (Fig. 3) of Ni(II) was studied in oxygen from 30 to 800 °C to assist in characterization of the precursor and to determine the temperature at which it is completely decomposed. The first decomposition starts from 30 to 130 °C and corresponds to the loss of one molecule of hydrated H2O (exp. 4%; calcd. 3.8%). The second step starts from 131 to 196 °C representing the loss of 2H2O (coordinated) and 3(OH) groups (exp. 17.8%; calcd. 18.5%). The third steps starts from 197 to 345 °C and represents the loss of 2(CO2) and 3(OH) (exp. 29.1%; Calcd. 29.7%). The last step begins from 345 to 408 °C due to the loss of the moiety (C10H8O2) (exp. 31.3%; calcd. 30.7%), leaving NiO as a residue (exp. 15.4%; calcd. 15.8%).
3.2 Characterization of NiO nanoparticles
3.2.1 XRD
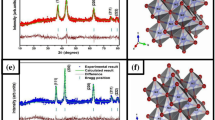
Ni(II) precursor was calcined at 700 °C, and the calcination product was characterized by XRD (Fig. 4). The pattern confirms the formation of cubic NiO, which crystallizes in Fm-3 m space group with lattice parameters a = b = c = 4.17 Ǻ and α = β = γ = 90°. Characteristic diffraction peaks appear at 2θ = 37.31, 43.36, 62.99, 75. 56 and 79.56 which represent the planes (111), (200), (220), (311) and (222), respectively, of NiO (JCPDS 01-075-0197), Fig. S1. About 1.9% of Ni metal exists in the sample as indicated from the peaks at 2θ = 44.6, 51.9 and 76.5 whch may be resulted from the reduction of Ni(II) by ascorbic acid.
The crystallite size was determined from the major diffraction peak by applying Debye Scherer formula D = Kλ/βcos θ[35], where D is the crystallite size and λ is the wavelength of the X-ray (Cu Ka, 1.54056 Å); θ is the diffraction angle of the peak; K is the shape factor which was taken to be 0.89 and K depends on the shape and shape and the index (hkl) of the crystals. β refers to the full width at half maximum (FWHM) of the peak expressed in radian and after subtracting the effect of the device broadening on the reflection peaks. The crystallite size was found to be 42 nm.
3.2.2 TEM and FESM microscopes
TEM images of NiO nanoparticles (Fig. 5a) exhibit crystals of NiO nanoparticles with average particle size 40–60 nm.
SAED of NiO nanoparticle pattern (Fig. 5b) exhibits diffraction rings representing the planes (111), (200), (220) and (311), respectively. The diffraction rings are made up of many diffraction spots, which indicate polycrystalline structure of the obtained NiO nanoparticles. SAED pattern also confirms that the NiO nanoparticles have cubic structure, which agrees with X-ray results. FESEM image in Figure 5c shows lumps of pores of NiO nanoparticles; the porosity may be attributed to the evolution of gases during the calcination process. Figure 5d indicates the pore diameter as obtained from FESEM. It is clear that the pore size is in the range of 97 to 237 nm indicating the macropore material where the material is microporous when the pore diameter is 0.2–2.0 nm, mesoporous (when the pore diameter range is 2.0–50.0 nm) and macroporous as the pore diameter exceeds 50.0 nm [36].
The estimated crystallite size is 42 nm. The particle size distribution curve is indicated in Fig. 6. The distribution curve indicates a narrow unimodel size distribution in the range from 20 to 58 nm with average size 39 nm.
3.3 Magnetic parameters
The magnetic properties of Ni(II) precursor and NiO nanoparticles were carried out at room temperature (25 °C) and are presented in Fig. 7 and the saturation magnetization (Ms), remanence (Mr) and coercivity (Hc) of the samples are shown in Table 1. From the results, it is clear that the saturated magnetization (Ms) equals 0.23 and 3.1 emug−1 for Ni precursor and NiO, respectively. Notably, these values are lower than bulk nickel (55 Oe) (where 1 Oe equals 79.6 Am−2). The values of coercivity field (Hc) are 24.2 and 18.2 Oe, for the precursor and NiO, respectively. In comparison with the bulk coercivity of nickel particles (0.8 Oe), the present Ni compounds have higher values. This increase in coercivity is due to the smaller particle size [37]. Also, these values confirm the higher magnetic properties of Ni precursor and NiO nanoparticles in comparison with nickel microstructures [38]. Notably, the ferromagnetic character of both NiO-NPs and its precursor comes from the presence of Ni(III) ion or superparamagnetic metallic Ni (0) as traces in NiO sample. The observation of traces of ferromagnetic metallic Ni in the XRD supports reason of the ferromagnetism of NiO-NPs [39].
3.4 Adsorption of ponceau xylidine dye
Ponceau xylidine dye belongs to azo dye category, and its adsorption onto NiO was studied at different parameters.
3.4.1 Effect of pH on adsorption
Figure 8 shows that the removal degree of ponceau xylidine decreases rapidly at higher pH value and the higher adsorption occurs at (pH 3).
3.4.1.1 Zeta potential at different pH
Zeta potential measurement of NiO at different pH values in Fig. 9 shows that NiO is negatively charged. This behavior can be explained according to the mechanism suggested by Tombasez [40] as follows: On the surface of NiO, some layers of adsorbed water are presented controlling the interactions, and the Ni2+ on the top layer of the oxide surfaces completes their coordination sphere by OH groups from water molecules forming surface OH. So, the surface of the oxide will be covered with (Ni-OH) sites. The surface hydroxyl groups are structurally and chemically non-equivalent and undergo association–dissociation mechanism forming (surface-O−).
Ponceau xylidine dye will be protonated and positively charged at pH 3, so the negatively charged NiO oxide can adsorb the positively charged Ponceau xylidine dye.
It is worth nothing from FESEM images that the dye may be occluded into the macropores of NiO.
3.4.2 Effect of mass of NiO-NPs
The adsorbent amount is also one of the most important factors that affects the adsorption. To investigate the correlation between ponceau xylidine removal and NiO-NPs amount, various amounts (0.1–0.5 g) of NiO-NPs at pH 3 and room temperature were added into 1 L of 50 ppm solutions of ponceau xylidine. The results are shown in Fig. 10 from which it can be seen that the removal percentage of the dye continuously increases with increasing the amount of NiO-NPs, reaching the maximum % removal of 97.2% on 0.5 g of adsorbent; after this concentration, there is no noticeable increase in the removal capacity of NiO-NPs, because of the unavailability of adsorbate [41].
3.4.3 Contact time
The effect of contact time on the removal of the dye by NiO-NPs at pH 3 was investigated in time interval between 10 and 120 min. The results (Fig. 11a) exhibited that the removal increases as the time increases until it reaches constant adsorption after 30 min. It is suggested that after 30 min, the active sites on the surface of NiO will saturate with the dye and there is no active sites available for adsorption.
3.4.4 Adsorption isotherms
The adsorption isotherms are mathematical models used to describe the distribution of adsorbed molecules between the liquid and the adsorbent surface. The adsorption isotherms models of Langmuir et al. were applied on the equilibrium adsorption data to find out the appropriate one. Langmuir adsorption isotherm suggests the formation of monolayer during the adsorption process without interaction between adsorbate molecules and is expressed by [42]
where Ce is the equilibrium concentration, qmax is the maximum adsorption capacity and KL is the Langmuir constant.
Freundlich adsorption isotherm describes multilayer adsorption on a heterogeneous surface. It is given by the following equation [43]:
where Kf is a Freundlich isotherm constant, n is intensity of adsorption, and Ce and qe are the concentration of the dye and the amount adsorbed at equilibrium, respectively.
Temkin adsorption isotherm describes the adsorption of heterogeneous systems and suggests that the heat of adsorption decreases linearly with coverage due to adsorbent–adsorbate interactions. Temkin isotherm describes the surface energy and is represented as [44]
where B is constant = RT/bT, AT and bT are the Temkin constants, R is the universal gas constant and T is the absolute temperature.
In order to study the adsorption isotherms, 0.5 g of NiO-NPs at pH 3 was added to 1 L solution of different ponceau xylidine concentrations (25, 50, 65, 75, 85, and 100 ppm), with stirring rate of 400 rpm. The remains dye in solution can be measured spectrophotometrically at 507 nm. The parameters obtained from the three models are illustrated in Table 2.
The experimental results were found to fit the Langmuir isotherm model.
The values of KL in the range of 0 to 1 indicate that the NiO-NPs are favorable for adsorption of ponceau xylidine dye. The Langmuir model shows the maximum theoretical adsorption capacities 165 mg/g. From the Temkin isotherm model, the higher value of bT demonstrates a strong interaction force between the dye and the absorbents.
3.4.5 Adsorption kinetics
In order to define the mechanism of the dye adsorption onto NiO-NPs, experimental kinetic data were applied to the pseudo-first-order and pseudo-second-order and intra-particle diffusion model.
[45, 46]. As Langmuir and Freundlich models cannot give definite mechanisms, intra-particle diffusion model was applied to analyze the experimental [47, 48] data, in which the adsorbate species are probably transferred from the bulk of the solution into the solid phase [49, 50].
For kinetic measurements, 0.5 g of NiO-NPs at pH 3 and room temperature was added into 1 L of 50 ppm solutions of ponceau xylidine, with stirring rate of 400 (rpm), then determining the absorbance at 507 nm wavelength at different time intervals; by using a calibration curve, the corresponding dye concentration (ppm) can be determined.
Figure 11a–d shows the effect of contact time curve, the pseudo-first-order model, pseudo-second-order kinetic model, and intra-particle diffusion model curves. The values of the rate constants (k1, k2 and ki), the calculated qe values and the corresponding linear regression correlation coefficient values are given in Table 3.
The results (Table 3) indicate that the intra-particle diffusion model is the most reliable way to explain this adsorption. These results confirm the presence of both surface adsorption (chemisorptions) and intra-particle diffusion mechanism during removal of ponceau xylidine by NiO nanoparticles.
The adsorption capacity of azo dyes onto the synthesized NiO was compared with other earlier reports (Table 4) and indicated higher performance than these reports [51,52,53].
3.5 Antimicrobial activity
NiO was tested against Escherichia Coli, Candida albicans and Staphylococcus aureus. NiO shows activity towards the tested microorganisms (Table 5). It exhibits higher activity against E. coli in comparison with the other two pathogens.
4 Conclusions
NiO nanoparticles were synthesized by simple benign solid-state decomposition of Ni-ascorbate precursor. It is demonstrated that the prepared NiO-NPs are an effective adsorbent for the removal of ponceau xylidine dye besides its efficacy as a disinfectant for contaminated water. The magnetic parameters of the NiO and its precursor were determined and found to be affected by the particle size. The adsorption isotherm parameters and kinetics study for the adsorption process have been investigated. The equilibrium adsorption data of ponceau xylidine showed excellent fitting with the Langmuir and Temkin models rather than Freundlich adsorption isotherm, indicating that the uptake of dye is favorable, monolayer and occurs on homogeneous surface sites. NiO and its precursor exhibited interesting magnetic properties. NiO exhibited antimicrobial activity against some organisms that may exist in water. The adsorption kinetic is best described by both pseudo-second-order (chemisorptions) and intra-particle diffusion through the pores of NiO nanoparticles, all of which may be operating simultaneously.
References
N.M. Hosny, Single crystalline Co3O4: synthesis and optical properties. J. Mater. Chem. Phys. 144, 247–251 (2014)
H. Derikvandi, A. Nezamzadeh-Ejhieh, Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 321, 629–638 (2017)
M. Karimi-Shamsabadi, A. Nezamzadeh-Ejhieh, Comparative study on the increased photoactivity of coupled and supported manganese-silver oxides onto a natural zeolite nano-particles. J. Mol. Catal A 418–419, 103–114 (2016)
N.M. Hosny, Synthesis, characterization and optical band gap of NiO nanoparticles derived from anthranilic acid precursors via a thermal decomposition route. Polyhedron 30, 470–476 (2011)
N.M. Hosny, E. Othman, F.I. ElDossoki, [Cd(anthranilate)2]H2O as a precursor of CdO nanoparticles. J. Mol. Struct. 1195, 723–732 (2019)
N.M. Hosny, M. Badr, F.I. El-Dossoki, Copolymer of m-phenylenediamine and Anthranilic Acid (P(mPDA-co-AA): new precursor of MnO nanoparticles. Polym. Plast. Technol. Mater. 58, 1178–1190 (2009)
D.S. Saidina, M.Z. Abdullah, M. Hussin, Metal oxide nanofluids in electronic cooling: a review. J. Mater. Sci. Mater. Electron. 31, 4381–4398 (2020)
I. Anastopoulos, A. Hosseini-Bandegharaei, J. Fu, A.C. Mitropoulos, G.Z. Kyzas, Use of nanoparticles for dye adsorption: review. J. Disper. Sci. Technol. 43, 836–847 (2018)
C.H. Wei, Q. Ru, X. Kang, H. Hou, C.H. Cheng, D. Zhang, Self-template synthesis of double shelled ZnS-NiS197 hollow spheres for electrochemical energy storage. Appl. Surf. Sci. 435, 993–1001 (2018)
C.H. Wei, Q. Chen, C.H. Cheng, R. Liu, Q. Zhang, L. Zhang, Mesoporous nickel cobalt manganese sulfide yolk-shell hollow spheres for high-performance electrochemical energy storage. Inorg. Chem. Front. 6, 1851–1860 (2019)
X. Peng, Mechanisms for the shape-control and shape-evolution of colloidal semiconductor nanocrystals. Adv. Mater. 15, 459–463 (2003)
M. Parashar, V.K. Shukla, R. Singh, Metal oxide nanoparticles via sol-gel method: a review on synthesis, characterization and applications. J. Mater. Sci: Mater. Electron. 31, 3729–3749 (2020)
N. Revaprasadu, S.N. Mlondo, Use of metal complexes to synthesize semiconductor nanoparticles. Pure Appl. Chem. 78, 1691–1702 (2006)
S.N. Mlondo, N. Revaprasadu, P. Christian, M. Helliwell, P. O’Brien, Cadmium thiosemicarbazide complexes as precursors for the synthesis of nanodimensional crystals of CdS. Polyhedron 28, 2097–2102 (2009)
H. Yang, Q. Tao, X. Zhang, A. Tang, J. Ouyang, Solid-state synthesis and eectrochemical property of SnO2/NiO nanomaterials. J. Alloys Compd. 459, 98–102 (2008)
C. Díaz-Guerra, A. Remón, J.A. García, J. Piqueras, Cathodoluminescence and photoluminescence spectroscopy of NiO. Phys. Status Solidi 163, 497–503 (1997)
J. Bahadur, D. Sen, S. Mazumder, S. Ramanathan, Effect of heat treatment on pore structure in nano-crystalline NiO: a small angle neutron scattering study. J. Solid State Chem. 181, 12271–21235 (2008)
T. Nathan, A. Aziz, A.F. Noor, S.R.S. Prabaharan, Nanostructured NiO for electrochemical capacitors: synthesis and electrochemical properties. J. Solid State Electrochem. 12, 1003–1009 (2008)
I. Bazin, A. Ibn Hadj Hassine, Y. Haj Hamouda, W. Mnif, A. Bartegi, M. Lopez Ferber, M. De Waard, C. Gonzalez, Estrogenic and anti-estrogenic activity of 23 commercial textile dyes. Ecotoxicol. Environ. Saf. 85, 131 (2012)
M. Hashem, E. Saion, N.M. Al-Hada, H.M. Kamari, A.H. Shaari, Z. Talib, S.B. Paiman, M.A. Kamarudeen, Fabrication and characterization of semiconductor nickel oxide (NiO) nanoparticles manufactured using a facile thermal treatment. Results Phys. 6, 1024–1030 (2016)
Y.C. Wong, Y.S.W. Szeto, H. Cheung, G. McKay, Adsorption of acid dyes on chitosan—equilibrium isotherm analyses. Process. Biochem. 39, 695–704 (2004)
Z. Ni, Sh Xia, L. Wang, F. Xing, G. Pan, Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: adsorption property and kinetic studies. J. Colloid Interface Sci. 316, 284–291 (2007)
N. Roya, R.B. Gholam, M.A. Mohammad, M. Hamed, Decolourization of synthetic wastewater by nickel oxide nanoparticles. Int. J. Environ. Health Eng. 1, 1–25 (2013)
K. Ravindhrnath, M. Ramamoorty, Nickel based nano particles as adsorbents in water purification methods—a review. Orient. J. Chem. 33, 1603–1613 (2017)
A.R. Shahverdi, A. Fakhimi, H.R. Shahverdi, S. Minaian, Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 3, 168–171 (2007)
A. Abbaszadegan, Y. Ghahramani, A. Gholami, B. Hemmateenejad, S. Dorostkar, M. Nabavizadeh, H. Sharghi, The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J. Nanomater. 8, 720654 (2015)
B.M. Lund, Foodborne diesease due to Bacillus and Clostridium species. Lancet 336, 982 (1990)
A.I. Vogel, A Text Book of Quantitative Inorganic Analyses, 2nd edn. (Longman, London, 1961)
R.H. Baltz, A.L. Demain, J.E. Davies, Manual of Industrial Microbiology and Biotechnology, 3rd edn. (ASM Press, Washington, DC, 2010)
K. Nakamoto, Infrared spectra of inorganic and coordination compounds, 2nd edn. (Wiley, New York, 1970)
H.A. Dabbagh, F. Azami, H. Farrokhpour, A.N. Chermahini, UV-vis, NMR and FT-IR spectra of tautomers of vitamin C experimental and DFT calculations. J. Chil. Chem. Soc. 59, 2588–2594 (2014)
T.V. Long, A.W. Herlinger, E.F. Epstein, I. Bernal, T.V. Long, A.W. Herlinger, E.F. Epstein, I. Bernal, Syntheses, structures, and laser Raman and infrared spectra of Co(NH3)6CuCl5, [Co(NH3)5OH2] CuCl5, Co(NH3)6CdCl5, Co(NH3)6ZnCl5, and Co(NH3)6ZnCl4(NO3). Inorg. Chem. 9, 459–464 (1970)
G. Gliemann, Polarized absorption spectra of tetracyanoplatinate(II) single crystals. Ber. Bunsenges. Für Phys. Chem. 89, 940–948 (1985)
A.B.P. Lever, Inorganic Electronic Spectroscopy, 1st edn. (Elsevier, Amsterdam, 1984)
H.P. Klug, L.E. Alexander, X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd edn. (Wiley, New York, 1974)
N.M. Hosny, Solvothermal synthesis, thermal and adsorption properties of metal-organic frameworks Zn and CoZn(DPB). J. Therm. Anal. Calorim. 122, 89–95 (2015)
J. Hwang, V. Dravid, M. Teng, J. Host, B. Elliott, D. Johnson, T. Mason, Magnetic properties of graphitically encapsulated nickel nanocrystals. J. Mater. Res. 12, 1076–1082 (1997)
T. Fardin, The study of structural and magnetic properties of NiO nanoparticles. Opt. Photon. J. 60, 164–169 (2016)
J.T. Richardson, D.I. Yiagas, B. Turk, K. Forster, M.V. Twigg, Origin of super paramagnetism in nickel oxide. J. Appl. Phys. 70, 6977–6982 (1991)
E. Tombácz, pH-dependent surface charging of metal oxides. Per. Pol. Chem. Eng. 53(2), 77–86 (2009)
M. Arshadi, F. SalimiVahid, J.W.L. Salvacion, M. Soleymanzadeh, Adsorption studies of methyl orange on an immobilized Mn-nanoparticle: kinetic and thermodynamic. RSC Adv. 4, 16005–16017 (2014)
I. Langmuir, The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 39, 1848–1906 (1917)
H.M.F. Freundlich, Over the adsorption in solution. Phys. Chem. 57, 385–471 (1906)
M.I. Temkin, V. Pyzhev, Kinetics of ammonia synthesis on promoted iron catalyst. Acta. Physicochim. URSS 12, 327–356 (1940)
S.K. Lagergren, About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 24, 1–39 (1898)
Y.S. Ho, G. McKay, Sorption of dye from aqueous solution by peat. Chem. Eng. J. 70, 115–124 (1998)
W.J. Weber, J.C. Morris, Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 89, 31–38 (1963)
G. McKay, H.S. Blair, J.R. Gardner, The adsorption of dyes onto chitin in fixed bed columns and batch adsorbers. J. Appl. Polym. Sci. 29, 1499–1514 (1984)
Y.S. Ho, G. McKay, A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process. Saf. Environ. Prot. 76, 332–340 (1998)
Y.S. Ho, G. McKay, Comparative sorption kinetic studies of dye and aromatic compounds onto fly ash. J. Environ. Sci. Heal. A 34, 1179–1204 (1999)
M.N. Zafar, Q. Dar, F. Nawaz, M.N. Zafar, M. Iqbal, M.F. Nazar, Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 8, 713–1725 (2019)
M. Batool, W.M.D. Daoush, F. Hashmi, N. Mehboob, Z. Qureshi, Kinetic isotherm studies of azo dyes by metallic oxide nanoparticles adsorbent. Arch. Org. Inorg. Chem. Sci. 3, 426–435 (2018)
G. Kheraldeen-Kara, M. Rabbani, Experimental study of methylene blue adsorption from aqueous solutions onto Fe3O4/NiO nano mixed oxides prepared by ultrasonic assisted co-precipitation. J. Nanostruct. 9, 287–300 (2019)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosny, N.M., Gomaa, I., Abd El-Moemen, A. et al. Synthesis, magnetic and adsorption of dye onto the surface of NiO nanoparticles. J Mater Sci: Mater Electron 31, 8413–8422 (2020). https://doi.org/10.1007/s10854-020-03376-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03376-w