Abstract
Recently, AgNbO3 antiferroelectric ceramics have attracted great attention by virtue of their characters of high energy storage density and environmental friendliness. To further optimize the electrical properties, in this work, Lu2O3 modified AgNbO3 ceramics were prepared via conventional solid state method. Crystal structure and element analysis indicated the Lu3+ ion preferred to enter the A-site when Lu2O3 content was lower than 2 mol%, otherwise, it was more likely to form LuNbO4 or Lu3NbO7-based solid solutions. Remarkably improved stability of antiferroelectric phase was observed once Lu3+ ions enter into the A-site, on account of the decrease of cell volume and tolerance factor. As a consequence, an enhanced recoverable energy storage density (Wrec) of 3.5 J/cm3 was achieved in 1 mol% Lu2O3 modified AgNbO3 ceramics at 210 kV/cm, which is superior to the other lead-free ceramics under moderate electric field (< 220 kV/cm). It is believed our study will provide a good reference for the development of AgNbO3-based dielectric capacitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of electronic industry, much attention has been concentrated on discovering novel energy storage materials to meet the sustainable development [1,2,3,4]. As an important branch of energy storage systems, dielectric capacitors are widely used in various kinds of high power equipment because of their fast charge/discharge rate and high power density [5,6,7,8]. However, the dielectric capacitors are greatly limited in practical application due to their low energy storage density (Wrec) [9,10,11,12]. Among all dielectrics, antiferroelectric (AFE) energy storage ceramics with the character of high maximum polarization (Pm) and low remnant polarization (Pr) have received significant attention. At present, lead-based antiferroelectric materials have been widely investigated because of their excellent energy storage performance [13,14,15,16]. However, with the improved social awareness of environmental protection, the lead-based materials will be gradually restricted in law. Novel lead-free dielectric capacitors with high energy storage density are urgently demanded.
As a member of perovskite family, AgNbO3 (AN) lead-free antiferroelectric ceramics have recently received considerable interest. The energy storage density of pure AN ceramic ranged from 1.5–2.0 J/cm3 depending on the applied electric field [6, 17, 18]. More importantly, this material system exhibited highly adjustable characters in energy storage performance through composition modification. Recently, aliovalent A-site cation substitution is widely used to tailoring the energy storage property of AgNbO3-based ceramics [19]. For examples, high energy storage densities were achieved to 2.3, 2.6 and 2.9 J/cm3 in Ca2+, Bi3+ and Sr2+ modified AgNbO3 ceramics, respectively [20,21,22]. Of particular importance, the lanthanide metal oxides modified AgNbO3 exhibited more attractive energy storage density. A high energy storage density of 4.5 J/cm3 was achieved in Ag0.88Gd0.04NbO3 ceramics [23]. Our previous investigations showed that high energy storage densities of 3.12 J/cm3 and 5.2 J/cm3 were realized in Ag0.94La0.02NbO3 and Ag0.91Sm0.03NbO3 ceramics, respectively [12, 19]. The inner mechanism of above-mentioned enhanced energy storage density through chemical modification is ascribed to the improved stability of antiferroelectric phase, as a consequence of reduced tolerance factor t. Generally, the stability of an antiferroelectric phase for perovskite structure can be evaluated by the following formula:
where RO, RA and RB, respectively, represent the average ionic radii of oxygen anion, A-site and B-site cations [12, 24, 25]. It’s generally believed that a low tolerance factor is beneficial to obtaining a highly stabilized antiferroelectric phase. The mechanism of this empirical equation is considered to lie on the decrease of cell volume and cation displacement [26], which weakens ferroelectricity and improves the antiferroelectricity.
As a last element in the lanthanide, the ion radius of Lu3+ (R = 0.92 Å) is smaller than those of most lanthanides with a coordination number of 12 [27, 28]. Once it enters into A-site to replace Ag+, the tolerance factor and cell volume will be remarkably reduced, which will, in turn, improve the stability of antiferroelectric phase. Furthermore, among the lanthanide oxides, Lu2O3 was reported to possess better insulating property, higher dielectric constant and lower leakage current than those of other lanthanide oxides [29,30,31,32]. Therefore, the addition of Lu2O3 into AgNbO3 may also enhance the breakdown field strength (Eb), which is beneficial to improving the energy storage density.
According to the aforementioned consideration, Lu2O3 was added into AgNbO3 in this work, with the aim to improve the stability of antiferroelectric phase and enhance the energy storage density. As expected, an enhanced energy storage density (Wrec) of 3.5 J/cm3 was achieved in 1 mol% Lu2O3 modified AgNbO3 ceramics. The crystal structure, microstructure and electrical properties of the as-prepared AgNbO3-based ceramics were systematically investigated.
2 Experimental procedure
The LuxAg1−3xNbO3 (LuANx) ceramic samples with x = 0, 0.01, 0.02, 0.03 and 0.04 (abbreviated as AN, LuAN1, LuAN2, LuAN3 and LuAN4) were prepared by using a conventional solid state reaction method, details of which can be found in our earlier works [19, 26]. X-ray diffractometer (Smartlab-3KW, Rigaku Ltd, Japan) with monochromatic Cu Kα radiation was used to detect the crystal structure, after the samples were crushed into powders. The microstructure and element content of the ceramics were examined by scanning electron microscopy and energy dispersive spectrometer (SEM–EDS, Pro X, Phenom, Eindhoven, Netherlands). The dielectric-temperature spectrum was obtained by using the LCR tester (Model 4294A, Hewlett-Packard Co.) as the temperature elevate from 30 to 500 °C. Ferroelectric analyzer (RT66, Radiant Technologies, USA) was employed to obtain the polarization versus electric field hysteresis loops (P–E loops) and the current versus electric field (I–E curves) at 1 Hz.
3 Results and discussion
3.1 Crystal structure and microstructure analysis
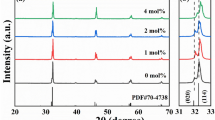
The compositional dependence of X-ray diffraction patterns are displayed in Fig. 1. Pure perovskite structure can be observed in undoped AgNbO3, as compared with JCPDS file No. 70-4738. However, once a certain amount of Lu2O3 is added, a few small extra peaks come across, as shown in the enlarged patterns with angles in the range of 29°–31°. When 1 mol% Lu2O3 is doped, an extra peak identified as LuNbO4 is observed, which may be associated with a higher reaction activity between Lu2O3 and Nb2O5 raw materials compared with that between Ag2O and Nb2O5. As the content of Lu increases to 2 mol%, other peaks identified as Lu3NbO7 are also observed. The XRD Rietveld refinement on the basis of Pbcm space group was obtained by using GSAS software to further study the crystal structure evolution of AgNbO3 with the increase of Lu2O3 doping amount, which was displayed in Fig. 2a–e. The low reliability factor values indicate the structural model is valid and the refinement results fit well with the experimental data. The Rietveld lattice parameters a, b, c and the cell volume V are calculated, as exhibited in Fig. 2f. The a and b slightly decrease with the increase of Lu2O3 doping amount, while the c changes very little. More interestingly, the cell volume V decreases remarkably at low Lu level (< 2 mol%), which then exhibits very small change by further adding Lu2O3. In general, the smaller Lu3+ (R = 0.92 Å) ions prefer to substitute for Ag+ (R = 1.48 Å) in the A-site, as described by the solid solution formula as follows:
The substitution of Ag+ by Lu3+ and the produced silver vacancies contribute to the sharp decrease of cell volume V, which is in favor of the stability of antiferroelectric phase. However, both lattice distortion and valance imbalance will increase with the Lu3+ ions successive diffusing into the crystal lattice, because of the large ionic radius and valance variations between Ag+ and Lu3+ ions. This will restrict extra Lu3+ ions from entering into AgNbO3 lattice, resulting into a limited solid solubility of Lu3+ ions. As a consequence, the cell volume V stays almost no change and secondary phases come to across gradually, which is the case of x > 2 mol%, as confirmed by the cell volume variations and the extra peaks in XRD patterns.
Figure 3a–e display the microstructure of the as-sintered LANx ceramics. Few porosities are observed on the surface morphology, which indicates a high relative bulk density. Some small grains with different contrast are also observed when x > 2 mol%, as marked with red arrows in Fig. 3d and e for LuAN3 and LuAN4, respectively. In order to explore the difference between the abnormal grains and the matrix, the EDS is applied on various grains of LuAN4 ceramics. The obtained EDS patterns and calculated atomic percentage are shown in Fig. 3f. The atomic ratio (Ag:Nb:O) of mother grains (point 1 in Fig. 3e) is near to 1:1:3, which is consistent with the chemical formula of AgNbO3. Besides, signature of Lu element with atomic percentage around 1.75% is also detected, indicating that a portion of Lu3+ diffuses into the AgNbO3 lattice and the solid solubility of Lu3+ is around 2 mol%. While the atomic percentage of Lu, Nb, O and Ag elements in abnormal grain (point 2 in Fig. 3e) are, respectively, found to be 14.63%, 18.35%, 58.87% and 8.15%, which is almost identical with the chemical formula LuNbO4 with a certain amount of Ag+ entering into the lattice. However, the Lu3NbO7 is not confirmed yet, which may because its amount is too low to be detected by EDS.
The breakdown strength is closely related to the grain size, so it’s very important to count the grain size distribution. Herein, the grain size variations of the AN-based ceramics before and after Lu2O3 modification are determined and shown in Fig. 4a–e. The statistical grain size generally obeys Gaussian distribution for all compositions. The relationship between average grain size and Lu2O3 doping amount is illustrated in Fig. 4f. The average grain size is about 6 μm for AN ceramics. The average grain size significantly reduces to ~ 2.3 μm after 1 mol% Lu2O3 addition, which may be resulted from the silver vacancies combined with the impurity ions (Lu3+ ions in the lattice) which inhibit the mass transport [33, 34]. However, the average grain size tends to increase with further increase of Lu2O3 content. This may be associated with the limited solid solubility of Lu3+ ions in the AgNbO3 lattice. The excess Lu3+ ions prefer to precipitate from the mother grain and exist in the grain boundary during the sintering process, which may accelerate the grain boundary mobility, thus leading to the growth of mother grain. Due to the hindrance of the secondary phase, the grain size is still smaller than that of pure AgNbO3.
3.2 Dielectric properties
In order to investigate dielectric properties and the stability of antiferroelectricity in the Lu2O3 modified AgNbO3 ceramics, the dielectric permittivity and loss are measured at different frequencies from 1 kHz to 1 MHz, as shown in Fig. 5a–e. Four dielectric anomalies are observed in AN, LuAN1 and LuAN2 ceramics, which is consistent with the existing reports [3, 8, 19].
It should be noticed that an extra dielectric anomaly at temperature around 270 °C is observed when x > 2 mol%, which may be associated with the secondary phase. All samples exhibit very low dielectric loss, indicating high electric resistance. In order to explore the influence of Lu2O3 modification on the phase transition, the phase diagram is constructed in Fig. 5f based on the dielectric-temperature spectrum. The TM1–M2 (M1–M2 phase transition temperature) is adjusted to below room temperature when Lu2O3 doping amount is over 2 mol%. The lower TM1–M2 indicates a much more stable antiferroelectric phase at room temperature for Lu2O3 doped compositions, which may benefit the improvement in energy storage density. The M2–M3 phase transition temperature (TM2–M3) is also found to decrease with increase of Lu2O3 level. Furthermore, the M3–O phase (TM3–O) and O–T (TO–T) transition temperatures increase slightly with the increase of Lu2O3 content. The temperatures for the extra dielectric anomaly stay stable with increasing Lu2O3 content, which further verify it may be caused by the secondary phases; more investigations need to be done to confirm this.
3.3 Electrical properties
To investigate the ferroelectric and energy storage properties of the Lu2O3 modified AgNbO3 ceramics, the P–E loops and I–E curves are shown in Fig. 6a–e. Typical double-like P–E loops are obtained when x < 3 mol%, which is consistent with a antiferroelectric feature. The four peaks in I–E curves are further confirmed the antiferroelectric feature [35]. Figure 6f gives a comparison of the P–E loops for LuANx system. The hysteresis becomes smaller with increase of the Lu2O3 doping amount, which is benefit to higher energy storage efficiency. In order to look into the effect of Lu2O3 modification on ferroelectric properties, the detailed evolution of the electrical parameters is also investigated, as displayed in Fig. 7a, b. The Pmax initially increases from 37.0 µC/cm2 for undoped AgNbO3 to 39.7 µC/cm2 for LuAN1 ceramic after 1 mol% Lu2O3 modification, which decreases continuously by further increasing Lu2O3 content. While the Pr exhibits a generally decrease with the increase of Lu2O3 doping amount, which is attributed to the enhanced stability of antiferroelectric phase. Furthermore, both EF (AFE to FE phase transition electric field) and EA (FE to AFE phase transition electric field) increase with the increase of Lu2O3 doping content when x < 2 mol%, as shown in Fig. 7b. Once the Lu2O3 doping level exceeds 2 mol%, the EA is relative stable, due to the limited solid solubility for Lu3+ in AgNbO3. The disappearance of EF at higher Lu2O3 doping level may be resulted from the higher antiferroelectric–ferroelectric phase transition electric field, which exceeds the breakdown field strength of the ceramics.
It is generally believed that the stability of antiferroelectricity is closely related to the tolerance factor t. Herein, the theoretical value of t is calculated on the basis of Eq. (1) and is displayed in Fig. 7c, which decreases linearly if all Lu3+ ions enter into A-site. In fact, due to the limited solid solubility of Lu3+ in AgNbO3 lattice, the possible value of t may be deviated to the theoretical value. As discussed previously, the solid solubility of Lu3+ in AgNbO3 lattice is around 2 mol%, so the possible value of t should decrease firstly (~ 2 mol%) and then become relatively stable with further increase of Lu2O3 content, similar to the dotted curve given Fig. 7c. The change of t and unit cell volume V may account for the complex evolution of EA and EF.
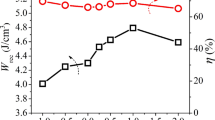
Figure 7d gives the Wrec and efficiency η of Lu2O3 modified AgNbO3 ceramics to evaluate the electric performance. The Wrec is up to 1.89 J/cm3 at 190 kV/cm for undoped AgNbO3, which increases to 3.5 J/cm3 accompanied with efficiency η of 53.1% after 1 mol% Lu2O3 addition at 210 kV/cm. With further increases of the Lu2O3 doping amount, the Wrec decreases due to the lower Pmax. The energy storage efficiency also increases from 37.9% (AN) to 66.5% (LuAN4) because of the decreased hysteresis of P–E loops. Furthermore, a comprehensive comparison of Wrec among other lead-free materials system are made in Table 1 [11, 17, 20, 36,37,38,39,40,41,42,43,44,45,46,47], which indicates that Lu2O3 doped AgNbO3 antiferroelectric ceramics are superior to the most of lead-free ceramics under moderate electric field (< 220 kV/cm).
4 Conclusion
In this work, Lu2O3 modified AgNbO3 lead-free antiferroelectric ceramics were synthesized via solid state reaction method. A certain amount of Lu2O3 (< 2 mol%) led a decrease in cell volume V and tolerance factor t, while second phase came to across with higher Lu2O3 level due to the limited solid solubility. The Lu3+ ions in the lattice benefited to the improvement of stability of antiferroelectric phase, thus leading to the enhanced EF and EA. As a consequence, high Wrec up to 3.5 J/cm3 accompanied with efficiency of 53.1% at 210 kV/cm was achieved in 1 mol% Lu2O3 doped AgNbO3 ceramics. The results indicate that Lu2O3 doped AgNbO3 antiferroelectric ceramics is promising for dielectric capacitors.
References
J. Cherusseri, N. Choudhary, K. Sambath Kumar, Y. Jung, J. Thomas, Recent trends in transition metal dichalcogenide based supercapacitor electrodes. Nanoscale Horiz. 4, 840–858 (2019)
X. Jian, H. Wang, G. Rao, L. Jiang, H. Wang, C.M. Subramaniyam, A. Mahmood, W. Zhang, Y. Xiang, S.X. Dou, Z. Zhou, D. Hui, K. Kalantar-Zadeh, N. Mahmood, Self-tunable ultrathin carbon nanocups as the electrode material of sodium-ion batteries with unprecedented capacity and stability. Chem. Eng. J. 364, 578–588 (2019)
B. Xu, J. Iniguez, L. Bellaiche, Designing lead-free antiferroelectrics for energy storage. Nat. Commun. 8, 15682 (2017)
Y. Guo, X. Jian, L. Zhang, C. Mu, L. Yin, J. Xie, N. Mahmood, S. Dou, R. Che, L. Deng, Plasma-induced FeSiAl@Al2O3@SiO2 core–shell structure for exceptional microwave absorption and anti-oxidation at high temperature. Chem. Eng. J. 384, 123371 (2020)
S. Aboubakr, A. Hajjaji, M. Rguiti, K. Benkhouja, C. Courtois, A high dielectric composite for energy storage application. Int. J. Hydrog. Energy 42, 19504–19511 (2017)
Y. Tian, L. Jin, H. Zhang, Z. Xu, X. Wei, E.D. Politova, S.Y. Stefanovich, N.V. Tarakina, I. Abrahams, H. Yan, High energy density in silver niobate ceramics. J. Mater. Chem. A 4, 17279–17287 (2016)
M. Zhou, R. Liang, Z. Zhou, S. Yan, X. Dong, Novel sodium niobate-based lead-free ceramics as new environment-friendly energy storage materials with high energy density, high power density, and excellent stability. ACS Sustain. Chem. Eng. 6, 12755–12765 (2018)
Y. Ahn, J. Seo, J.Y. Son, Ferroelectric domain switching kinetics of a lead-free AgNbO3 thin film on glass substrate. Appl. Surf. Sci. 357, 429–432 (2015)
M. Zhou, R. Liang, Z. Zhou, C. Xu, X. Nie, X. Chen, X. Dong, High energy storage properties of (Ni1/3Nb2/3)4+ complex-ion modified (Ba0.85Ca0.15) (Zr0.10Ti0.90)O3 ceramics. Mater. Res. Bull. 98, 166–172 (2018)
Y. Xie, J. Wang, Y. Yu, W. Jiang, Z. Zhang, Enhancing breakdown strength and energy storage performance of PVDF-based nanocomposites by adding exfoliated boron nitride. Appl. Surf. Sci. 440, 1150–1158 (2018)
L. Zhao, J. Gao, Q. Liu, S. Zhang, J.F. Li, Silver niobate lead-free antiferroelectric ceramics: enhancing energy storage density by B-site doping. ACS Appl. Mater. Interfaces 10, 819–826 (2018)
N. Luo, K. Han, L. Liu, B. Peng, X. Wang, C. Hu, H. Zhou, Q. Feng, X. Chen, Y. Wei, Lead-free Ag1−3xLaxNbO3 antiferroelectric ceramics with high-energy storage density and efficiency. J. Am. Ceram. Soc. 102, 4640–4647 (2019)
B. Peng, Q. Zhang, X. Li, T. Sun, H. Fan, S. Ke, M. Ye, Y. Wang, W. Lu, H. Niu, X. Zeng, H. Huang, Large energy storage density and high thermal stability in a highly textured (111)-oriented Pb0.8Ba0.2ZrO3 relaxor thin film with the coexistence of antiferroelectric and ferroelectric phases. ACS Appl. Mater. Interfaces 7, 13512–13517 (2015)
Y. Liu, Y. Wang, X. Hao, J. Xu, Preparation and energy-storage performance of PLZT antiferroelectric thick films via sol–gel method. Ceram. Int. 39, S513–S516 (2013)
X. Hao, Y. Wang, L. Zhang, L. Zhang, S. An, Composition-dependent dielectric and energy-storage properties of (Pb, La) (Zr, Sn, Ti)O3 antiferroelectric thick films. Appl. Phys. Lett. 102, 163903 (2013)
X. Yang, C. He, Y. Liu, X. Long, Structure and properties of (NaxLaxPb1−2x) (Lu1/2Nb1/2)O3 antiferroelectric ceramics. Mater. Des. 92, 330–334 (2016)
L. Zhao, Q. Liu, S. Zhang, J.-F. Li, Lead-free AgNbO3 anti-ferroelectric ceramics with an enhanced energy storage performance using MnO2 modification. J. Mater. Chem. C 4, 8380–8384 (2016)
Y. Tian, L. Jin, Q.Y. Hu, K. Yu, Y.Y. Zhuang, G. Viola, I. Abrahams, Z. Xu, X.Y. Wei, H.X. Yan, Phase transitions in tantalum-modified silver niobate ceramics for high power energy storage. J. Mater. Chem. A 7, 834–842 (2019)
N. Luo, K. Han, F. Zhuo, C. Xu, G. Zhang, L. Liu, X. Chen, C. Hu, H. Zhou, Y. Wei, Aliovalent A-site engineered AgNbO3 lead-free antiferroelectric ceramics toward superior energy storage density. J. Mater. Chem. A 7, 14118–14128 (2019)
K. Han, N. Luo, Y. Jing, X. Wang, B. Peng, L. Liu, C. Hu, H. Zhou, Y. Wei, X. Chen, Q. Feng, Structure and energy storage performance of Ba-modified AgNbO3 lead-free antiferroelectric ceramics. Ceram. Int. 45, 5559–5565 (2019)
Y. Tian, L. Jin, H. Zhang, Z. Xu, X. Wei, G. Viola, I. Abrahams, H. Yan, Phase transitions in bismuth-modified silver niobate ceramics for high power energy storage. J. Mater. Chem. A 5, 17525–17531 (2017)
K. Han, N. Luo, S. Mao, F. Zhuo, X. Chen, L. Liu, C. Hu, H. Zhou, X. Wang, Y. Wei, Realizing high low-electric-field energy storage performance in AgNbO3 ceramics by introducing relaxor behaviour. J. Materiomics 5, 597–605 (2019)
S. Li, H. Nie, G. Wang, C. Xu, N. Liu, M. Zhou, F. Cao, X. Dong, Significantly enhanced energy storage performance of rare-earth-modified silver niobate lead-free antiferroelectric ceramics via local chemical pressure tailoring. J. Mater. Chem. C 7, 1551–1560 (2019)
H. Shimizu, H. Guo, S.E. Reyes-Lillo, Y. Mizuno, K.M. Rabe, C.A. Randall, Lead-free antiferroelectric: xCaZrO3–(1–x)NaNbO3 system (0 ≤ x ≤ 0.10). Dalton Trans. 44, 10763–10772 (2015)
R. Xu, Q. Zhu, J. Tian, Y. Feng, Z. Xu, Effect of Ba-dopant on dielectric and energy storage properties of PLZST antiferroelectric ceramics. Ceram. Int. 43, 2481–2485 (2017)
K. Han, N. Luo, S. Mao, F. Zhuo, L. Liu, B. Peng, X. Chen, C. Hu, H. Zhou, Y. Wei, Ultrahigh energy-storage density in A-/B-site co-doped AgNbO3 lead-free antiferroelectric ceramics: insight into the origin of antiferroelectricity. J. Mater. Chem. A 7, 26293–26301 (2019)
L.A. Xue, Y. Chen, R.J. Brook, The effect of lanthanide contraction on grain growth in lanthanide-doped BaTiO3. J. Mater. Sci. Lett. 7, 1163–1165 (1988)
L.A. Xue, Y. Chen, R.J. Brook, The influence of ionic radii on the incorporation of trivalent dopants into BaTiO3. Mater. Sci. Eng. BI, 193–201 (1988)
C.L. Yuan, P. Darmawan, M.Y. Chan, P.S. Lee, Leakage conduction mechanism of amorphous Lu2O3 high-k dielectric films fabricated by pulsed laser ablation. Europhys. Lett. 77, 67001 (2007)
P. Darmawan, P.S. Chia, P.S. Lee, Rare-earth based ultra-thin Lu2O3 for high-k dielectrics. J. Phys. Conf. Ser. 61, 229–233 (2007)
G. Scarel, E. Bonera, C. Wiemer, G. Tallarida, S. Spiga, M. Fanciulli, I.L. Fedushkin, H. Schumann, Y. Lebedinskii, A. Zenkevich, Atomic-layer deposition of Lu2O3. Appl. Phys. Lett. 85, 630–632 (2004)
S. Ohmi, M. Takeda, H. Ishiwara, H. Iwai, Electrical characteristics for Lu2O3 thin films fabricated by E-beam deposition method. J. Electrochem. Soc. 151, G279–G283 (2004)
Q. Lin, M. Jiang, D. Lin, Q. Zheng, X. Wu, X. Fan, Effects of La-doping on microstructure, dielectric and piezoelectric properties of Ba0.85Ca0.15Ti0.90Zr0.10O3 lead-free ceramics. J. Mater. Sci. Mater. Electron. 24, 734–739 (2012)
B.W. Lee, E.J. Lee, Effects of complex doping on microstructural and electrical properties of PZT ceramics. J. Electroceram. 17, 597–602 (2006)
G. Viola, T. Saunders, X. Wei, K.B. Chong, H. Luo, M.J. Reece, H. Yan, Contribution of piezoelectric effect, electrostriction and ferroelectric/ferroelastic switching to strain-electric field response of dielectrics. J. Adv. Dielectr. 03, 1350007 (2013)
M. Wei, J. Zhang, K. Wu, H. Chen, C. Yang, Effect of BiMO3 (M=Al, In, Y, Sm, Nd, and La) doping on the dielectric properties of BaTiO3 ceramics. Ceram. Int. 43, 9593–9599 (2017)
H. Yang, F. Yan, Y. Lin, T. Wang, F. Wang, Y. Wang, L. Guo, W. Tai, H. Wei, Lead-free BaTiO3–Bi0.5Na0.5TiO3–Na0.73Bi0.09NbO3 relaxor ferroelectric ceramics for high energy storage. J. Eur. Ceram. Soc. 37, 3303–3311 (2017)
Y. Lin, D. Li, M. Zhang, S. Zhan, Y. Yang, H. Yang, Q. Yuan, Excellent energy-storage properties achieved in BaTiO3-based lead-free relaxor ferroelectric ceramics via domain engineering on the nanoscale. ACS Appl. Mater. Interfaces 11, 36824–36830 (2019)
T. Wang, L. Jin, Y. Tian, L. Shu, Q. Hu, X. Wei, Microstructure and ferroelectric properties of Nb2O5-modified BiFeO3–BaTiO3 lead-free ceramics for energy storage. Mater. Lett. 137, 79–81 (2014)
D. Zheng, R. Zuo, D. Zhang, Y. Li, X. Tan, Novel BiFeO3–BaTiO3–Ba(Mg1/3Nb2/3)O3 lead-free relaxor ferroelectric ceramics for energy-storage capacitors. J. Am. Ceram. Soc. 98, 2692–2695 (2015)
D. Wang, Z. Fan, D. Zhou, A. Khesro, S. Murakami, A. Feteira, Q. Zhao, X. Tan, I.M. Reaney, Bismuth ferrite-based lead-free ceramics and multilayers with high recoverable energy density. J. Mater. Chem. A 6, 4133–4144 (2018)
Z. Chen, X. Bai, H. Wang, J. Du, W. Bai, L. Li, F. Wen, P. Zheng, W. Wu, L. Zheng, Y. Zhang, Achieving high-energy storage performance in 0.67Bi1−xSmxFeO3–0.33BaTiO3 lead-free relaxor ferroelectric ceramics. Ceram. Int. (2020). https://doi.org/10.1016/j.ceramint.2020.01.181
L. Zhang, Y. Pu, M. Chen, T. Wei, W. Keipper, R. Shi, X. Guo, R. Li, X. Peng, High energy-storage density under low electric fields and improved optical transparency in novel sodium bismuth titanate-based lead-free ceramics. J. Eur. Ceram. Soc. 40, 71–77 (2020)
L. Zhang, Y. Pu, M. Chen, T. Wei, X. Peng, Novel Na0.5Bi0.5TiO3 based, lead-free energy storage ceramics with high power and energy density and excellent high-temperature stability. Chem. Eng. 383, 123154 (2020)
F. Li, R. Si, T. Li, C. Wang, J. Zhai, High energy storage performance and fast discharging speed in dense 0.7Bi0.5K0.5TiO3–0.3SrTiO3 ceramics via a novel rolling technology. Ceram. Int. 46, 6995–6998 (2020)
F. Li, T. Jiang, J. Zhai, B. Shen, H. Zeng, Exploring novel bismuth-based materials for energy storage applications. J. Mater. Chem. C 6, 7976–7981 (2018)
Z. Yang, F. Gao, H. Du, L. Jin, L. Yan, Q. Hu, Y. Yu, S. Qu, X. Wei, Z. Xu, Y.-J. Wang, Grain size engineered lead-free ceramics with both large energy storage density and ultrahigh mechanical properties. Nano Energy 58, 768–777 (2019)
Acknowledgements
This work was supported by National Nature Science Foundation of China (Grant 11864004), Nature Science Foundation of Guangxi (Grant 2017GXNSFBA198132), Science and Technology Major Project of Guangxi (Grant AA17204110), and Innovation Project of Guangxi Graduate Education (Grant YCSW2019053).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mao, S., Luo, N., Han, K. et al. Effect of Lu doping on the structure, electrical properties and energy storage performance of AgNbO3 antiferroelectric ceramics. J Mater Sci: Mater Electron 31, 7731–7741 (2020). https://doi.org/10.1007/s10854-020-03309-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03309-7