Abstract
(1 − x)[0.9(Bi0.5Na0.5)TiO3–0.1BiScO3]–xBaTiO3 (BNT–BS–xBT) ceramics are prepared by the traditional solid-state sintering. The structure, morphology, ferroelectricity, strain, energy storage, dielectricity, and impedance of the BNT–BS–xBT ceramics are investigated. XRD shows that all ceramics have pseudo-cubic structures. The results also show that BT can refine the grain size of the ceramics and reduce the corresponding density. At x = 0.20, the energy storage performance of the ceramics is optimum (Wrec = 0.563 J/cm3, η = 63%). At x = 0.10, the electrostriction coefficient (Q33) of the ceramics reaches 2.72325 × 10−2 m4/C2. Dielectric and impedance spectroscopies show that the ceramics are relaxor ferroelectrics and have good insulation properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the development of science and technology, traditional devices are developing towards miniaturization and multifunction, which needs materials with multiple properties at the same time. PbZrTiO3 (PZT) is used in piezoelectric ceramic field owing to its excellent energy storage density (Wrec = 63.7 J/cm3) and efficiency (η = 81.3%) [1] and electrostrictive coefficient (Q33 = 0.033–0.036 m4/C2) [2]. However, the volatilization of toxic PbO during high-temperature sintering not only causes environmental pollution but also generates instability in the composition and electrical properties of the products [3, 4]. Therefore, the development of lead-free piezoelectric materials has attracted much attention and many research programs for them have been carried out [5,6,7,8,9].
In 1961, Smolensky first synthesized (Bi1−xNax)TiO3 (BNT) lead-free ceramics, and they have attracted a lot of attentions due to its strong ferroelectricity (Pr = 38 μC/cm2), large piezoelectric coefficient (K33 = 40–50%), and other advantages [10, 11]. However, it is difficult to be polarized because of its high coercive field (Ec = 73 kV/cm); the high Ec also leads to large energy loss and affects energy storage performance. The defects make BNT ceramics difficult to replace Pb-based ceramics. Multi-component solid solution is one of the commonly used optimization methods for BNT ceramics. Some researchers have reported that the energy storage of BNT ceramics can be effectively improved by the method. For example, the introduction of SrTiO3 and Sr (Ti0.85Zr0.15) O3 into BNT ceramics can make the energy storage density of BNT ceramics reach 3.13 J/cm3, and the energy storage efficiency is 91.14% [12]; The energy storage density of solid solution of BNT and Ba0.94La0.04Zr0.02Ti0.98O3 under the action of SrTiO3 and Li2CO3 is also as high as 2.72 J/cm3 [13]. When SrY0.5Nb0.5O3 is introduced into BNT-BT ceramics, the energy storage density of the ceramics is also 1.36 J/cm3 [14]; In BNT–SrTiO3 ceramics, the introduction of Bi (Mg2/3Nb1/3)O3 makes the energy storage density reach 3.45 J/cm3 [15]. These results show that multi-component solid solution can effectively optimize the energy storage performance of BNT-based ceramics.
As for the electrostrictive properties of BNT-based ceramics, there are also many literature reports that (1 − x)(Bi0.5Na0.5)TiO3−xBaTiO3 (BNT–BT) piezoelectric ceramics have morphotropic phase boundaries between the rhombohedral and tetragonal phases, where the piezoelectric property of BNT–BT ceramics is the best (d33 = 125 pC/N), the Ec of the ceramics is also significantly reduced to 30 kV/cm, which makes the ceramics easier to polarize and reduces the energy loss in the energy storage [8, 9], and the electrostrictive coefficient (Q33) of the ceramics also reached 0.022 m4/C2 [16]. Further, researchers found that adding K0.5Na0.5NbO3 (KNN) or SrTiO3 to BNT–BT ceramics can optimize the Q33 of the ceramics, the results showed that the Q33 of the ceramics all reached 0.026 m4/C2 [17, 18]. We have found that the BiScO3 (BS) could improve the energy storage and the ferroelectric properties of BNT ceramics, in which the Ec of 0.9BNT–0.1BS ceramics can be effectively reduced to 15 kV/cm, and the energy storage density of the ceramics can reach 0.17 J/cm3 [Details in the Supporting Information]. It also has been reported that BT can also optimize the Q33 of BNT ceramics reported in other works [16,17,18]. Therefore, we want to further optimize the BNT-based ceramics to have better energy storage and electrostrictive properties at the same time. This is a very interesting attempt; the 0.9BNT–0.1BS ceramic was selected as the matrix to investigate the effect of BT doping on the structure, surface morphology, and electrical properties of the 0.9BNT–0.1BS ceramics. The results indicated that the (1 − x)[0.9(Bi0.5Na0.5)TiO3–0.1BiScO3]–xBaTiO3 (BNT–BS–xBT) ceramics can achieve an excellent energy storage efficiency (η = 63%) and a high electrostrictive coefficient (Q33 = 2.72325 × 10−2 m4/C2).
2 Experimental
Six different compositions with x = 0.00, 0.05, 0.10, 0.15, 0.20, and 0.25 in the BNT–BS–xBT ceramic system were designed to prepare the samples. Bi2O3, Na2CO3, BaCO3, Sc2O3, and TiO2 of purities 99.0%, 99.8%, 98.0%, 99.9%, and 99.0%, respectively, were used as raw materials. The required quantity of each powder was computed according to the corresponding chemical formula, and the raw material powders were weighed using an electronic balance with a precision of 0.1 mg. The weighed raw material powders were added to absolute ethanol and the resulting mixture was further poured into a ball mill jar which contained zirconia balls. Mechanical milling was performed for 24 h to ensure full and uniform mixing. The mixture of the raw material after ball grinding was then dried at 90 °C and further calcinated at 880 °C for 2 h. An appropriate amount of 7% polyvinyl alcohol (PVA) binder was added to the formula powder obtained after pre-sintering, and this was further followed by uniform mixing. The formula powder was dried and passed through a 100 mesh sieve to obtain granulated powder. The granulated powder was pressed into green bodies with a dimension of 13 mm and a thickness of 1 mm at 40 MPa. Subsequently, the PVA binder were released from green bodies at 600 °C, heated at 1150 °C for 2 h, and cooled inside the furnace to 30 °C. The sintered samples were polished and cleaned on both sides, and a silver paste with a diameter of 8 mm was printed and held at 600 °C for 30 min to obtain a silver electrode. After cooling to room temperature, the samples were taken out for electrical performance testing.
X-ray diffraction (XRD, Empyrean Pixcel3D) was used to analyze the phase structure of the ceramics, while field emission scanning electron microscopy (FESEM, Quanta 450 FEG, FEI) was used to observe the surface morphology and grain size of the samples. A computer-controlled impedance analyzer (4294A, Agilent) was used to determine the temperature-dependent dielectric properties from ambient temperature to 600 °C. An impedance analyzer (Agilent 4294) was used to measure the temperature-dependent impedance spectrum from 40 Hz to 1 MHz. The current–electric field (I–E) curves and polarization–electric field (P–E) hysteresis loops of the samples were measured by a ferroelectric tester (TF Analyzer 2000, aixACCT).
3 Results and discussion
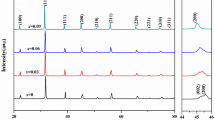
The XRD patterns of the BNT–BS–xBT ceramics are shown in Fig. 1. The XRD patterns of all compositions have a single perovskite structure, which indicates the dissolution of BT into the 0.9BNT–0.1BS matrix to form a new solid solution. Figure 1b and c are the enlarged images of the (111) and (200) diffraction peaks, respectively.
As we known, pure BNT is rhombohedral phase and pure BT is tetrahedral phase [19]. Peak splitting of (111) peak at 40° implies that there is rhombohedral phase, and the splitting at 46.5° for (200) peak indicates the tetrahedral phase [20]. In Fig. 1b and c, all peaks have non-splitting peak characteristics, which suggests that the BNT–BS–xBT ceramics have pseudo-cubic structures. The related phenomena have been reported by Hiruma, in which the crystal structure of BNT–KNbO3 and BNT–NaNbO3 ceramics transforms from rhombohedral to pseudo-cubic indicating a ferroelectric transformed into antiferroelectric [21]. However, the (111) and (200) peaks are asymmetric due to the existence of shoulder peaks, which implies that the ceramics are not complete pseudo-cubic structure, that is the rhombohedral and tetrahedral phases don’t completely transform into pseudo-cubic phase. It should be noted that the symmetry of (111) and (200) peaks increases gradually with the increase of BT content, which indicates that BT makes the phase structure of these ceramics transform into pseudo-cubic more thorough. Furthermore, it is observed that the (111) and (200) diffraction peaks shift to low angles with an increase in BT content. This shift can be ascribed to the crystal lattice expansion of the 0.9BNT–0.1BS ceramics. Such enlargement of the crystal lattice occurs because the ionic radius of Ba2+ is greater than that of the A-site and the ionic radius of Ti4+ is similar to that of the B-site in the 0.9BNT–0.1BS matrix.
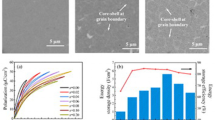
Figure 2 shows the microscopic morphologies of the BNT–BS–xBT ceramics. The 0.9BNT–0.1BS ceramics have a compact and square structure. The average grain size of ceramics is 3.2 μm at x = 0 in Fig. 3a. After doping BT component, the grain size of the BNT–BS–xBT ceramics gradually decreased. At x = 0.25, shown in Fig. 3f, the average grain size is 1.71 μm and the grain shape changed from a cubic to spherical configuration along with a decrease in compactness. This may be due to the substitution of Na+ ions by Ba2+ ions, which are similar in radii to Na+ ions. When Ba2+ ions replace Na+ ions, VNa' is produced in the ceramics cell in order to maintain the balance of chemical valence, which decreases the ceramic density and further makes the lattice structure incompact [22].
Figure 4a shows the P–E hysteresis loops of the BNT–BS–xBT ceramics at an electric field lower than 70 kV/cm. The hysteresis loops of all samples have slender curves with small Ec and Pr, indicating that the BNT–BS–xBT ceramics possessed strong relaxor characteristics. With an increase in BT content (Fig. 4b), the maximum polarization (Pmax) first increases and then decreases, but Pr does not show any variation. When x = 0.15, the difference between Pmax and Pr reaches a maximum value; this can be beneficial in obtaining good energy storage properties. The composition dependence of the Wrec and η of the BNT–BS–xBT ceramics is plotted in Fig. 4c. Wrec and η show a trend similar to that of Pmax and Pr. When x = 0.20, Wrec and η can reach the peak value of 0.563 J/cm3 and 63%, respectively. The critical point of x = 0.15 can be observed when the difference between Pmax and Pr is becomes larger; however, this composition does not suggest that the energy storage density reaches a maximum value. As shown in Fig. 4d, P1 and P2 are polarization differences at x = 0.15 and 0.20, respectively. Although the polarization difference at x = 0.15 is greater than that at x = 0.20, the P–E loops are much flatter at x = 0.2 due to the BT component doping, which enhances the relaxor properties of the BNT–BS–xBT ceramics. The relaxor state is conducive for increasing the energy storage density. Therefore, BT can improve the energy storage performance of BNT–BS matrix.
The relaxor characteristics of the BNT–BS–xBT ceramics were also reflected in the strain curves. Figure 5a shows the field-induced strain (S–E) curves of the BNT–BS–xBT ceramics at 70 kV/cm. Assuming that the BNT–BS–xBT ceramics are in an relaxor state, the S–E curves are shaped as a sprout without the negative strain, which indicates low ferroelectric domain movement [23,24,25,26]. With an increase in the BT content of the solid solution, the maximum field-induced strain gradually increased from 0.02 to 0.15% and began to appear slightly asymmetrical. BNT–BS–xBT ceramics are relaxor ferroelectrics with strong electrostriction behavior. Hence, the electrostriction coefficient (Q33) was analyzed in this study. The S–P2 curves can be linearly fitted according to the formula S = Q33P2, and the results are shown in Fig. 5c. The relationships between S and P2 at all composition points are generally linear. Thus, the field-induced strain of the BNT–BS–xBT ceramics is mainly due to the electrostriction effect [27,28,29]. Table 1 shows the electrostriction coefficients (Q33) of BNT–BS–xBT ceramics. Owing to the asymmetric strain of the ceramics, Q33 is calculated under positive and negative electric fields to derive the average value. When x = 0.1, the negative Q33 of the ceramics reaches the maximum value of 2.72325 × 10−2 m4/C2 with an average value of 2.41909 × 10−2 m4/C2. Compared with other BNT-based ceramics, for example, BNT–BT–KNN ceramics, obtained a high electrostrictive coefficient of 2.4 × 10−2 m4/C2 at an electric field of less than 60 kV/cm [30], the BNT–BS–xBT ceramics were not only able to withstand a higher electric field (70 kV/cm), but also had a higher electrostriction coefficient of 2.72325 × 10−2 m4/C2. This may be attributed to the high electrostriction effect of the relaxor in the disordered state due to the large vibration space [31, 32].
Figure 6 shows the temperature dependence of the dielectric constant (εr) and dielectric loss (tanδ) of the BNT–BS–xBT ceramics at frequencies of 1, 10, and 100 kHz. As shown in Fig. 6a, the Curie temperature (Tm) of the ceramics is 372 ℃ at x = 0. The Tm peak shifts to the low-temperature region with an increase in the BT content of the solid solution. When x = 0.25 (Fig. 6f), the Tm peak decreases to 232 ℃, indicating that the increase in the BT content inhibited the ferroelectric stability of the 0.9BNT–0.1BS ceramics [33]. Moreover, the maximum dielectric constant (εr) of the ceramics was also affected by the BT content of the solid solution. In particular, the maximum dielectric constant (εr) of the ceramics was 1670 at the test frequency of 100 kHz at x = 0. With the introduction of BT, the maximum dielectric constant (εr) of the ceramics increased significantly. When x = 0.15, the maximum dielectric constant (εr) reached the maximum value of 4185. Although the BT content continued to increase, the maximum dielectric constant (εr) of the ceramics remained stable at approximately 4000. Oxygen vacancies and ionic polarizability can be used to analyze the increase of dielectric constant [34]. As the ionic radius of Ba2+ (0.135 nm) is closer to that of Bi3+ (0.103 nm) than that of Na+ (0.102 nm), Ba2+ ions mainly replace Bi3+ ions. The substitution process generates oxygen vacancies inside the ceramics. The Ti element in BNT-based ceramics will transform from Ti4+ to Ti3+ during sintering process, which produces the oxygen vacancies [35,36,37]. The oxygen vacancies inside the ceramic cause the distortion of ceramic grain cells and an increase in the active area of Ti4+ within the Ti–O6 octahedron; further, all such changes are beneficial to the relative displacement of the positive and negative ions inside the ceramic at 90° domain steering [34]. The ionic polarizability of Ba2+ ion is 1.31 C m2/V, which is larger than that of Na+ (0.199 C m2/V) and Bi3+ (0.387 C m2/V), Ba2+ ions are easier to be polarized than Bi3+ and Na+ ions [34, 38]. Hence, the dielectric constant of BNT–BS–xBT ceramics is increased because of the above two reasons. Moreover, the dielectric loss (tanδ) of the ceramics increased sharply at high temperatures due to the introduction of BT in the solid solution, which may be related to the polarization of the space charge within the ceramics by the Shockley–Read mechanism [39, 40]. At the same time, the dielectric coat temperatures less thnstant (εr) of the ceramics depended largely on frequency. When the temperature was lower than Tm, the frequency dispersion decreased; however, when the temperature became higher than Tm, the frequency dispersion increased. This phenomenon is due to the change in symmetry of the polar nanoregions (PNRs) within the ceramics and the relaxation of these PNRs at high temperatures [41]. The BNT–BS–xBT ceramics are relaxor ferroelectrics.
Figure 7a shows the impedance spectrum of the BNT–BS–0.2BT ceramic from 100 to 550 °C (40 Hz–1 MHz). Only single semi-circular arc is observed for the impedance spectrum at different temperatures in the test frequency range. The semi-circular arc of the BNT–BS–0.2BT ceramics is essentially a Debye-like semicircle without distortion, and its impedance behavior can be derived from the internal grain effect of the material. The impedance spectra show strong linear characteristics at temperatures less than 450 °C, and a complete semicircle appears gradually when the temperature increases to 500 °C, which indicates that the ceramic material has good insulation properties [42]. Figure 7b shows the relationship between the imaginary part of the impedance spectra (− Z") and the frequency (f) of the BNT–BS–0.2BT ceramics. Only one characteristic peak at different test temperatures can be observed, which corresponds to the semicircle in the temperature range of 450–550 °C in the impedance spectra. The grains of the BNT–BS–0.2BT ceramic are ferroelectric, and the grain boundary is amorphous and non-ferroelectric. Thus, the grains of the BNT–BS–0.2BT ceramic play a major role in the impedance of these materials. With an increase in temperature, the maximum value of the imaginary part of impedance decreased, which indicated that the BNT–BS–0.2BT ceramics had negative temperature coefficient characteristics [43]. Meanwhile, the characteristic peak shifted to the high-temperature region, indicating the extension of the relaxation time of polarizable grains and the existence of heat-induced relaxation in the ceramics [44,45,46].
4 Conclusion
The structure, ferroelectricity, strain, energy storage, dielectric, and impedance properties of 0.9BNT–0.1BS ceramics were significantly affected by doping BT. XRD shows that the phase structure is pseudo-cubic. The BT results in the lattice expansion, inhibits the grain growth, and increases the porosity of these ceramics. The average grain size is 3.2 μm at x = 0 and decreases gradually to 1.71 μm. The ceramic at x = 0.20 has a high η of 63%, and its Wrec reaches 0.563 J/cm3. The field-induced strain increases with the increase in BT content. The strain obtains the maximum value of 0.15% at x = 0.25, and a high electrostriction coefficient of 2.72325 × 10−2 m4/C2 is achieved at x = 0.10. The Tm peaks decreases from 372 to 232 °C with the increase of BT content, indicating the decreasing of the ferroelectric stability. The maximum permittivity increases first and then decreases and reaches max value of 4185 at x = 0.15. The ceramics have good insulation properties, and the impedance behavior can be derived from the internal grain effect.
References
T.D. Zhang, W.L. Li, Y. Zhao, Y. Yu, W.D. Fei, Adv. Funct. Mater. 28, 1706211 (2018)
V.D.N. Tran, T.H. Dinh, H.-S. Han, W. Jo, J.-S. Lee, Ceram. Int. 39, S119 (2013)
X. Wang, L.W. Chan, C.L. Choy, Solid State Commun. 125, 395 (2003)
W. Li, Z.J. Xu, R.Q. Chu, P. Fu, G.Z. Zang, J. Am. Ceram. Soc. 93, 2942 (2010)
X.Y. Zhao, H. Wang, C.L. Yuan, J.W. Xu, Y.R. Cui, J.F. Ma, J. Mater. Sci. - Mater. Electron. 24, 1480 (2013)
H. Wang, X.Y. Zhao, J.W. Xu, L. Yang, J. Mater. Sci. Electron. 26, 6129 (2015)
H. Wang, X.Y. Zhao, J.W. Xu, X. Zhai, L. Yang, J. Mater. Sci. Mater. Electron. 27, 2036 (2016)
W.J. Wu, D.Q. Xiao, J.G. Wu, J. Li, W.F. Liang, C. Zhang, Z. Wang, Z.Y. Li, J.G. Zhu, J. Funct. Mater. 11, 2050 (2011)
M. Chandrasekhar, P. Kumar, Ceram. Int. 41, 5574 (2015)
T. Takenaka, K. Maruyama, K. Sakata, Jpn. J. Appl. Phys. 30, 2236 (1991)
H.P. Liu, X.Y. Huang, C.H. Gao, Chin. Ceram. Soc. 24, 70 (2005)
D. Li, Y. Lin, M. Zhang, H.B. Yang, Chem. Eng. J. S1385, 33144 (2019)
H.B. Yang, P.F. Liu, F. Yan, Y. Lin, T. Wang, J. Alloys Compd. S0925, 33500 (2018)
X.Y. Liu, H.B. Yang, F. Yan, Y. Qin, Y. Lin, T. Wang, J. Alloys compd. 778, 97 (2019)
Y. Lin, D. Li, M. Zhang, H.B. Yang, J. Mater. Chem. C. https://doi.org/10.1039/c9tc06218a (2020)
S.T. Zhang, A.B. Kounga, W. Jo, C. Jamin, K. Seifert, T. Granzow, J. Rodel, D. Damjanovic, Adv. Mater. 21, 4716 (2009)
S.T. Zhang, F. Yan, B. Yang, W.W. Cao, Appl. Phys. Lett. 97, 122901 (2010)
F.F. Wang, C.C. Jin, Q.R. Yao, W.Z. Shi, J. Appl. Phys. 114, 027004 (2013)
C.G. Xu, D.M. Lin, K.W. Kwok, Soild. State. Sci. 10, 934 (2008)
Q. Xu, T.M. Li, H. Hao, S.J. Zhang, Z.J. Wang, M.H. Cao, Z.H. Yao, H.X. Liu, J. Eur. Ceram. Soc. 35, 545 (2015)
Y. Hiruma, H. Nagata, T. Takenaka, J. Appl. Phys. 104, 124106 (2008)
Y. Qu, D. Shan, J. Song, Mater. Sci. Eng. B 121, 148 (2005)
W.G. Ma, Y.W. Zhu, M.A. Marwat, P.Y. Fan, B. Xie, D. Salamon, Z.G. Ye, H.B. Zhang, J. Mater. Chem. C 7, 281 (2019)
D.S. Yin, Z.H. Zhao, Y.J. Dai, Z. Zhao, X.W. Zhang, S.H. Wang, J. Am. Ceram. Soc. 99, 2354 (2016)
N. Kumar, T.Y. Ansell, D.P. Cann, J. Appl. Phys. 115, 154104 (2014)
M.L. Liu, D.A. Yang, Y.F. Qu, J. Alloys Compd. 496, 449 (2010)
S.J. Pang, L. Yang, J.Y. Qin, H. Qin, H. Xie, H. Wang, C.R. Zhou, J.W. Xu, Appl. Phys. A 125, 119 (2019)
S. Nomura, K. Uchino, Ferroelectrics 41, 117 (1982)
J.G. Hao, Z.J. Xu, R.Q. Chu, W. Li, J. Du, J. Mater. Sci. 50, 5328 (2015)
J.G. Hao, C.G. Ye, B. Shen, J.W. Zhai, J. Appl. Phys. 114, 054101 (2013)
L. Jin, R.J. Huo, R.P. Guo, F. Li, D.W. Wang, Y. Tian, Q.Y. Hu, X.Y. Wei, Z.B. He, Y. Yan, G. Liu, ACS Appl. Mater. Interfaces. 8, 31109 (1944)
S.T. Zhang, A.B. Kounga, W. Jo, C. Jamin, K. Seifert, T. Granzow, R. Jürgen, D. Damjanovic, Adv. Mater. 21, 4716 (2009)
C. Wang, X.J. Lou, T.D. Xia, S.T. Tian, Ceram. Int. 43, 9253 (2017)
W.F. Mu, H.L. Du, X. Shi, S.M. Du, J. Chin. Ceram. Soc. 39, 1941 (2011)
W.B. Hu, Y. Liu, R.L. Withers, T.J. Frankcombe, L. Noren, A. Snashall, M. Kitchin, P. Smith, B. Gong, H. Chen, J. Schiemer, F. Brink, W.-L. Jennifer, Nat. Mater. 12, 821 (2013)
W. Dong, W.B. Hu, T.J. Frankcombe, D.H. Chen, C. Zhou, Z.X. Fu, C. Ladir, G.Q. Hai, H. Chen, Y.X. Li, R.L. Withers, Y. Liu, J. Mater. Chem. A 5, 5436 (2017)
Z.T. Li, H. Liu, H.C. Thong, Z. Xu, M.H. Zhang, J. Yin, J.F. Li, K. Wang, J. Chen, Adv. Electron. Mater. 5, 1800756 (2018)
Y.B. Feng, S.Y. Zhang, H.T. Sun, Y. Li, Y.Y. Zhu, J. Dalian Inst. Light Ind. 19, 98 (2000)
R.R. Neurgaonkar, J.R. Oliver, W.K. Cory, L.E. Cross, Mater. Res. Bull. 18, 735 (1983)
R.R. Neurgaonkar, J.G. Nelson, J.R. Oliver, L.E. Cross, Mater. Res. Bull. 25, 959 (1990)
C. Jiang, X.F. Zhou, K.C. Zhou, C. Chen, H. Luo, X. Yuan, D. Zhang, J. Eur. Ceram. Soc. 36, 1377 (2016)
J.R. Cheng, G.Y. Shi, Y.F. Qi, J.G. Chen, Y.S. Wen, J. Shanghai Univ. (Natural Science Edition) 17, 213 (2011)
G.B. Liu, H. Wang, H. Xie, S.J. Pang, C.R. Zhou, J.W. Xu, J. Inorg. Mater. 33, 1331 (2018)
G.C. Liu, H.Q. Fan, G.Z. Dong, J. Shi, Q. Chang, J. Alloys Compd. 664, 632 (2016)
S.N. Das, A. Pattanaik, S. Kadambini, S. Pradhan, S. Bhuyan, R.N.P. Choudhary, J. Mater. Sci. Mater. Electron. 27, 10099 (2016)
M.A. Rafiq, M. Rasheed, Q.K. Muhammad, M. Waqar, M. Zubair, J. Mater. Sci. Mater. Electron. 28, 15009 (2017)
Acknowledgements
This work is supported by the National Nature Science Foundation of China (Grant Nos. 11664006, 61965007), Guangxi Nature Science Foundation (Grant No. 2018GXNSFDA281042), and Guangxi Key Laboratory of Information Materials (Grant No. 171009-Z).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, Y., Wang, H., Liu, G. et al. High energy storage efficiency and high electrostrictive coefficients in BNT–BS–xBT ferroelectric ceramics. J Mater Sci: Mater Electron 31, 5546–5553 (2020). https://doi.org/10.1007/s10854-020-03119-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03119-x