Abstract
Nickel foam (NF) was used as sacrificial template to synthesize graphene foam (GF) by chemical vapor deposition (CVD). ZnO NSAs were grown on the GF by hydrothermal synthesis to fabricate ZnO NSA/GF, which was used to detect dopamine in the presence of ascorbic acid and uric acid. The inter-connected ZnO NSAs with the sheet size of ~ 10 μm and thickness of ~ 500 nm uniformly distributed on the GF. The ZnO NSA/GF shows a sensitivity of 0.95 μA μM−1 in the concentration range of 0–80 μM and a measured limit of detection of 1 μM for determination of dopamine. Furthermore, the prepared electrode displays a reliable anti-interference to ascorbic acid and uric acid. It also shows an excellent reproducibility and long-time stability. The ZnO NSA/GF electrode was applied in human urine samples and exhibited satisfied recoveries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As an important neurotransmitter, dopamine (DA) is used to help cells transmit pulses. It plays an important role in the central nervous system of human body. Any deviation in DA levels in the human body is associated with neurological diseases [1,2,3,4,5]. So it is significant to precisely determine the DA level in human body.
The electrochemical method has received considerable interest for detecting biomolecules due to fast response, convenient operation, simplicity, high sensitivity and reproducibility [6,7,8]. How to improve the sensitivity and selectivity of modified electrodes is an urgent problem for researchers [9]. Some electrochemical electrodes have been reported for the detection of DA [9,10,11,12,13,14,15,16,17,18,19,20,21]. However, several serious problems are still existed, including narrow linear dynamic range, low sensitivity and poor selectivity.
Graphene is a two-dimensional material with single sp2-hybridized carbon atoms, has been applied in sensors, liquid crystal devices and field effect transistors owing to the unique mechanical, thermal, and electronic properties [22]. Graphene foam (GF) has shown a promising platform for electrochemical biosensors. Three-dimensional (3D) GF synthesized by chemical vapor deposition (CVD) is a seamlessly continuous graphene network with few defects and high conductivity [23, 24]. In addition, the 3D multi-porous structure of GF is an ideal carrier for combining with other nanomaterials [25].
Zinc oxide (ZnO), an environmental-friendly semiconductor, has attracted much attention due to the excellent optical, electrocatalytic and sensing properties. ZnO is a unique metal oxide with several scientific and engineering applications including field emission displays, ultraviolet (UV) lasers, dye-sensitized solar cells and gas/bio sensors [26,27,28]. In these years, the abundant nanostructures of ZnO with various morphologies have been successfully synthesized, for instance, nanoparticles, nanowires, nanorods, nanotubes, nanobelts, nanoplates, nanowire/nanosheet arrays, nanoflowers and so on [29,30,31]. Among them, the ZnO nanosheet arrays (ZnO NSAs) are of great interests due to the nanometer-scale thickness, large specific surface area and great potential for fabricating facile biosensors. There are many ways to synthesize ZnO NSAs, such as CVD, electrodeposition, thermal evaporation and hydrothermal synthesis [32,33,34,35]. Comparatively, the hydrothermal synthesis is a versatile approach for its simplicity, convenience and large-scale production.
Herein, nickel foam (NF) was used as the sacrificial template to prepare 3D GF by CVD and ZnO NSAs were vertically grown on the GF by hydrothermal synthesis. The obtained ZnO NSA/GF was used as working electrode to detect the content of dopamine under the interference of ascorbic acid (AA) and uric acid (UA). Because of the synergistic effect of the two materials, the ZnO NSA/GF composite shows good electrochemical properties and has great prospects in clinical application.
2 Experimental
2.1 Preparation of the ZnO NSA/GF
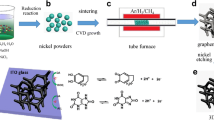
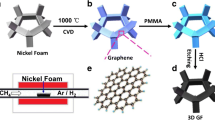
The GF was synthesized by CVD using CH4 as carbon source and NF as sacrificial template [36]. The obtained GF was transferred onto ITO coated glass and freeze-dried in vacuum.
Zinc oxide seed layer was prepared by dissolving Zn(CH3COO)2·2H2O in methanol (0.01 M) solution, then dropping it into the GF above and annealed at 250 °C for 1 h to obtain ZnO seed layer. Then, ZnO-seeded GF was immersed in Zn(NO3)2·6H2O (0.05 M), NH3·H2O (0.05 M) and HMTA (0.05 M) and hydrothermal reaction was conducted at 100 °C for 12 h. Finally, the prepared ZnO NSA/GF was annealed at 450 °C for 1 h.
2.2 Characterizations
The morphology of ZnO NSA/GF was observed by scanning electron microscopy (SEM) of JSM700F. The ZnO NSA/GF were characterized by Rigaku Ratoflex D/MAX X-ray diffractometer (XRD). Raman spectra were recorded on a micro-Raman system of RM-1000 in via.
2.3 Electrochemical measurements
All electrochemical measurements were carried out using VMP3 electrochemical workstation in phosphate buffer saline solution (0.01 M, pH 7.0) using three-electrode system. The ZnO NSA/GF was the working electrode, Ag/AgCl and platinum wire were used as referance and counter electrode, respectively. The responses of cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were recorded in the range of 0.2 to 0.6 V. The effective area of ZnO NSA/GF was 0.7 cm2.
3 Results and discussion
3.1 Characterizations of the ZnO NSA/GF
The redox reaction diagram of AA, DA and UA in the electrochemical process of ZnO-NSA/GF is shown in Fig. 1. The biomolecules are oxidized at the ZnO NSA/GF accompanying the generation of electrons and protons. The electrons directly transfer to the ITO coated glass. The electrochemical reactions of DA and UA are reversible, while the reaction of AA is irreversible.
Figure 2 is the SEM morphologies of the GF and ZnO NSA/GF. The GF shows the sililar porous structure of nickel foam after eching nickel and the pore size is ~ 200 μm, as shown in Fig. 2a. The ZnO NSAs with the average sheet size of ~ 10 μm and thickness of ~ 500 nm are fully covered on the GF (Fig. 2b–d). The formation of ZnO NSAs can be summarized as the following equations:
In the synthesis process, the ammonia releases OH− continually (Eq. 1). Then, the hydroxylation reaction between Zn2+ and OH− occurs to form Zn(OH)2 (Eq. 2). Finally, the Zn(OH)2 is decomposed to ZnO (Eq. 3). The Al slice plays an important role in the growth system, since the formation of \({{\text{Al(OH}})_{4}}^{ - }\) ions (Eq. 4) may effectively block the growth of ZnO along [0001] direction and the lateral growth is relatively enhanced to obtain ZnO nanosheets [37, 38].
Figure 3 represents the structures of the ZnO NSA/GF. The XRD pattern of ZnO NSA/GF is shown in Fig. 3a. From the graph, two diffraction peaks (labeled with *) can be found at 2θ = 26° and 54°, which is the (002) and (004) planes of graphite (JCPDS75-1621), respectively. The peaks at (100), (002), (101), (102), (110), (103) and (112) plane are consistent with hexagonal ZnO (JCPDS36-1451) [39].
The Raman spectrum shown in Fig. 3b shows that there are mainly three obvious peaks. The peak of ZnO at ~ 428 cm−1 is the Raman active optical phonon E2 mode of Wurtzite ZnO [40]. The other two peaks G (at ~ 1586 cm−1) and 2D (at ~ 2725 cm−1) correspond to the coplanar vibration of the SP2 carbon atom orbital hybridization and the dual resonance of Raman scattering [41, 42]. The strength ratio of G peak to 2D peak proves that GF is mainly multilayered graphene. The absence of D peak shows the high quality of GF. All the above results indicate the successful integration of GF and ZnO NSAs.
3.2 Electrochemical properties
CV responses of the ZnO NSA/GF composite in 0.1 mM AA, DA and UA are depicted in Fig. 4. The oxidation peak potentials of AA, DA and UA are − 0.03, 0.18 and 0.34 V, respectively. Furthermore, the oxidation peaks of AA, DA and UA are higher than that of ITO electrodes, which shows that ZnO NSA/GF electrodes have good electrochemical properties. The enhanced electrochemical prosperities are related with the high conductivity of 3D porous GF and large specific areas provided by ZnO NSAs.
Figure 5 shows the effect of different pH values on DA oxidation peaks. The pH value of the solution was regulated by 0.1 M H3PO4 and NaOH. The oxidation peak current of DA increases gradually from 3.0 to 7.0 and then decreases from 7.0 to 9.0, the maximum peak current is found at the pH value of 7.0. Also the neutral pH value is in accordance with the physiological conditions, therefore, the optimal pH value for the electrochemical measurement is chosen as 7.0.
Figure 6 shows the CV responses of the ZnO NSA/GF in 0.1 mM AA, DA and UA. The oxidation peak currents of AA, DA and UA increases with increasing the scanning rate (Fig. 6a, b and d). The electrochemical reactions for UA and DA are reversible, and the oxidation peak current value is larger than that of the reduction peak current. The reduction peak for AA is not clearly visible due to the completely irreversible reaction. The oxidation peak current is linearly proportional to the square-root of the scanning rate for AA, DA, and UA in the range from 10 to 100 mV s−1. This linear relationship of Ip and ν1/2 suggests that the electrochemical reactions of these three biomolecules primarily are diffusion-controlled process [43].
CV curves of the ZnO NSA/GF electrode in 0.1 mM AA, DA and UA at different scanning rates. a–c CV curves of the ZnO NSA/GF electrode in 0.1 mM AA, DA and AA, respectively, at scanning rates of 10, 20, 40, 60, 80 and 100 mV s−1. d–f The oxidation peak current of the ZnO NSA/GF electrode in 0.1 mM AA, DA and UA versus square-root of the scan rates, respectively, in the range of 10–100 mV s−1
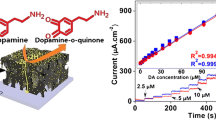
The DPV can detect biomolecules more accurately than CV. The DPV curves of the ZnO NSA/GF electrode in different concentrations of DA are depicted in Fig. 7. The oxidation peak current of DA increases linearly with its concentration and the equation is IDA = (5.81 ± 0.64) + (0.95 ± 0.04) CDA (R2 = 0.9919). The sensitivity is 0.95 μA μM−1 and the measured limit of detection is 1 μM. Compared with other previous electrode, the ZnO NSA/GF electrode exhibits superior electrochemical performance (Table 1).
DPV curves of the ZnO NSA/GF with different concentrations of DA in the range of 0–80 μM. a The concentration of DA from bottom to top is 0, 1, 5, 20, 35, 50, 65 and 80 μM, respectively. b Relationship between the oxidation peak current of DA and its concentration. DPV conditions: pulse height is 50 mV, pulse width is 200 ms, step height is 4 mV and step time is 500 ms
It is significant important to investigate whether the other biomolecules can influence the detection of DA because of the coexistence in human fluids, so the interference measurements are paramount necessary. The AA and UA are chosen as interference biomolecules due to the near oxidation potential. Figure 8 shows the DPV curves of ZnO NSA/GF in different concentrations of DA under the interference of 50 μM AA or 10 μM UA. The oxidation peak current of DA increases linearly as its concentration and the addition of AA does not have too much impact on the oxidation peak current and potential of DA (Fig. 8a) according to the equation of IDA = (4.19 ± 0.26) + (0.93 ± 0.03) CDA (R2 = 0.9952) (Fig. 8b). The similar trend is occured under the interference of UA. The oxidation peak current of DA increases linearly as its concentration (Fig. 8c) and the corresponding equation is IDA = (5.59 ± 1.07) + (0.92 ± 0.04) CDA (R2 = 0.9948) (Fig. 8d). The results indicate that the ZnO NSA/GF electrode is feasible to detect DA under the interference of AA or UA. However, DA cannot be detected precisely when it coexists with neurotransmitters, such as, levodopa (LD), norepinephrine (NE) and epinephrine (E) due to the same oxidation potential.
DPV curves of the ZnO NSA/GF electrode in DA with different concentrations in the presence of AA or UA. a in 50 μM AA and DA concentration from bottom to top is 0, 1, 10, 20, 30 and 40 μM, respectively. b Relationship between the oxidation peak current of DA and its concentration. c in 10 μM UA and DA concentration from bottom to top is 0, 1, 10, 20, 30 and 40 μM, respectively. d Relationship between the oxidation peak current of DA and its concentration. The DPV conditions are the same as in Fig. 7
3.3 Reproducibility and stability analysis
The reproducibility and stability of biosensors is of great significance for clinical application. The oxidation peak value of ZnO NSA/GF electrode decreased by 2.5% after DPV experiments for 5 time in the presence of 50 μM DA, indicating that it has good reproducibility. Then the ZnO NSA/GF electrode was tested every 3 days. After 2 weeks, the initial peak current decreased by less than 6%, which proved that ZnO-NSA/GF has practical clinical value.
3.4 Determination of DA in real urine
The feasibility of ZNO NSA/GF was verified by the determination of DPV response in human urine. First, 0.01 M phosphate buffer saline diluted urine sample was used as the actual detection liquid. Table 2 shows the results and the recoveries are 103.0%, 102.0% and 99.5%, respectively, with the standard deviations of less than 2.4%. It is proved that ZnO NSA/GF electrode is feasible for the detection of DA in the human urine.
4 Conclusions
In this study, a novel structure of ZnO NSA/GF composite was prepared by CVD and in situ hydrothermal synthesis. The inter-connected ZnO NSAs with the sheet size of ~ 10 μm and thickness of ~ 500 nm uniformly distributed on the GF. Because of the synergistic effect of these two nanomaterials, the new type of ZnO NSA/GF can be used to determine the DA level in the presence or absence of AA or UA. In addition, the electrode has low detection limit, excellent selectivity and good repeatability. In clinical research, it is possible to detect DA with ZnO NSA/GF electrode.
References
T.R. Silva, I.C. Vieira, A biosensor based on gold nanoparticles stabilized in poly(allylamine hydrochloride) and decorated with laccase for determination of dopamine. Analyst 141, 216–224 (2016)
M. Noroozifar, M. Khorasani-Motlagh, R. Akbari, P.M. Bemanadi, Simultaneous and sensitive determination of a quaternary mixture of AA, DA, UA and Trp using a modified GCE by iron ion-doped natrolite zeolite-multiwall carbon nanotube. Biosens. Bioelectron. 28, 56–63 (2011)
I.M. Taylor, E.M. Robbins, K.A. Catt, P.A. Cody, C.L. Happe, X.T. Cui, Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 89, 400–410 (2017)
Y. Xin, Z. Li, W. Wu, B. Fu, H. Wu, Z. Zhang, Recognition unit-free and self-cleaning photoelectrochemical sensing platform on TiO2 nanotube photonic crystals for sensitive and selective detection of dopamine release from mouse brain. Biosens. Bioelectron. 87, 396–403 (2017)
X. Yan, Y. Gu, C. Li, L. Tang, B. Zheng, Y. Li, Z. Zhang, M. Yang, Synergetic catalysis based on the proline tailed metalloporphyrin with graphene sheet as efficient mimetic enzyme for ultrasensitive electrochemical detection of dopamine. Biosens. Bioelectron. 77, 1032–1038 (2016)
Y. Li, H. Song, L. Zhang, P. Zuo, B. Ye, J. Yao, W. Chen, Supportless electrochemical sensor based on molecularly imprinted polymer modified nanoporous microrod for determination of dopamine at trace level. Biosens. Bioelectron. 78, 308–314 (2016)
Y. Ma, M. Zhao, B. Cai, W. Wang, Z. Ye, J. Huang, 3D graphene network@WO3 nanowire composites: a multifunctional colorimetric and electrochemical biosensing platform. Chem. Commun. 50, 11135–11138 (2014)
Y.X. Chen, K.J. Huang, L.L. He et al., Tetrahedral DNA probe coupling with hybridization chain reaction for competitive thrombin aptasensor. Biosens. Bioelectron. 100, 274–281 (2018)
T. Yang, H. Chen, C. Jing, S. Luo, W. Li, K. Jiao, Using poly(m-aminobenzenesulfonic acid)-reduced MoS2 nanocomposite synergistic electrocatalysis for determination of dopamine. Sens. Actuators B 249, 451–457 (2017)
H.Y. Yue, P.F. Wu, S. Huang et al., Electrochemical determination of dopamine in the presence of uric acid using WS2 nanospheres-carbon nanofibers. J. Electroanal. Chem. 833, 427–432 (2019)
S. Huang et al., Highly selective and sensitive determination of dopamine in the presence of ascorbic acid using a 3D graphene foam electrode. Electroanalysis 26, 184–190 (2014)
S. Huang et al., ZnO nanosheet balls anchored onto graphene foam for electrochemical determination of dopamine in the presence of uric acid. Sens. Actuators B 277, 381–387 (2018)
W. Zhao et al., Synthesis of the 3D graphene foam by chemical vapor deposition using nickel powders and application for simultaneous electrochemical detection of dopamine and uric acid. Ionics 254, 1813–1823 (2019)
H.Y. Yue et al., Preparation of three-dimensional hollow graphene balls and simultaneous electrochemical determination of dopamine and uric acid. J. Mater. Sci. 2914, 12330–12339 (2018)
H.Y. Yue et al., Highly sensitive and selective uric acid biosensor based on a three-dimensional graphene foam/indium tin oxide glass electrode. Anal. Biochem. 488, 22–27 (2015)
H.Y. Yue et al., A novel non-enzymatic dopamine sensors based on NiO-reduced graphene oxide hybrid nanosheets. J. Mater. Sci. 305, 5000–5007 (2019)
H.Y. Yue et al., Golf ball-like MoS2 nanosheet arrays anchored onto carbon nanofibers for electrochemical detection of dopamine. Microchim. Acta 2019, 378–385 (1866)
Z.H. Sheng, X.Q. Zheng, J.Y. Xu, W.J. Bao, F.B. Wang, X.H. Xia, Electrochemical sensor based on nitrogen doped graphene: simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 34, 125–131 (2012)
X. Zhang, Y.C. Zhang, L.X. Ma, One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B 227, 488–496 (2016)
X. Chen, G. Zhang, L. Shi, S. Pan, W. Liu, H. Pan, Au/ZnO hybrid nanocatalysts impregnated in N-doped graphene for simultaneous determination of ascorbic acid, acetaminophen and dopamine. Mater. Sci. Eng. C 65, 80–89 (2016)
A. Roychoudhury, S. Basu, S.K. Jha, Dopamine biosensor based on surface functionalized nanostructured nickel oxide platform. Biosens. Bioelectron. 84, 72–81 (2016)
H. Li, Y. Wang, D. Ye, J. Luo, B. Su, S. Zhang, J. Kong, An electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan based on MWNTs bridged mesocellular graphene foam nanocomposite. Talanta 127, 255–261 (2014)
E. Lee, H.C. Lee, S.B. Jo, H. Lee, N.S. Lee, C.G. Park, S.K. Lee, H.H. Kim, H. Bong, K. Cho, Heterogeneous solid carbon source-assisted growth of high-quality graphene via CVD at low temperatures. Adv. Funct. Mater. 26, 562–568 (2016)
X. Wang, D. Gao, M. Li, H. Li, C. Li, X. Wu, B. Yang, CVD graphene as an electrochemical sensing platform for simultaneous detection of biomolecules. Sci. Rep. 7, 7044 (2017)
X. Dong, X. Wang, L. Wang, H. Song, H. Zhang, W. Huang, P. Chen, 3D graphene foam as a monolithic and macroporous carbon electrode for electrochemical sensing. ACS Appl. Mater. Interfaces 4, 3129–3133 (2012)
L. Zhu, Y. Li, W. Zeng, Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl. Surf. Sci. 427, 281–287 (2018)
T. Rakshit, S.P. Mondal, I. Manna, S.K. Ray, CdS-decorated ZnO nanorod heterostructures for improved hybrid photovoltaic devices. ACS Appl. Mater. Interfaces 4, 6085–6095 (2012)
R. Ahmad, N. Tripathy, N.K. Jang, G. Khang, Y.B. Hahn, Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuators B 206, 146–151 (2015)
B. Rezaei, L. Shams-Ghahfarokhi, E. Havakeshian, A.A. Ensafi, An electrochemical biosensor based on nanoporous stainless steel modified by gold and palladium nanoparticles for simultaneous determination of levodopa and uric acid. Talanta 158, 42–50 (2016)
S.Y. Gao, H.D. Li, J.J. Yuan, Y.A. Li, X.X. Yang, J.W. Liu, ZnO nanorods/plates on Si substrate grown by low-temperature hydrothermal reaction. Appl. Surf. Sci. 256, 2781–2785 (2010)
M. Arvand, N. Ghodsi, A voltammetric sensor based on graphene-modified electrode for the determination of trace amounts of L-dopa in mouse brain extract and pharmaceuticals. J. Solid State Electrochem. 17, 775–784 (2013)
H. Beitollahi, N.F. Garkani, Graphene oxide/ZnO nano composite for sensitive and selective electrochemical sensing of levodopa and tyrosine using modified graphite screen printed electrode. Electroanalysis 28, 2237–2244 (2016)
X. Wang, L. Huang, Y. Zhao, Y. Zhang, G. Zhou, Synthesis of mesoporous ZnO nanosheets via facile solvothermal method as the anode materials for lithium-ion batteries. Nanoscale Res. Lett. 11, 37 (2016)
Y. Sun, S. Ge, H. Huang, H. Zheng, Z. Jin, J. Shan, C. Gu, X. Huang, F. Meng, Novel volatile organic compound (VOC) sensor based on Ag-decorated porous single-crystalline ZnO nanosheets. Mater. Express. 6, 191–197 (2016)
L. Wang, K. Tang, M. Zhang, J. Xu, Facile synthesis of Mn-doped ZnO porous nanosheets as anode materials for lithium ion batteries with a better cycle durability. Nanoscale Res. Lett. 10, 983 (2015)
H.Y. Yue, S. Huang, J. Chang, C.J. Heo, F. Yao, S. Adhikari, F. Gunes, L.C. Liu, T.H. Lee, E.S. Oh, B. Li, J.J. Zhang, T.Q. Huy, N.V. Lua, Y.H. Lee, ZnO nanowire arrays on 3D hierachical graphene foam: biomarker detection of Parkinson’s disease. ACS Nano 8, 1639–1646 (2014)
T. Yang, M. Chen, Q. Kong, X. Luo, K. Jiao, Toward DNA electrochemical sensing by free-standing ZnO nanosheets grown on 2D thin-layered MoS2. Biosens. Bioelectron. 89, 538–544 (2017)
H. Huang, H. Wang, B. Li, X. Mo, H. Long, Y. Li, H. Zhang, D.L. Carroll, G. Fang, Seedless synthesis of layered ZnO nanowall networks on Al substrate for white light electroluminescence. Nanotechnology 24, 315203 (2013)
J.B. Raoof, N. Teymoori, M.A. Khalilzadeh, R. Ojani, A high sensitive electrochemical nanosensor for simultaneous determination of glutathione, NADH and folic acid. Mater. Sci. Eng. C 47, 77–84 (2015)
X.C. Dong, Y.F. Cao, J. Wang, M.B. Chan-Park, L.H. Wang, W. Huang, P. Chen, Hybrid structure of zinc oxide nanorods and three dimensional graphene foam for supercapacitor and electrochemical sensor applications. RSC Adv. 2, 4364–4369 (2012)
Z.P. Chen, W.C. Ren, L.B. Gao, B.L. Liu, Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 10, 424–428 (2011)
C.D. Wang, J.L. Xu, M.F. Yuen, J. Zhang, Y.Y. Li, X.F. Chen, W. Zhang, Hierarchical composite electrodes of nickel oxide nanoflake 3D graphene for high-performance pseudocapacitors. Adv. Funct. Mater. 24, 6372–6380 (2014)
Q. Lian, Z. He, Q. He, A. Luo, K. Yan, D. Zhang, X. Lu, X. Zhou, Simultaneous determination of ascorbic acid, dopamine and uric acid based on tryptophan functionalized graphene. Anal. Chim. Acta 823, 32–39 (2014)
Acknowledgements
This work is supported by the Postdoctoral Initial Founding of Heilongjiang Province (LBH-Q14117), the Foundation for Selected Overseas Chinese Scholar, Ministry of Personal of Heilongjiang Province (2018383) and the fundamental research foundation for University of Heilongjiang province (LGYC2018JQ012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, S., Wu, P.F., Yue, H.Y. et al. ZnO nanosheet arrays/graphene foam: voltammetric determination of dopamine in the presence of ascorbic acid and uric acid. J Mater Sci: Mater Electron 30, 16510–16517 (2019). https://doi.org/10.1007/s10854-019-02027-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02027-z