Abstract
A series of blue–green Ba3Gd(1−x)P3O12: xTb3+ nanocrystals has been successfully prepared via the urea-assisted solution-combustion method. Its structure, morphology, energy-transfer mechanism, photoluminescent (PL) excitation-emission and decay time behavior were investigated in detail employing powder X-ray diffraction (XRD), Transmission Electron Microscopy (TEM), Diffuse Reflectance (DR) and PL spectroscopy. The Rietveld analysis exposed the cubic phase of all the nanophosphors with I-43d (220) space group and infers that Gd3+ ions can be well substituted by Tb3+ ions without any major alteration in the crystal prototype of the host lattice. The optical band-gap of the host was calculated to be 4.9 eV, unveiling the high potential as a host for lanthanide activators. Under the excitation at λex = 224 nm, the photoluminescent emission spectra exhibited the two main characteristic peaks at 545 nm and 487 nm as a result of 5D4 → 7F5 (green and magnetic-dipole) and 5D4 → 7F6 (blue and electric-dipole) transitions, respectively. The decay analysis showed that the activator occupies a single crystallographic site, a fact that is also supported by the Rietveld refinement. The critical distance of the energy transfer (19.87 Å) integrated with Dexter’s modeling inferred about the energy migration (dipole–dipole). The PL result showed that the blue-to-green tunable emission can be achieved simply via varying the dopant concentration, with 7 mol% as the optimum concentration for standard CIE coordinates of green emission. All the results suggest that Ba3Gd(1−x)P3O12: xTb3+ crystals may find their use as a green phosphor component in display devices and solid-state lighting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the recent years, scientists all around the world have been making efforts in producing nanophosphor materials as these are considered as very valuable asset in the field of optical applications such as solid-state laser devices, optical amplifiers, light emitting diodes (LED’s), solar cells, X-ray medical radiography, cathode ray-tubes, sensor technology, optical markers, and plasma display panels [1,2,3,4]. The motive is to design and develop a near-ultraviolet (NUV) excited but eco-friendly phosphor material with better luminescence, long emission lifetime, high thermal stability, narrow emissions bands and with reasonable product reliability [5,6,7,8,9]. In this context, rare-earth trivalent ions play an important role when incorporated in a suitable host matrix; which is obviously due to their outstanding luminescent properties, arising from the intra-configurational 4f–4f and 4f–5d transitions [10]. Furthermore, it is a quite well-known fact that Tb3+ ions can act as activators for green-emitting nanophosphor in various suitable host matrixes. Many researchers are paying attention to grow the Tb3+-based green-emitting phosphor crystals. The reason being for the high popularity of Tb3+ ions as an activator is a dominant peak at around 545 nm (5D0 → 7F5 transition) which can be tweaked to a great extent due to the large electric-dipole character [11,12,13,14]. Nonetheless, other transitions originating from the 5DJ excited state are also of great significance as far as visible emission is concerned.
Though there are some reports on the single Tb3+-doped Ba3GdP3O12compositions via conventional solid-state method [15, 16]; however, to the best of the author knowledge, concentration-dependent PL properties, Rietveld analysis and synthesis via other chemical routes are unavailable to date. This paper covers the fabrication of a series of Ba3Gd(1−x)P3O12: xTb3+ (x = 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08) via urea-based solution combustion method for the first time. The structural prototype, phase purity, surface morphology, emission dynamics and optical properties of the synthesized nanophosphor were characterized by X-ray diffraction technique, Rietveld refinement using FULLPROF program, transmission electron microscopy, decay time and photoluminescence spectroscopy, respectively. The corresponding critical distance (Rc) for energy transfer was determined experimentally and then employed to demonstrate the mechanism responsible for the concentration quenching arising from energy-migration among the neighboring Tb3+ ions. The assessed outcomes display that the solution-combustion approach for the synthesis of a blue–green tunable nanophosphor provided a fast and economic technique which diminished the sintering temperature as well as the duration of the heat treatment. The synthesized phosphor samples were found with high purity, single-phased crystalline nanostructure, better luminescent intensity than the sample prepared from the solid-state method. So, the obtained nanophosphors can be utilized as a solid-state lighting nanomaterial.
2 Experimental setup
2.1 Material and synthesis
A series of Ba3Gd(1−x)TbxP3O12 (x = 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08) nanophosphors was prepared from urea-assisted solution combustion technique. The initial materials used were Gd(NO3)3·6H2O, Tb(NO3)3·5H2O, Ba(NO3)2, (NH4)2HPO4 and NH2CONH2 were of analytical grade. In a typical procedure, all the nitrates in stoichiometric quantities were dissolved in a minimal amount of deionized water. The quantity of urea used as a fuel for the propellant reaction was calculated by using total oxidizing and reducing valencies of oxidizer and fuel [17]. After obtaining the homogenous solution, the final solution was placed in a preheated furnace kept at 500 °C for 15–20 min. Initially, the solution boils with the liberation of huge amounts of gases. These gases burnt and ignited with a blaze yielding a foamy solid. Further, the obtained voluminous sample was effortlessly milled to get fine precursor powder by using an agate mortar and annealed at 1200 °C for 5 h in the air to attain better crystallinity.
2.2 Materials characterization
Powder X-ray diffraction (PXRD) patterns of all the samples were evaluated by a Rigaku make (Ultima-IV) powder X-ray diffractometer from 2θ = 10°–80° at a scanning rate of 2°/min using Cu Kα as radiation source, while the tube voltage and tube current were kept constant at 40 kV and 40 mA, correspondingly. Furthermore, the phase analysis and refinement of parameters of the synthesized nanophosphor sample was achieved with the Rietveld method in FULLPROF software package. The isotropic displacement and fractional coordinates of all the mixed atoms were constrained during the refinement. Technai-G2 transmission electron microscope (TEM) was used to study the surface morphology and crystallite size. The Diffuse Reflectance Spectrum (DRS) of all the powder samples were recorded in the wavelength ranging from 200 to 800 nm using Shimadzu UV-3600 accessorized with an integrated sphere and BaSO4 as reference standard was used. Edinburgh fluorescence spectrophotometer (FLS-980) equipped with 450 W Xenon lamp as the excitation source was used to study the photoluminescence spectra, color coordinates and decay curves of terbium doped Ba3GdP3O12 nanophosphors. The photo-multiplier tube (PMT) voltage was set at 400 V while the excitation-emission bandwidths were fixed at 2.5 nm. The whole process was performed at room temperature and atmospheric pressure.
3 Result and discussion
3.1 Structural analysis
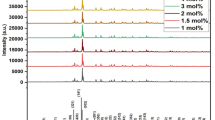
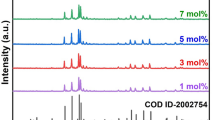
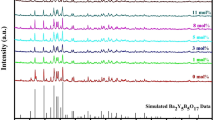
The analysis of the crystal structure and corresponding phase purity for all the samples was performed by powder X-ray diffraction (PXRD) technique. Figure 1 displays the XRD patterns of the complete Ba3Gd(1−x)TbxP3O12 (x = 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08) nanophosphor series along with the standard JCPDS card No. 29-0163. It can be seen that all the peaks of the XRD patterns are well-matched with the standard data. The cubic phase with a space group of I-43d without any other impurity peak indicates that Tb3+ ions substitute the Gd3+ ions without disturbing the crystal structure prototype. In support of the above-mentioned statement, Rietveld refinement in FULLPROF software was achieved for Ba3Gd0.93Tb0.07P3O12 nanocrystal using its PXRD profile. Figure 2 displays the calculated (red line) and observed (black line) patterns; the deviation of experimental and theoretical data (blue); and the corresponding Bragg positions (pink). The refinement converged to a reasonable fit with Rp = 9.03(%), Rwp = 11.7(%), Rexp = 8.43(%) and χ2 = 1.94. The refinement process yielded various structural parameters such as a = b = c = 10.445 Å, V = 1111 Å3 and Z = 4. Table 1 shows the comparative study of the crystal structure data of the host and of Ba3Gd0.93Tb0.07P3O12 system. The comparison clarifies that there is a slight decrease in the unit cell volume from 1151.009 to 1148.95 Å3 upon substitution of Gd3+ ions by Tb3+ ions, which can be explained on the account of smaller ionic radius of Tb3+ (92.3 pm) than Gd3+ (93.8 pm), and hence decreases the density of the doped nanophosphor [18]. The refined atomic positions and their occupancies are tabularized in Table 2; whilst Table 3 displays the several interatomic distances (Å) in Ba3Gd0.93Tb0.07P3O12 nanocrystal.
Moreover, the average crystallite size was evaluated by using the full-width at half-maxima (FWHM) of the most prominent diffraction peak for Ba3Gd0.93Tb0.07P3O12 nanophosphor from Scherrer’s equation [19, 20]:
where D is the average crystallite size, λ the X-ray wavelength (0.15406 nm), θ is the diffraction angle; βSi(2θ) and β0(2θ) are the full-width at half-maximum (FWHM, in radian) of the standard silicon and of the synthesized sample, correspondingly. The results of the particle size suggested a range of 40–70 nm. Additionally, to study the surface morphology along with particle size of as-prepared Ba3Gd1−xTbxP3O12 nanophosphor samples Transmission Electron Microscopy (TEM) was carried out. Figure 3 displays the TEM micrograph of the Ba3Gd0.93Tb0.07P3O12 nanophosphor, showing well-dispersed particles with minute agglomeration magnified at 100 nm. Therefore, it is quite safe to say that the results from Scherrer’s equation and TEM micrography, are found to be in good agreement with each other. Later on, the Strain (\(\varepsilon\)) and Dislocation density (\(\delta\)) were calculated for all the synthesized nanophosphors and are reported in Table 4 utilizing the following equations [21, 22]:
Furthermore, the variance of the absorption coefficient against photon energy, along with the reliable diffuse reflectance spectra (inset), for the Ba3GdP3O12 host is shown in Fig. 4. The Kubelka–Munk function is utilized to evaluate the magnitude of the optical band gap (Eg) for solids. So, the values of various kinds of band-gap can be implicated by using the below relation [23]:
where C is a proportionality constant, hν denotes the energy of the photon and Eg signifies the band gap (eV). The symbol n is 0.5, 2, 1.5 and 3 for allowed indirect, allowed direct, forbidden direct and forbidden indirect transitions, respectively. F(R∞) implies the Kubelka–Munk function whose standards is well-defined as [24]:
where K and S are the absorption and scattering coefficients, correspondingly; while R∞ represents the ratio of Rsample to Rstandard. The extrapolation of the line with F(R∞) hν = 0 exposed a band-gap of 4.88 eV.
3.2 Optical properties
Figure 5 displays the photoluminescence excitation (PLE) spectrum of Tb3+ ions doped Ba3GdP3O12 nanophosphor obtained when monitored at λem of 545 nm due to (5D4 → 7F5) transition. The PLE revealed that the spectrum consists of a broad band ranging from 200 to 240 nm (centered at 224 nm), which obviously arises due to the transition from ground state 4f8 to the excited state 4f7 5d1 (f–d inter-configurational transition) in trivalent terbium ions [25, 26]. Along with this, weak absorptions at 273 nm and 311 nm are also found and are assigned as the intra-configurational 4f–4f transitions of the Tb3+ ions in Ba3GdP3O12 host. Among these peaks, the broad-band at 224 nm has been chosen to evaluate the emission spectra of Ba3GdP3O12: Tb3+ (2–8 mol%).
The photoluminescence emission spectrum of Tb3+ doped nanophosphors mainly consists of several prominent peaks in the wavelength region of 500–700 nm. The PL emission spectra shows the peaks at 415 nm, 436 nm and 456 nm are arising from 5D3 → 7FJ (J = 5, 4, 3) transitions, whereas the transitions at 487 nm, 545 nm, 582 nm and 620 nm appear from 5D4 → 7FJ (J = 6, 5, 4, 3), respectively [27,28,29,30]. Among these peaks, transitions from 5D3 are responsible for the blue color of the nanophosphors while transitions from the 5D4 state are responsible for the characteristic green emission in the case of trivalent terbium ions. It can be seen from Fig. 6, that the most intense peak located at 545 nm (5D4 → 7F5) will be primarily responsible for the emission color of the nanophosphors. Furthermore, the PL emission intensity of the peaks from the 5D4 excited state shows remarkable enhancement on increase in activator concentration in the host matrix up to 7 mol% but decreases afterward as shown in Fig. 7. This decrease in the PL intensity for 5D4 → 7FJ transitions can be ascribed to the concentration quenching effect. The reason for the concentration quenching in PL spectra can be explained on the basis of decreasing distance between the neighboring Tb3+ ions in the host matrix, resulting in a considerable increase in the energy transfer via non-radiative process [31, 32].
Besides this, it is also worthy to note that the intensity of the emission peaks arising from 5D3 → 7FJ transitions decreases with the increase in the Tb3+ ions concentration as shown in Fig. 8. This leads to the shift in the emission color from light blue to light green. The energy level plot for the Tb3+-doped Ba3GdP3O12 also includes the DC (down-conversion) energy transfer mechanism through radiative as well as non-radiative paths. Upon excitation, the Tb3+ ions get excited from the 7F6 ground state to higher energy state (224 nm); which in turn gets depopulated non-radiatively to enrich the lower energy levels (5D3, 5D4) of Tb3+ ions. The tunability of the emission color for terbium ions may be attributed to the cross-relaxation phenomenon. The same energy gap between the excited energy states 5D3–5D4 and ground state 7F6–7F0 levels elucidates the cross-relaxation via resonant energy transfer as shown in schematic energy diagram (Fig. 9) [33].
Generally, the transfer of the resonant energies between the two adjacent Tb3+ ions can occur via three mechanisms i.e., exchange interaction, electric multipolar interaction and radiation re-absorption. Out of these three possibilities, radiation re-absorption can be discarded as there is no spectral overlapping of excitation and emission bands. Therefore, it is necessary to figure out the critical distance (Rc) to assess the potential mechanism for energy transfer. So, for the 5D4 emission in the Ba3Gd0.93Tb0.07P3O12 (critical concentration) nanophosphor Rc can be evaluated by using the equation reported by Blasse and Grabmaier and expressed as [34, 35]:
where V is the volume of the unit cell (Å3), N is the number of replaceable cations in the unit cell and xc is the critical concentration. After putting N = 4, xc = 0.07 and V = 1148.95 Å3 for the Rc is measured to be 19.87 Å; suggesting that the resonant energy transfer occurs via multipolar interaction among the adjacent trivalent terbium ions in this case. Furthermore, dominating electric multipolar interactions are categorized as dipole–dipole (d–d), dipole–quadrupole (d–q) and quadrupole–quadrupole (q–q) interactions. So, the relationship between the dopant ions concentrations (x) and luminescence intensity (I) can be expressed as [36, 37]:
where A is a constant, x is the concentration of activator ion greater than the critical concentration, Q denotes the type of multipolar interaction value of which varies as 6, 8 or 10 for dipole–dipole (d–d), dipole-quadrupole (d-q) and quadrupole–quadrupole (q–q) interactions, respectively. As the graph demonstrated in Fig. 10 shows the relationship between log(I/x) and log(x) with the slope value − 2.2568 ± 0.11516 results in the value of Q ≈ 6; indicating that the dipole–dipole interactions are the main cause for the cross-relaxation energy transfer in Tb3+ doped Ba3GdP3O12 nanophosphors.
Also, the decay behavior of 5D4 state in Ba3GdP3O12:Tb3+, at λex = 224 nm and λem = 545 nm, was evaluated. The obtained curves for all Ba3Gd1-xTbxP3O12 nanophosphors are well fitted by the first exponential decay by utilizing the equation [38]:
where I and Io are the luminescence intensity at time t and 0 respectively, τ is the fluorescence lifetime and A is a constant. The values of photoluminescent lifetime for different Tb3+-doped samples reveal that the value of τ declines with the increase in the activated concentration (Table 5). Figure 11 represents the single exponential decay of the Ba3Gd0.93Tb0.07P3O12 which can be viewed in terms of homogenous dispersal of all terbium ions in the host matrix with similar local environment returning at the same rate.
The Commission Internationale de I’Eclairage (CIE) chromaticity coordinates depicts the emission color of the nanophosphors. Figure 12 shows the calculated chromaticity coordinates (x, y) of the Ba3Gd1−xP3O12: xTb3+ (x = 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08) on the CIE 1931 color space chromaticity diagram obtained from emission spectra under the excitation of 224 nm wavelength and charted in Table 5. The chromaticity coordinates for all the synthesized nanophosphors clearly show the tunability from blue to green region on the intensification of Tb3+ ions in the host lattice. The luminescent tunability in Ba3GdP3O12: Tb3+ (2–8 mol%) finds its use in the wider range of display devices or in solid state lighting.
4 Conclusion
A series of blue–green Ba3Gd(1−x)P3O12: xTb3+ nanocrystals has been efficaciously synthesized via urea-assisted solution-combustion technique. Its structural morphology, energy-transfer mechanism, photoluminescent (PL) excitation-emission and decay time behavior were examined in detail by means of powder X-ray Diffraction (XRD), Transmission Electron Microscopy (TEM), Diffuse Reflectance (DR) and PL spectroscopy. The Rietveld investigation shows the cubic phase formation of all the nanophosphors with I-43d (220) space group and concludes that Gd3+ ions can be well replaced by Tb3+ ions without any major modification in the crystal structure of the host matrix. The optical band-gap of the host was assessed to be 4.9 eV, offering high potential as a host for lanthanide activators. Upon excitation at λex = 224 nm, the photoluminescent emission spectra showed the two main characteristic peaks at 545 nm and 478 nm as a result of 5D4 → 7F5 (green and magnetic-dipole) and 5D4 → 7F6 (blue and electric-dipole) transitions, respectively. The decay analysis exhibited that the activator resides in a single crystallographic site, a fact that is also supported by the Rietveld refinement. The Rc of the energy transfer (19.87 Å) combined with Dexter’s modeling provides information about energy migration via dipole–dipole interactions. The PL result displayed that the blue-to-green tunable emission can be attained simply via varying the activator concentration, with 7 mol% as the optimal concentration for standard CIE coordinates of green emission. All the results propose that Ba3Gd(1−x)P3O12: xTb3+ crystals can find their usage as a green phosphor component in display gadgets and solid-state lighting.
References
D. Kima, B.K. Moona, S.H. Parka, J.H. Kima, J.H. Jeonga, Full-color tuning in europium doped phosphosilicate phosphors via adjusting crystal field modulation or excitation wavelength. J. Alloys Compd. 770, 411–418 (2019)
T.M. Katona, P.M. Pattison, S. Paolini, Status of solidstate lighting product development and future trends for general illumination. Rev. Cell. Biol. 7, 263–281 (2016)
H. Zhu, C.C. Lin, W. Luo, S. Shu, Z. Liu, Y. Liu, J. Kong, E. Ma, Y. Cao, R. Liu, X. Chen, Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Commun. 5, 4312 (2014)
N. Salah, S.S. Habib, Z.H. Khan, Quantum effect on the energy levels of Eu2+doped K2Ca2(SO4)3 nanoparticles. J. Fluoresc. 20, 1009–1015 (2010)
L.M. Becerra, R.V. Arreguın, U. Balderas, S.C. Tellez, H.M. Sanchez, C. Falcony, Luminescent characteristics of layered yttrium oxide nano-phosphors doped with europium. J. Appl. Phys. 121, 125111 (2017)
C. Manjunatha, M.S. Rudreshaa, R.H. Krishnab, B.M. Nagabhushanab, B.M. Walshc, K.R. Nagabhushanad, B.S. Panigrahi, Spectroscopic studies of strong red emitting Sr2SiO4:Eu3+ nanophosphors with high color purity for application in WLED using Judd-Ofelt theory and TL glow curve analysis. Opt. Mater. 85, 363–372 (2018)
E. Pavitra, G.S.R. Raju, J.H. Oh, L.K. Bharath, J.H. Jeong, Y.S. Huh, J.S. Yu, (BaSr)2SiO4:Eu2+ nanorods with enhanced luminescence properties as green-emitting phosphors for white LED applications. Dyes Pigments 142, 447–456 (2017)
M. Jiao, N. Guo, W. Lü, Y. Jia, W. Lv, Q. Zhao, B. Shaoa, H. You, Synthesis, structure and photoluminescence properties of europium-, terbium-, and thulium-doped Ca3Bi(PO4)3 phosphors. Dalton Trans. 42, 12395 (2013)
Z. Fua, L. Maa, S. Sahia, R. Halla, W. Chen, Influence of doping concentration on valence states of europium in SrAl2O4: Eu phosphors. J. Lumin. 143, 657–662 (2013)
V. Singha, S. Watanabeb, T.K.G. Rao, H.Y. Kwak, Luminescence and defect centres in Tb3+ doped LaMgAl11O19 phosphors. Solid State Sci. 12, 1981–1987 (2010)
G.H. Dieke, Spectra and Energy Levels of Rare Earth Ions in Crystals (Inter-science Publishers, Olney, 1968), p. 253
B. Ramesh, G.R. Dillip, B. Rambabu, S.W. Joo, B.D. Raju, Structural studies of a green-emitting terbium doped calcium zinc phosphate phosphor. J. Mol. Struct. 1155, 568–572 (2018)
V.R. Panse, N.S. Kokode, S.J. Dhoble, Tb3+ doped Sr2(BO3)Cl green emitting phosphor for solid state lighting. Adv. Mater. Lett. 5, 604–610 (2014)
A.G.B. Jr, S.A.M. Lima, A.M. Pires, Energy transfer between terbium and europium ions in barium orthosilicate phosphors obtained from sol-gel route. J. Lumin. 199, 372–378 (2018)
N. Guo, W. Lu, Y. Jia, W. Lv, Q. Zhao, H. You, Eu2+ & Mn2+-coactivated Ba3Gd(PO4)3 orange-yellow emitting phosphor with tunable color tone for UV-excited white LEDs. Chem. Phys. Chem. 14, 192–197 (2013)
Q. Di, Q. Xu, L. Han, J. Sun, Synthesis and luminescence properties of Eu3+-doped Ba3Gd(PO4)3 phosphors for light-emitting diodes. Opt. Eng. 54, 035104 (2015)
S. Ekambaram, K.C. Patil, J. Alloys Compd. 448, 7 (1997)
N.N. Greenwood, A. Earnshaw, Chemistry of the Elements (Elsevier, Amsterdam, 1997), pp. 1230–1242
H. Dahiya, M. Dalal, A. Siwach, M. Dahiya, D. Kumar, Cool white light emitting Ba5Zn4Y8O21:Dy3+ nanophosphors for single phased WLEDs. J. Mater. Sci. 29, 20750–20758 (2018)
M. Dalal, V.B. Taxak, J. Dalal, A. Khatkar, S. Chahar, R. Devi, S.P. Khatkar, Crystal structure and Judd-Ofelt properties of a novel color tunable blue-white-red Ba5Zn4Y8O21:Eu3+ nanophosphor for near-ultraviolet based WLEDs. J. Alloys Compd. 698, 662–672 (2017)
D.S. Kshatri, A. Khare, Characterization and optical properties of Dy3+ doped nanocrystalline SrAl2O4: Eu2+ phosphor. J. Alloys Compd. 588, 488–495 (2014)
A. Chowdhury, B. Biswas, M. Majumder, M.K. Sanyal, B. Mallik, Studies on phase transformation and molecular orientation in nanostructured zinc phthalocyanine thin films annealed at different temperatures. Thin Solid Films 520, 6695–6704 (2012)
J. Qiao, Z. Xia, Z. Zhang, B. Hu, Q. Liu, Near UV-pumped yellow-emitting Sr9MgLi(PO4)7:Eu2+ phosphor for white-light LEDs. Sci. China Mater. 61, 985–992 (2018)
S. Devi, A. Khatkar, V.B. Taxak, M. Dalal, S. Chahar, J. Dalal, S.P. Khatkar, Optical properties of trivalent samarium-doped Ba5Zn4Y8O21 nano-diametric rods excitable by NUV light. J. Alloys Compd. 767, 409–418 (2018)
K. Thomas, D. Alexander, S. Sisira, P.R. Biju, N.V. Unnikrishnan, M.A. Ittyachen, C. Joseph, NUV/blue LED excitable intense green emitting terbium doped lanthanum molybdate nanophosphors for white LED applications. J. Mater. Sci. 28, 17702–17709 (2017)
X. Gaoa, C. Lia, S. Lib, H. Zhanga, Z. Lia, Y. Honga, J. Sun, Intense green/blue light emission of Zn2GeO4 nanophosphors doped Tb3+ ions under 265-nm excitation. J. Lumin. 190, 457–461 (2017)
D. Kumar, M. Sharma, D. Haranath, O.P. Pandey, Facile route to produce spherical and highly luminescent Tb3+ doped Y2O3 nanophosphors. J. Alloys Compd. 695, 726–736 (2017)
S.J. Motloung, S.K.K. Shaat, K.G. Tshabalalaa, O.M. Ntwaeaborwa, Structure and photoluminescent properties of green-emitting terbium-doped GdV1-xPxO4 phosphor prepared by solution combustion method. Luminescence 31, 1069–1076 (2016)
X. Yang, B. Zhang, T. Xu, L. Wang, J. Shen, Q. Zhang, Enhanced luminescent intensity of Sr2SiO4:Tb3+ phosphors by charge compensation (Li+) addition. J. Mater. Sci. 27, 9448–9453 (2016)
B. Li, X. Huang, H. Guo, Y. Zeng, Energy transfer and tunable photoluminescence of LaBWO6:Tb3 + , Eu3 + phosphors for near-UV white LEDs. Dyes Pigments 150, 67–72 (2018)
X. Huang, B. Li, H. Guo, Synthesis, photoluminescence, cathodoluminescence, and thermal properties of novel Tb3+-doped BiOCl green-emitting phosphors. J. Alloys Compd. 695, 2773–2780 (2017)
L. Wang, L. Shi, N. Liao, H. Jia, P. Du, Z. Xi, L. Wang, D. Jin, Photoluminescence properties of Y2O3:Tb3+ and YBO3:Tb3+ green phosphors synthesized by hydrothermal method. Mater. Chem. Phys. 119, 490 (2010)
X. Zhou, S. Jiang, G. Xiang, X. Tang, X. Luo, L. Li, X. Zhou, Tunable emission color of Li2SrSiO4:Tb3+ due to cross-relaxation process and optical thermometry investigation. J. Am. Ceram. Soc. 101, 3076–3085 (2018)
G. Blasse, Energy transfer in oxidic phosphors. Phys. Lett. 28, 444–445 (1968)
G. Ramakrishnaa, H. Nagabhushana, R.B. Basavaraj, S.C. Prashantha, S.C. Sharma, R. Naik, K.S. Anantharaju, Green synthesis, structural characterization and photoluminescence properties of Sm3+ co-doped Y2SiO5:Ce3+ nanophosphors for wLEDs. Optik 127, 5310–5315 (2016)
D. Dexter, J. Schulman, Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 22, 1063–1070 (1954)
Y. Fang, X. Huang, Y. Juang, S. Chu, Color-tunable blue to green Ca4-1.5xTa2O9: xTb3+ phosphor: cross relaxation mechanism and thermal stability. J. Am. Ceram. Soc. 95, 1613–1618 (2012)
A. Prasad, A.S. Rao, M. Gupta, G.V. Prakash, Morphological and luminescence studies on KGdF4:Yb3+/Tb3+ up-conversion nanophosphors. Mater. Chem. Phys. 219, 13–21 (2018)
Acknowledgements
This work was financially supported in the form of Senior Research Fellowship (SRF) from Department of Science & Technology, Haryana by The Haryana State Council for Science and Technology (HSCST) as HSCST Fellowships (HSCST Award No.: HSCST/1797) One of the authors, Dinesh Kumar gratefully acknowledges the financial support in form of Minor Research Project granted from Deenbandhu Chhotu Ram University of Science & Technology, Murthal (Reg. No.-DCRUST/DR/11/2018), India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dahiya, H., Dalal, M., Siwach, A. et al. A blue to green tunable Ba3GdP3O12:Tb3+ nanophosphor: structural and opto-electronic analysis. J Mater Sci: Mater Electron 31, 3750–3758 (2020). https://doi.org/10.1007/s10854-019-02009-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02009-1