Abstract
NbOx thin films have been deposited on silicon (100) and quartz substrates by magnetron sputtering using a metallic Nb target in an optimized argon–oxygen atmosphere. This work investigates the dependence of structure–property relations on the key deposition parameters towards establishing optimum deposition conditions for the growth of NbOx polycrystalline films. It is found that a sputtering condition corresponding to DC power density 9.87 W cm−2, a substrate temperature of 720 °C, low gas pressures of 8 mtorr, a target to substrate distance of 45 mm gives thin films with good homogeneity and a high degree of crystallinity in the case of both NbO2 and Nb2O5. X-ray diffraction (XRD) and Raman spectroscopy confirmed the tetragonal phase of NbO2 and orthorhombic phase of Nb2O5 for similar deposition temperatures. Scanning electron microscopy (SEM) observations indicate that NbO2 has a unique nanoslice structure while Nb2O5 has a flake-like structure. The optical transmittance of the films has been investigated and found to be dependent on the oxygen gas content during deposition; the optical transmittance decreases with increasing O2 gas content. Optical constants of the films were calculated by fitting a suitable thin film transmittance model to experimental transmittance spectra using a modified Swanepoel technique. The nanohardness and stress in the films were measured by nanoindentation and an optical profilometer respectively. Nanohardness and stress in the film show no large dependence on the oxygen gas content except at high oxygen gas content. The nanohardness value of NbO2 films is approximately 6 GPa, and the Young’s modulus is 150 GPa. The Nb2O5 films exhibit a nanohardness of 5.8–13 GPa and a Young’s modulus of 137–161 GPa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The niobium–oxygen phase diagram comprises a variety of different stable and metastable niobium oxide phases, which make the niobium–oxygen system highly complex. Three dissimilar charge states of niobium exist in its oxides: + 2 (in NbO), + 4 (in NbO2) and + 5 (in Nb2O5). Niobium monoxide shows typical metallic behavior and below the critical temperature of 1.38 K, NbO is superconductive [1]. Niobium dioxide is a semiconductor at room temperature and a conductor at high temperatures with an electrical conductivity on the order of 103 S cm−1 [2]. A reversible second-order phase transition (metal–insulator transition), along with a change of the crystal structure from distorted rutile to a regular rutile lattice, is observed at 808 °C. The metal–insulator transition can also be induced by a suitable applied field [3]. The sharp changes in the electrical and optical properties through the metal–insulator transition region makes this material system particularly favorable for optical and electrical switching applications. Moreover, excellent dielectric and switching properties of NbO2 make it a useful candidate for electronic device applications [4]. Despite many attractive characteristics of NbO2, it is technically challenging to prepare pure NbO2 thin films, as stable NbO2 exists only in a small range of stoichiometry [5]. Niobium pentoxide is a useful optical material with a higher refractive index (approximately 2.55 at a wavelength of 400 nm) than those for many glasses [6]. It has a wide bandgap (varies between 3.2 and 4 eV) [7] and good corrosion resistance [8]. Nb2O5 has nine different polymorphs [9]. Being the most stable oxidation state of the niobium–oxygen system, niobium pentoxide (amorphous or crystalline) has a wide range of electronic and optical applications, e.g. intelligent windows, electrodes in solar cells, gas sensors, photocatalysis etc. Thus, both NbO2 and Nb2O5 can be used in optoelectronics or electronic device applications. For applications that involve microelectromechanical system (MEMS) devices and micro-optical devices, the mechanical properties of niobium oxides are also important in addition to their optical and electrical properties [10].
Niobium oxide thin films can be prepared by a variety of methods, including thermal oxidation [4], ion beam sputtering [5], chemical vapour transport [11], sol–gel [12], chemical vapor deposition [13] etc. In recent years, the magnetron sputtering (DC or RF) has become widely popular for thin film deposition because of its high process control and higher homogeneity on large area deposition [14]. The structural characteristics of NbOx films can vary significantly with different deposition techniques as well as with the process parameters. During magnetron sputtering, Nb2O5 continuously forms while there is adequate oxygen present, and the formation of other oxides is limited. Under limited oxygen supply, other oxides can also form. Therefore, the tuning of the precise oxygen flow with the right power density is a major challenge for synthesising specific stoichiometric niobium oxides.
There are only a limited number of research papers on the preparation of phase-pure crystalline NbO2 deposited by magnetron sputtering. Recently, Wang et al. [5] synthesized epitaxial NbO2 thin films on Al2O3 (0001) (sapphire) substrates via reactive bias target ion beam deposition to find a suitable range of conditions for the epitaxial growth of NbO2 films on the Al2O3 substrate. Wong et al. [15] have prepared epitaxial NbO2 thin films on Al2O3 (sapphire) substrate by reactive sputtering for the optical spectra analyses in their insulating state. However, there is no reported literature regarding the crystal formation and physical properties of phase-pure tetragonal NbO2 on Si (100) or quartz substrates. There have been various contradictions and inconsistencies in the information reported in the literature on various phases and polymorphs of NbOx [16] Graça et al. [17], Yoshimura et al. [18] and Venkataraj et al. [19] used different oxygen flow rates and found only Nb2O5 phase. They did not report on the formation of NbO2 and NbO in their films. Foroughi-Abari et al. [20] reported work on the formation of amorphous NbO, NbO2 and Nb2O5 in their films, identified by XPS depth profile measurements. There is limited information on crystalline NbO2 and Nb2O5 film formation by DC magnetron sputtering and their respective structural and physical properties.
Thus, the current paper first experimentally considers suitable deposition parameters for phase-pure NbO2 and Nb2O5 crystal structures and then evaluates their functional properties. The DC magnetron sputtering deposition technique is used for thin film preparation and the relevant properties are investigated systematically since the physical properties are highly dependent on the particular phase or mixture of phases. Overall, this study will expand our knowledge on the effects of substrate temperature and oxygen content in the gas (during deposition) on the composition, structural, mechanical and optical properties of the oxide films. The results are expected to advance their potential for applications in electronics and optoelectronics; as well as photocatalysis.
2 Material and method
2.1 Film deposition
NbO2 and Nb2O5 thin films were prepared by reactive magnetron sputtering using metallic Nb target (99.95% purity) that is 50.8 mm in diameter and 6.35 mm in thickness. Ar (99.99% purity) and O2 (99.99% purity) gases were used as a working and reactive gas respectively. Before deposition, the stainless-steel vacuum chamber was pumped down to a base pressure lower than 5 × 10−4 Pa. The deposition pressure was controlled by an adjustable throttle valve and the total chamber pressure for each deposition was kept at 1.07 Pa. The argon and oxygen flow rates were adjusted accordingly to obtain the desired O2 volume content of oxygen in the gas mixture that is, \( \frac{{{\text{O}}_{2} }}{{{\text{O}}_{2} + {\text{Ar}}}} \times 100\% . \) The distance between the target and substrate was 45 mm and substrate was rotated at a speed of 3 rpm to obtain uniform films. The deposition was performed at different temperatures with a constant DC power density of 9.87 W cm−2. The thin films were deposited on p-type single crystal Si (100) wafers and polished optical grade Quartz substrates for the measurement of optical properties. All substrates were rectangular in geometry.
2.2 Film characterization
The optical profilometer (Zygo Corporation) with a 50× Mirau objective was used to measure the mean roughness (Ra) and the thickness of the coatings. Average values for the thickness were calculated from three different measurements. Thickness values were also verified by an SEM cross section measurement. The crystallinity of the films was evaluated by X-ray diffraction using a Co Kα (λ = 1.78 Å) radiation source operating at 30 kV and 20 mA. The measured XRD patterns were matched with the Joint Committee on Powder Diffraction Standards (JCPDS) database, using X’Pert HighScore Plus software to identify the niobium oxide peaks. The peak positions and widths of two highest intensity first order Bragg diffraction peaks were used to estimate the crystalline size using the Scherrer formula (discussed below). Renishaw Invia Reflex Raman microscope with an Argon laser source at a wavelength 514 nm was used at room temperature to identify the oxide phases.
X-ray Photoelectron Spectroscopy (XPS) equipment with a 500 mm Rowland circle monochromated Al K-α (1486.6 eV) source and an accelerating voltage of 15 keV and an emission current of 15 mA was used to determine the core level lines of niobium and oxygen, relative atomic percentages of existing elements and their chemical states in the thin films. All the XPS measurements were collected using a Kratos (Manchester, UK) AXIS Supra system at the Saskatchewan Structural Sciences Centre (SSSC). A spot size of hybrid slot (300 × 700 μm) was used and Argon sputtering was done on each spot using a 4 keV source for 1 min to eliminate the surface contaminants.
Scanning electron microscopy (SEM) was employed to observe the surface and cross-sectional morphologies of the thin films.
Hardness and Young’s modulus were measured using a nanoindentation equipment (according to ISO 14577-1:2015 standard and Oliver–Pharr method) with a three-face pyramid Berkovich indenter. The indentation depth was kept within the optimum range so that the surface roughness and substrate hardness have no impact on the measured values [21]. The total stress in the thin films was assessed using the Stoney formula by measuring the sample curvature before and after film deposition in an optical profilometer [22]. The total stress was then calculated by the following formula [22].
where Es is Young’s Modulus and νs is the Poison ratio of the substrate (Si), ds and df are the thickness of the substrate and film respectively, R is the net change of curvature of the substrate before and after deposition [22]. The curvature measurement was done multiple times along two perpendicular directions, (parallel to the substrate edges) on the surface, and average values were taken for a standard sized sample.
The optical transmittance of the thin films was measured using a UV–IR spectrophotometer (Perkin Elmer Lambda 900 UV/VIS/NIR spectrometer) under normal beam incidence. The wavelength range of the spectrophotometer was 260 nm and 2500 nm. Optical constants were then extracted by fitting model calculations using a modified Swanepoel method, which is a well established technique [23,24,25].
3 Results and discussion
3.1 Deposition characteristics
Niobium is highly reactive with oxygen and a niobium oxide compound is usually formed on the metallic target surface during deposition, which reduces the deposition rate. To avoid target “poisoning”, a mixture of Ar and O2 gases with a suitably designed ratio is required. Moreover, the identification of elemental and compound mode sputtering region is also required to prepare a stoichiometric or fully oxidized niobium oxide phases. Figure 1a shows the effect of oxygen gas content on the cathode (target) voltage under the conditions of constant power density of 9.87 W cm−2 and total pressure of 1.08 Pa. Figure 1b shows the dependence of the overall rate of deposition on the oxygen gas content under the same conditions. The overall deposition rate is taken as the film thickness divided by the duration of deposition. As can be seen from Fig. 1a there is a slight hysteresis in the cathode potential vs. O2 gas content behavior for increasing and decreasing oxygen gas content, which is the biggest challenge for compound niobium oxide deposition by sputtering. It is clear that the cathode potential increases rapidly up to 5% of oxygen gas content, which corresponds to the oxidation of the target. For oxygen gas content ratios greater than 10%, there is only a slight increase in the cathode potential with oxygen gas content. This regime represents the slow oxidization of the Nb target. For 20% oxygen gas content (not shown in Fig. 1a), there is no change in the cathode voltage, indicating that the target is fully oxidised, and the deposition is in full oxidic mode. Thus, an oxide layer is formed on the surface of the Nb target that leads to a decrease in the sputtering yield and therefore the deposition rate decreases. The target converts to the metallic state again at lower oxygen gas content that shows the hysteresis in Fig. 1a.
Figure 1b illustrates the variation of the overall deposition rate as a function of oxygen gas content inside the chamber under identical deposition conditions (film thicknesses were measured by cross-section SEM images). The dependence of the overall deposition rate on the O2 gas content, as expected, correlates well with rate of change (the slope) of the cathode potential vs. O2 gas content behavior in Fig. 1a. The deposition rate increases and reaches a maximum at around 5% O2 gas content which is the “knee” region in the cathode potential versus O2 gas content curve in Fig. 1a; and then decreases sharply. When the O2 gas content is above 8.5%, the changes become insignificant, indicating that the samples are close to be oxidized fully, which is also in agreement with the cathode potential curve in Fig. 1a. Higher oxygen gas contents are linked with lower sputtering yield and lead to form thinner films. Among various deposition parameters, the oxygen gas content is the most important factor for optimising the chemistry of NbOx thin films and controlling the growth and morphology of sputtered films during magnetron sputtering. Nevertheless, the formation of specific NbOx also depends on the surface condition of Nb target, power density and vacuum pressure during deposition. Depending on the oxygen gas contents, the visual color and transmittance of the thin film changes, and these changes can be distinguished by the naked eye.
The transparency and visual color of the samples are observed in Fig. 2 (on Quartz substrates) with different oxygen gas content. Actual optical properties will be described in Sect. 3.5. The amorphous Nb2O5 film has very good transmittance due to large bandgap and negligible scattering. The NbO2 and Nb2O5 films are not as transparent to the naked eye as the amorphous film, due to the presence of some scattering. In the NbO2 formation range, the color of the films turns to bluish-black and black with decreasing oxygen gas content.
3.2 Structural properties
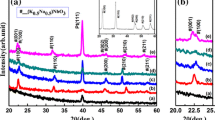
Niobium oxides can exhibit several different polymorphs depending on the deposition temperature or oxygen gas content during sputtering or different post deposition annealing temperatures. In our case, all deposited NbO2 and Nb2O5 films show tetragonal and orthorhombic structures, respectively, as confirmed by X-ray diffraction (XRD) results shown in Fig. 3a and b. Figure 3a illustrates the XRD pattern of the phase-pure body centered tetragonal (BCT) NbO2 (PDF code: 00-009-0235) with space group I41/a. The common diffraction angle of the NbO2 structures with highest intensities are 29.52°, 62.36° and 93.33°. Lower oxygen gas contents show few more diffraction peaks at 37.66°, 42.53°, 60.62°, 70.89° diffraction angles. Figure 3b shows the XRD pattern of the orthorhombic phase of Nb2O5 (PDF code: 01-071-0336) with space group Pbam. The major diffraction angles for the Nb2O5 structures are observed at 26.33°, 33.7°, 59.92°. The peaks with the highest intensity for the tetragonal NbO2 and orthorhombic Nb2O5 structures are in agreement with XRD Powder Diffraction Data. Mismatch of few lower intensity peaks might be due to the preferred orientation (note, both NbO2 and Nb2O5 crystals have numerous peaks and it is hardly possible to match all the peaks). XRD results point out that the variation of oxygen gas content does not significantly affect the crystal structures when the oxygen gas contents are in the formation range of NbO2 or Nb2O5. As can be seen from Fig. 3a that high purity BCT NbO2 is formed with 1.5–5% oxygen gas content. Figure 3b shows that films grown with 8.5% or higher oxygen gas content exhibit the orthorhombic Nb2O5 phase. The film grown under 6.5% oxygen gas content is primarily Nb2O5 but also has other phases such as NbO2, orthorhombic Nb12O29 and monoclinic Nb2O5. The structural difference of Nb2O5 at this oxygen gas content will be further discussed below in terms of XPS and SEM studies.
The deposition temperature and oxygen gas content are the most important experimental parameters for the formation of particular phases. It is found that the samples deposited below 700 °C, regardless of other deposition parameters, show only one very broad peak at 30° (= 2θ), indicating amorphous structure within the detection limit of XRD, results are not shown here. It is also observed that XRD peaks appear for samples deposited at 700 °C or above but the peaks are broadened, indicating nano scale size of the grains. The average crystallite size (D) is estimated (as an order of magnitude) from the Scherrer equation,
considering the two highest intensity Bragg diffraction peaks [26]. In Eq. (2), K is an empirical constant, so-called the Scherrer constant, λ is the x-ray wavelength and β is the full width at half maxima (FWHM) of the diffraction peak (in radians). The estimated crystalline sizes by Scherrer equation are summarised in Table 1. Only the first two XRD peaks were used and D averaged. The Scherrer equation itself does not include any strain in the film. However, the films are actually strained, and we can assume that the strain present in NbO2 and Nb2O5 is isotropic. In this case we can use the Williamson–Hall-isotropic strain model (WH-ISM) analysis for the total peak [26, 27].
where ε is the strain in the film. A plot is drawn taking 4sinθ along X-axis and βcosθ along Y-axis and the lattice strain values and the crystallite size values are respectively extracted from the slopes and the intercepts of the respective linear fits [26]. The projected values of the lattice strain and the crystallite size of NbO2 and Nb2O5 by WH-ISM method are summarized in Table 1. Note that K, the empirical constant was taken as 0.89, \( \lambda \) is wavelength of Co source (1.78 Å), θ is the Bragg angle (diffraction angle/2) in radians and β is the full width at half maximum (FWHM) in radians. XRD peaks are fitted by a Gaussian equation to calculate the FWHM (considering the instrumental peak broadening to be negligible).
Crystallite size calculated by the two analyses model are very close and the W–H analysis results are probably more accurate for crystallite size estimation [26]. The obtained crystallite size can be equal or larger than the actual crystallite size as the stacking faults, dislocations and instrumental errors were not considered in calculating the FWHM, which further broaden the diffraction peaks. In addition, it is well-known that Eqs. (2) and (3) have limitations and strictly not appropriate to use to estimate the average size of grains with anisotropic shape.
We have also carried out Raman spectroscopic studies to complement the XRD results. To date, studies on Raman spectra of NbOx thin films have been limited. Typical Raman spectra of nanostructured NbO2 and Nb2O5 thin films are illustrated in Fig. 4. Raman shifts at 163 cm−1, 247 cm−1, 342 cm−1, 401 cm−1, 631 cm−1 and 815 cm−1 are assigned to the Raman mode of tetragonal NbO2 [5, 11]. The Raman shifts at 126 cm−1, 228 cm−1, 310 cm−1, 685 cm−1 are for Nb2O5 [12] phase whereas the main vibration mode at ~ 685 cm−1 is assigned for low distorted NbO6 octahedral coordination and other modes at ~ 228 cm−1 and ~ 310 cm−1 are assigned for the tetragonal Nb2O5 phase [12]. These Raman results are in good agreement with the XRD data for the phase identifications. The oxygen gas content during deposition has only a small effect on the formation range for both NbO2 and Nb2O5, based on Raman spectra analysis. At about 5% oxygen gas content, there is a small and broad peak emerging at ~ 685 cm−1 that probably corresponds to Nb2O5. No notable shifts in the Raman spectrum are observed for Nb2O5, while the intensity difference is attributed to different film thickness.
The oxidation state and stoichiometric composition of the niobium oxides are further corroborated by XPS measurements. Survey scans with a wide range of binding energies are taken in multiple points before and after the removal of impurities from the film by Ar sputtering. Typical survey scans of niobium oxides after Ar sputtering and high resolution Nb 3d spectrum for 17.5, 6.5 and 1.5% oxygen gas contents are presented in Fig. 5a and b respectively. Inset of Fig. 5a presents the relative peak heights of Nb 4d and O 2p peaks. Binding energy in the range of − 3 eV to 580 eV is presented and the characteristic peaks are denoted as Nb 4d, O 2p, Nb 4p, Nb 4s, O 1s, C 1s, Nb 3p, Nb 3s and Nb 3d. Atomic concentrations can be calculated from the area and sensitivity factor of Nb 3d (Relative Sensitivity Factor, RSF = 2.92) and O 1 s (RSF = 0.78) peaks. The highly surface sensitive survey scan shows the O/Nb ratio over 2.8 before Ar sputtering that indicates the surface as over oxidised. After Ar cleaning the O/Nb ratio displays 1.85, 2.25 and 2.32 for 1.5, 6.5 and 17.5% oxygen gas contents in deposition respectively. Relative peak positions of Nb 3d line shape for these oxygen gas contents are shown in Fig. 5b. Even though there are not any significant discrepancies among the line shapes of these three samples, relative peak positions and intensities have slight differences. Peak position of the Nb 3d doublets can ascribe the thin films as NbO2 and Nb2O5 according to reference standards [28, 29]. Slight shift of binding energies are due to the Ar sputtering of the surface, which is consistent with Kang et al. [29]. For the same reason, the 3d doublets show comparable shapes for different oxygen gas contents. Negligible shoulder in Fig. 5b (ii) and (iii) can be attributed to Nb2O5, however, standard Nb 3d core level peak shape for NbO2 is still unresolved in the literature.
Difference of Δ(O − Nb) is more stable parameter for describing the electrical properties of niobium oxides as the variations of Δ(O − Nb) depend on O–Nb bonding [30]. Oxidation of niobium leads to alteration of Nb 3d binding energy to higher energy level and O 1s binding energy to lower energy level. Therefore, lesser difference of Δ(O − Nb) binding energy attributes to more oxidation of niobium. Energy differences of Δ(O 1s − Nb 3d) observed in Fig. 5a (i–iii) are 325 eV, 323.3 eV and 323 eV that can be assigned as NbO2, Nb2O5 and Nb2O5 respectively [30]. In addition, the valence band ratio, ratio of maximum height of Nb 4d to maximum height of O 2p, can also be utilised for the determination of Nb oxidation state. The inset of Fig. 5a presents the relative intensities of Nb 4d and O 2p peaks. Fully oxidized Nb has typically broader O 2p peak with very insignificant Nb 4d peak whereas the metallic Nb show sharp Nb 4d peak. Relative differences of valence band ratio in the inset of Fig. 5a signify the oxidation states of the thin films.
XPS analyses also carried out for the amorphous samples deposited at room temperature. The results confirm that the amorphous film with 8.5% oxygen gas content also shows Nb2O5 composition. (Results are not shown here.)
3.3 Roughness and surface morphology
Figure 6 illustrates the SEM cross section (Fig. 6b, e) and surface morphology (Fig. 6a, c, d, f) of crystalline NbO2 and Nb2O5 respectively. NbO2 shows nanoslice structure while Nb2O5 shows two different kinds of “spongy” structures (Fig. 6d and f). NbO2 with 1.5% oxygen gas content (in deposition) shows multiple orientations whereas NbO2 with 5% oxygen gas content have roughly two orientations which are in agreement with XRD peaks. The surface morphology of Nb2O5 has different structures as shown in Fig. 6d and f for 17.5% and 6.5% oxygen gas contents respectively. For 17.5% oxygen gas content, the growth of crystals is visible (reminiscent to blooming flowers) whereas for 6.5% oxygen gas content flakes are fully-fledged in different directions. Thus, the surface structure transforms from nanoslices to flake-like structure for increasing oxygen gas content.
Nanoslices are unique nanostructures of the niobium oxide thin films having superior electric properties and low surface energies which have been reported by two researchers earlier [4, 31]. Having an approximate length of 100–1000 nm, these nanoslices can be aligned or randomly accumulated, where the random accumulation has been attributed to thermal growth [4]. The exact mechanism that leads to alignment or random orientation has not been resolved, but one would expect the surface diffusion velocities of atoms on the substrate (caused by disproportionate kinetic energies during sputtering) to play a role. In contrast, Nb2O5 shows flake-like surface morphology as in Fig. 6d–f which arises from crystallization at a higher deposition temperature compared with other work in the literature. These observed structures are fairly different from those deposited crystalline structures reported by other researchers [32, 33]. SEM analyses of these thin films are done at different spots and it is found that the structure of the films is uniform throughout the sample surface.
Figure 7 shows the effects of oxygen gas contents, deposition temperature and film thickness on the surface roughness of niobium oxide films as measured by the optical profilometer. It can be seen that the films are relatively smooth with a roughness of ~ 1 nm for deposition at 650 °C. Oxygen gas content has negligible effect for amorphous thin films which is consistent with results reported by Foroughi-Abari et al. [20]. However, there is an observable impact of oxygen gas content on the surface roughness for crystalline films with a higher thickness (above 2 µm). It can be concluded that higher deposition rate, higher deposition pressure, greater thickness and moreover crystal growth at higher temperature lead to increased surface roughness.
3.4 Mechanical properties
3.4.1 Total stress
Stress is generated in a thin film during the deposition; the substrate is thick comparatively and has negligible stress. Excessive stress can form cracking, deformation and delamination of film that makes the device practically unusable. Stress can be measured in several ways. In our case, we have used a straightforward technique to measure the surface curvature by an optical profilometer. The total residual stress of thin films can be determined by the Stonye equation, as shown in Eq. (1) given in Sect. 2.2. The amount of curvature in the Si wafer is measured before and after deposition. The beam bending formula is the basic principle of this method in which the deposition of the film modifies the substrate curvature. The whole sample is scanned by using the stitching mode of the profilometer, that is, using the curvature measurements before and after deposition. The curvature measurements are taken on a Si substrate horizontally and vertically before and after the thin film’s deposition at several positions. Measurements are taken on the same spots before and after depositions to reduce the errors. Two differently shaped rectangular substrates were considered including (6 mm × 4 mm) and (4 mm × 40 mm) for each of the NbO2 and Nb2O5 thin films. Slight substrate offsets in the substrate positions can create a large error therefore, substrate positions and focusing are done very carefully. The stress behaviour of sputtered thin films usually depends on the mobility of the depositing atoms and becomes compressive due to densification of impinged atoms. The total stress of metal oxide thin films does not solely depend on oxygen gas content, and it is also influenced by film density, surface roughness and film thickness. According to previously reported data, the average stress of niobium oxides is usually compressive and it is below 480 MPa [34]. However, the stress can also be tensile for films annealed at high temperature [35]. In our case, the stress for both NbO2 and Nb2O5 are compressive, as shown in Table 2, and the values are 107 ± 15 MPa to 385 ± 20 MPa respectively, where the stated errors have been determined from multiple measurements. At higher deposition temperatures, the residual stress becomes lower. Nix et al. [36] have shown that the average stress decreases with increasing grain size which is supportive in our case, comparing the grain sizes of NbO2 and Nb2O5 films.
3.4.2 Hardness and Young’s modulus
Nanohardness and Young’s modulus were measured by using the nanoindentation technique under low loads. The nanohardness of NbO2 thin films, as shown in Table 2, is 4.8–6.0 GPa. There is no readily available direct comparison with any previous work on NbO2 thin films. The nanohardness of Nb2O5 thin films, as shown in Table 2, is 5.8–7.9 GPa, which is in agreement with the reported nanohardness of Nb2O5 thin films [35]. However, Nb2O5 with 21.5% oxygen gas contents show very high hardness of 13 GPa which is very close to that of bulk Nb2O5. Although, in general, it is difficult to correlate the oxygen gas content with mechanical properties such as hardness and Young’s modulus, nonetheless, the higher oxygen gas content during deposition leads to more homogenous surface and better mechanical properties. The measurements are taken by the nanoindentation using low loads (from 3 to 6 mN) to avoid the influence of the substrate. Due to thinness of the coatings, the nanoindenter penetration depths in this work were below 15–20% of the thickness, even though widely used rule of thumb restricts the penetration depth to less than 10% of film thickness for soft films [21]. In this work, the error due to the substrate is negligible as all of the samples have almost similar thicknesses and all have been deposited on the same substrate, and the surface roughness is negligibly small compared with the thickness. Different surface topography and roughness can also affect the hardness of thin films. The highest hardness (13 GPa) was exhibited by the Nb2O5 thin film deposited under an oxygen gas content of 21.5%. For this oxygen gas content, the film structure is still orthorhombic Nb2O5, and the film morphology is more densely packed, and hence shows the higher hardness.
3.5 Optical properties
Refractive index and absorption coefficient as well as the light scattering coefficient of niobium oxide thin films were determined by fitting model calculations to the experimental transmission spectrum data obtained on thin films deposited on quartz substrates, as shown in Fig. 8. In this calculation, the modified Swanepoel method has been used [24, 25, 37]. The following description is the core of the method and modifications required in this particular case. According to Swanepoel technique, the spectral dependence of optical transmittance T(λ) of a non-uniform thin film with average thickness d and thickness variation over the field of illumination Δd may be expressed as,
where λ is a light wavelength, φ = 4πnd/λ, φ1 = 4πn(d − Δd)/λ and φ2 = 4πn(d + Δd)/λ, n(λ) is the wavelength dependent refractive index. The functions A(λ), B(λ), C(λ) and D(λ) are defined as.
and
where s is the refractive index of substrate which is assumed to be independent of λ, and X(λ) as,
where α(λ) is an optical absorption coefficient.
Optical transmittance of niobium oxide films. Points are experimental data. Lines are results of calculations using the model described in paper. No fit could be achieved for sample c-NbO2 (1.5). Explanation of notation: “c-” stands for crystalline and “a-” for amorphous samples, number in brackets are oxygen gas contents (in %) (see also Table 3)
However, some of the niobium oxide films have been found to be structurally non-uniform that leads to significant light scattering. Formally, this effect may be taken into account by an attenuation coefficient that includes both absorption and scattering of light, i.e. αext = αabs + αscat and thus modifying X(λ) as,
To fit Eq. (4) to experimental data, we need to choose the appropriate values of d and Δd as well as appropriate functions n(λ), αabs(λ) and αscat(λ) for the refractive index, absorption and scattering coefficients, respectively. Figure 8 shows that in most cases this problem is solvable within reasonable values for the optical parameters that are discussed below.
There are many possible approximations for n(λ). Among the most popular are the Sellmeier or Cauchy dispersion formulae [25, 38]. The latter worked the best in our case in a truncated form as,
where n0, C2 and C4 are numerical adjustable parameters. Obviously, n0 is the limiting refractive index as λ → ∞.
The general theory of light scattering on spherical particles with size L was developed by Mie et al. [39]. For small particles with L < λ, the scattering is reduced to the Rayleigh λ−4 law i.e. \(\alpha_{\text{scat}}(\lambda) \propto \lambda^{-4}\) [40]. In the opposite case \( L \gg \lambda \), the approximation of geometrical optics is valid and scattering does not depend on wavelength, i.e. \(\alpha_{\text{scat}}(\lambda) \propto \lambda^{0}\). Based on this extreme case, in the present paper, we use a generalized approximation.
with as and m being formal adjustable parameters. Parameter m varies from 0 to 4, pointing on the size of structural deformities in niobium oxide films that are scattering the light.
The final choice is the function Xabs(λ) = exp [− αabs(λ)d], which is made to be as flexible as possible. First, we notice that Xabs(λ) = 0 at small λ corresponding to strong absorption. Oppositely, Xabs(λ) = 1 at large λ, manifesting the absence of absorption. In the present approach we try Xabs(λ) as a smooth function with smooth derivatives passing through four nodes with coordinates (λ0, 1), (λ1, x1), (λ2, x2) and (λ3, 0). The parameters x1 and x2 are chosen manually around 0.8 and 0.2, respectively. The wavelengths λ0 and λ4 are chosen manually to guarantee the absence of optical absorption above λ0 and strong absorption below λ4. Overall, the model has 9 adjustable parameters d, Δd, n0, C2, C4, αs, n, λ2, and λ4 which in most cases (with moderate light scattering) allow a good fit to experimental data as illustrated in Fig. 8.
When the fitting is done, aabs(λ) or aabs(hν) is calculated. The dependence of the absorption coefficient as a function of photon energy is presented in Fig. 9. Further derivation of the optical band gap uses different approaches for crystalline and amorphous materials.
The dependence of optical absorption coefficient in niobium oxide films versus photon energy. a Semilogarithmic plots of the absorption coefficient versus hν. b Square root of the absorption coefficient versus hν. c Sokolov and Tauc pots for amorphous thin films. The notation is explained in Fig. 8 caption
Figure 9b shows the determination of optical gap Eg in crystalline Nb2O5 which is known to be an indirect bandgap semiconductor where the optical absorption is accompanied with absorption or emission of phonons [41]. As a result the optical absorption may be represented as a sum of two processes corresponding to phonon absorption and phonon emission [25].
and
here Eg is optical bandgap, A is a constant, hϑ is phonon energy, fBE(hϑ) is the Bose–Einstein distribution function at the phonon energy hϑ, i.e. fBE(hϑ) = [(exp(hϑ/kBT) − 1]−1, where kB is the Boltzmann constant and T is the temperature. The sum of (13) and (14) will give two straight lines in α1/2 versus hν coordinates with onsets at (Eg − hϑ) and (Eg + hϑ) as shown in Fig. 9b. Eg is obviously the average between these two onsets. The values of Eg are close 2.30 eV and are shown in Table 3.
The values of so-called optical bandgap for amorphous solids may substantially vary depending on the definition adopted. The most popular method is the Tauc rule [42] which relates the optical absorption α and photon energy hν as,
where BTauc is a constant, independent of the photon energy, and ETauc is a constant called Tauc bandgap. The best fit using the Tauc relation is shown in Fig. 9c and the value of ETauc = 3.53 eV is obtained for a-Nb2O5 film. Other expressions α versus hν are known as the Cody and Sokolov [43] relationships.
Both of these give better results than the Tauc relation, and the Sokolov expression in Eq. (17) gives the best near-perfect fit which is shown in Fig. 9c with the value of ESokolov = 3.28 eV.
The oxygen gas content in the reaction chamber during deposition seems to play the major role in properties of niobium oxide films. Films with a higher oxygen gas content are prone to be higher oxides Nb2O5 while lower oxygen gas content leads to the NbO2 formation. Films with a higher oxygen gas content seem to be more optically uniform with small or undetectable light scattering. Figure 8 shows reasonable agreement with model predictions for all samples excluding the sample with the lowest oxygen gas contents (1.5%) where the scattering was so severe that the model failed completely. However, even in the best samples, the power m in the scattering expression [αscat(λ) = asλ−m] was substantially lower than 4, pointing to scattering on objects with typical sizes larger than the wavelength. It may be connected with the non-uniformity of structure.
At higher oxygen gas content, Nb2O5 is predominantly formed. Once oxygen gas content is sufficiently high, there is not much difference in the optical properties of the films with different oxygen gas content Fig. 9a, b and Fig. 10). Amorphization of Nb2O5 at 8.5% of oxygen gas content leads to the theoretically predicted increase of the optical bandgap (Eg) and a reduction of n0 [44].
Refractive index of niobium oxide films. The notation is explained in Fig. 8 caption
According to XRD and Raman scattering data, the reduction of oxygen gas content to 3.5% and below leads to the prevalent formation of NbO2. However, information on NbO2 is scarce, and it seems to have an indirect Eg around 0.9 eV and a direct Eg around 1.3 eV [45] so that that is well below our results shown in Table 3.
In our opinion this disagreement may be related to the nonuniformity of the film which may consist of a mixture of NbO2 and Nb2O5 oxides with Nb2O5 forming “transparency windows” that lead to the measured optical properties. This model would explain also the strong scattering of light observed in low oxygen content films. It is worth noting that similar scenario was also observed by Foroughi-Abari et al. [20].
4 Conclusions
The formation of niobium oxide thin films by magnetron DC sputtering was examined with different oxygen gas content in the chamber. The structure and properties of the films were investigated as a function of the oxygen gas content. It is observed that the deposition rate decreases significantly in the NbO2 to Nb2O5 transition region. The XRD and Raman spectroscopy and XPS measurements confirmed that tetragonal NbO2 thin films always form for specific oxygen gas content (< 5%) and orthorhombic Nb2O5 films form for a wide range of oxygen gas content (> 6.5%). The SEM studies illustrate two distinct structures of NbO2 and Nb2O5. The films exhibit compressive residual stress, which has been measured. The mechanical and optical properties of the films have been investigated systematically. The nanohardness value of NbO2 films is approximately 6 GPa, and the Young’s modulus is 150 GPa. The Nb2O5 films exhibit a nanohardness 5.8–13 GPa and a Young’s modulus of 137–161 GPa. The optical properties were extracted from the transmission spectra by using a modified Swanepoel technique and introducing a degree of scattering along with absorption. The refractive index dispersion curves have been extracted for all the films. The refractive index at a photon energy of 2 eV (620 nm) is approximately 2.38 for NbO2 and 2.21 for NbO5 films. A bandgap energy of 3.42 eV has been determined for polycrystalline Nb2O5 films, and 3.50 for amorphous NbO5 films. The bandgap determination for NbO2 films is less reliable due to light scattering, but a value of 3.5 eV was estimated. In the latter case, the sample may not be homogeneous.
References
J.K. Hulm, C.K. Jones, R.A. Hein, J.W. Gibson, Superconductivity in the TiO and NbO systems. J. Low Temp. Phys. 7, 291–307 (1972). https://doi.org/10.1007/BF00660068
R.F. Janninck, D.H. Whitmore, Electrical conductivity and thermoelectric power of niobium dioxide. J. Phys. Chem. Solids 27, 1183–1187 (1966). https://doi.org/10.1016/0022-3697(66)90094-1
C. Funck, S. Menzel, N. Aslam, H. Zhang, A. Hardtdegen, R. Waser, S. Hoffmann-Eifert, Multidimensional simulation of threshold switching in NbO2 based on an electric field triggered thermal runaway model. Adv. Electron. Mater. 2(7), 1600169 (2016). https://doi.org/10.1002/aelm.201600169
Y. Zhao, Z. Zhang, Y. Lin, Optical and dielectric properties of a nanostructured NbO2 thin film prepared by thermal oxidation. J. Phys. D 37, 3392–3395 (2004). https://doi.org/10.1088/0022-3727/37/24/006
Y. Wang, R.B. Comes, S. Kittiwatanakul, S.A. Wolf, J. Lu, Epitaxial niobium dioxide thin films by reactive-biased target ion beam deposition. J. Vac. Sci. Technol. A 33, 021516 (2015). https://doi.org/10.1116/1.4906143
M. Vinnichenko, A. Rogozin, D. Grambole, F. Munnik, A. Kolitsch, W. Möller, O. Stenzel, S. Wilbrandt, A. Chuvilin, U. Kaiser, Highly dense amorphous Nb2O5 films with closed nanosized pores. Appl. Phys. Lett. 95, 081904 (2009). https://doi.org/10.1063/1.3212731
M.A. Aegerter, Sol-gel niobium pentoxide: a promising material for electrochromic coatings, batteries, nanocrystalline solar cells and catalysis. Sol. Energy Mater. Sol. Cells 68(3–4), 401–422 (2001). https://doi.org/10.1016/S0927-0248(00)00372-X
M.F. Pillis, G.A. Geribola, G. Scheidt, E.G. de Araújo, M.C.L. de Oliveira, R.A. Antunes, Corrosion of thin, magnetron sputtered Nb2O5 films. Corros. Sci. 102, 317–325 (2016). https://doi.org/10.1016/j.corsci.2015.10.023
C. Nico, M.R.N. Soares, J. Rodrigues, M. Matos, R. Monteiro, M.P.F. Graça, M.A. Valente, F.M. Costa, T. Monteiro, Sintered NbO powders for electronic device applications. J. Phys. Chem. C 115, 4879–4886 (2011). https://doi.org/10.1021/jp110672u
H. Kupfer, T. Flügel, F. Richter, P. Schlott, Intrinsic stress in dielectric thin films for micromechanical components. Surf. Coatings Technol. 116–119, 116–120 (1999). https://doi.org/10.1016/S0257-8972(99)00114-0
S. Lee, H. Yoon, I. Yoon, B. Kim, Single crystalline NbO2 nanowire synthesis by chemical vapor transport method. Bull. Korean Chem. Soc. 33, 839–842 (2012). https://doi.org/10.5012/Bkcs.2012.33.3.839
M.P.F. Graça, A. Meireles, C. Nico, M.A. Valente, Nb2O5 nanosize powders prepared by sol–gel—structure, morphology and dielectric properties. J. Alloys Compds. 553, 177–182 (2013). https://doi.org/10.1016/j.jallcom.2012.11.128
J.P. Masse, H. Szymanowski, O. Zabeida, A. Amassian, J.E. Klemberg-Sapieha, L. Martinu, Stability and effect of annealing on the optical properties of plasma-deposited Ta2O5 and Nb2O5 films. Thin Solid Films 515, 1674–1682 (2006). https://doi.org/10.1016/j.tsf.2006.05.047
G. Bräuer, B. Szyszka, M. Vergöhl, R. Bandorf, Magnetron sputtering—milestones of 30 years. Vacuum 84(12), 1354–1359 (2010). https://doi.org/10.1016/j.vacuum.2009.12.014
F.J. Wong, N. Hong, S. Ramanathan, Orbital splitting and optical conductivity of the insulating state of NbO2. Phys. Rev. B 90, 1–8 (2014). https://doi.org/10.1103/physrevb.90.115135
C. Nico, T. Monteiro, M.P.F. Graça, Niobium oxides and niobates physical properties: review and prospects. Prog. Mater Sci. 80, 1–37 (2016). https://doi.org/10.1016/j.pmatsci.2016.02.001
M.P.F. Graça, M. Saraiva, F.N.A. Freire, M.A. Valente, L.C. Costa, Electrical analysis of niobium oxide thin films. Thin Solid Films 585, 95–99 (2015). https://doi.org/10.1016/j.tsf.2015.02.047
K. Yoshimura, T. Miki, S. Iwama, S. Tanemura, Characterization of niobium oxide electrochromic thin films prepared by reactive d.c. magnetron sputtering. Thin Solid Films 281–282, 235–238 (1996). https://doi.org/10.1016/0040-6090(96)08640-3
S. Venkataraj, R. Drese, O. Kappertz, R. Jayavel, M. Wuttig, Characterization of niobium oxide films prepared by reactive DC magnetron sputtering. Phys. Status Solidi Appl. Res. 188, 1047–1058 (2001). https://doi.org/10.1002/1521-396X(200112)188:3%3c1047:AID-PSSA1047%3e3.0.CO;2-J
A. Foroughi-Abari, K.C. Cadien, Growth, structure and properties of sputtered niobium oxide thin films. Thin Solid Films 519(10), 3068–3073 (2011). https://doi.org/10.1016/j.tsf.2010.12.036
D.E. Kramer, A.A. Volinsky, N.R. Moody, W.W. Gerberich, Substrate effects on indentation plastic zone development in thin soft films. J. Mater. Res. 16(11), 3150–3157 (2001). https://doi.org/10.1557/JMR.2001.0434
X. Feng, Y. Huang, A.J. Rosakis, On the stoney formula for a thin film/substrate system with nonuniform substrate thickness. J. Appl. Mech. 74(6), 1276–1281 (2007). https://doi.org/10.1115/1.2745392
R. Swanepoel, Determination of the thickness and optical constants of amorphous silicon. J. Phys. E 16(12), 1214–1222 (1983). https://doi.org/10.1088/0022-3735/16/12/023
R. Swanepoel, Determination of surface roughness and optical constants of inhomogeneous amorphous silicon films. J. Phys. E 17(10), 896 (1984). https://doi.org/10.1088/0022-3735/17/10/023
J. Mistrik, S. Kasap, H.E. Ruda, C. Koughia, J. Singh, Optical properties of electronic materials, in Springer Handbook of Electronic and Photonic Materials, 2nd edn., ed. by S. Kasap, P. Capper (Springer, Heidelberg, 2017), pp. 47–83. https://doi.org/10.1007/978-3-319-48933-9. Chapter 3
P. Muhammed Shafi, A. Chandra Bose, Impact of crystalline defects and size on X-ray line broadening. AIP Adv. 5(5), 057137 (2015). https://doi.org/10.1063/1.4921452
G.K. Williamson, W.H. Hall, X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1(1), 22–31 (1953). https://doi.org/10.1016/0001-6160(53)90006-6
S. Kim, J. Park, J. Woo, C. Cho, W. Lee, J. Shin, G. Choi, S. Park, D. Lee, B.H. Lee, H. Hwang, Threshold-switching characteristics of a nanothin-NbO2-layer- based Pt/NbO2/Pt stack for use in cross-point-type resistive memories. Microelectron. Eng. 107, 33–36 (2013). https://doi.org/10.1016/j.mee.2013.02.084
M. Kang, S. Yu, J. Son, Voltage-induced insulator-to-metal transition of hydrogen-treated NbO2 thin films. J. Phys. D 48(9), 095301 (2015). https://doi.org/10.1088/0022-3727/48/9/095301
V.V. Atuchin, I.E. Kalabin, V.G. Kesler, N.V. Pervukhina, Nb 3d and O 1s core levels and chemical bonding in niobates. J. Electron Spectrosc. Relat. Phenom. 142, 129–134 (2005). https://doi.org/10.1016/j.elspec.2004.10.003
D. Music, R.W. Geyer, Theoretical and experimental study of NbO2 nanoslice formation. J. Phys. D 48(30), 305302 (2015). https://doi.org/10.1088/0022-3727/48/30/305302
R. Ghosh, M.K. Brennaman, T. Uher, M.-R. Ok, E.T. Samulski, L.E. McNeil, T.J. Meyer, R. Lopez, Nanoforest Nb2O5 photoanodes for dye-sensitized solar cells by pulsed laser deposition. ACS Appl. Mater. Interfaces 3(10), 3929–3935 (2011). https://doi.org/10.1021/am200805x
S.A. O’Neill, I.P. Parkin, R.J.H. Clark, A. Mills, N. Elliott, Atmospheric pressure chemical vapour deposition of thin films of Nb2O5 on glass. J. Mater. Chem. 13(12), 2952–2956 (2003). https://doi.org/10.1039/B307768n
C.C. Lee, C.L. Tien, J.C. Hsu, Internal stress and optical properties of Nb2O5 thin films deposited by ion-beam sputtering. Appl. Opt. 41(10), 2043–2047 (2002). https://doi.org/10.1364/AO.41.002043
E. Cetinörgü-Goldenberg, J.E. Klemberg-Sapieha, L. Martinu, Effect of postdeposition annealing on the structure, composition, and the mechanical and optical characteristics of niobium and tantalum oxide films. Appl. Opt. 51(27), 6498–6507 (2012). https://doi.org/10.1364/AO.51.006498
W.D. Nix, B.M. Clemens, Crystallite coalescence: a mechanism for intrinsic tensile stresses in thin films. J. Mater. Res. 14(8), 3467–3473 (1999). https://doi.org/10.1557/JMR.1999.0468
S.O. Kasap, W.C. Tan, J. Singh, A.K. Ray, Fundamental optical properties of materials. In J. Singh (ed.) Optical Properties of Condensed Matter and Applications, 2nd edn (Wiley, Chichester, 2019)
W. Sellener, Zur Erkarung der abnormen Farbenfolge im Spectrum einiger. Substanzen. Ann. Phys. Chem. 219, 272–282 (1871). https://doi.org/10.1002/andp.18712190612
G. Mie, Contributions to the optics of turbid media, especially colloidal metal solutions. Ann. Phys. 330(3), 377–445 (1908). https://doi.org/10.1002/andp.19083300302
S.O. Kasap, Optoelectronics and Photonics: Principles & Practices, 2nd edn. (Pearson, Upper Saddle River, 2013)
G. Ramírez, S.E. Rodil, S. Muhl, D. Turcio-Ortega, J.J. Olaya, M. Rivera, E. Camps, L. Escobar-Alarcón, Amorphous niobium oxide thin films. J. Non-Cryst. Solids 356(50–51), 2714–2721 (2010). https://doi.org/10.1016/j.jnoncrysol.2010.09.073
J. Tauc, Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 3(1), 37–46 (1968). https://doi.org/10.1016/0025-5408(68)90023-8
A.P. Sokolov, A.P. Shebanin, O.A. Golikova, M.M. Mezdrogina, Structural disorder and optical gap fluctuations in amorphous silicon. J. Phys. 3(49), 9887–9894 (1991). https://doi.org/10.1088/0953-8984/3/49/005
N.F. Mott, E.A. Davis, Electronic processes in non-crystalline materials (Oxford University Press, New York, 1971)
A. O’Hara, T.N. Nunley, A.B. Posadas, S. Zollner, A.A. Demkov, Electronic and optical properties of NbO2. J. Appl. Phys. 116(21), 213705 (2014). https://doi.org/10.1063/1.4903067
Acknowledgements
This project was made possible by a research grant from Cisco Systems and a CRD from NSERC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hossain, N., Günes, O., Zhang, C. et al. Structural and physical properties of NbO2 and Nb2O5 thin films prepared by magnetron sputtering. J Mater Sci: Mater Electron 30, 9822–9835 (2019). https://doi.org/10.1007/s10854-019-01319-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01319-8